Abstract
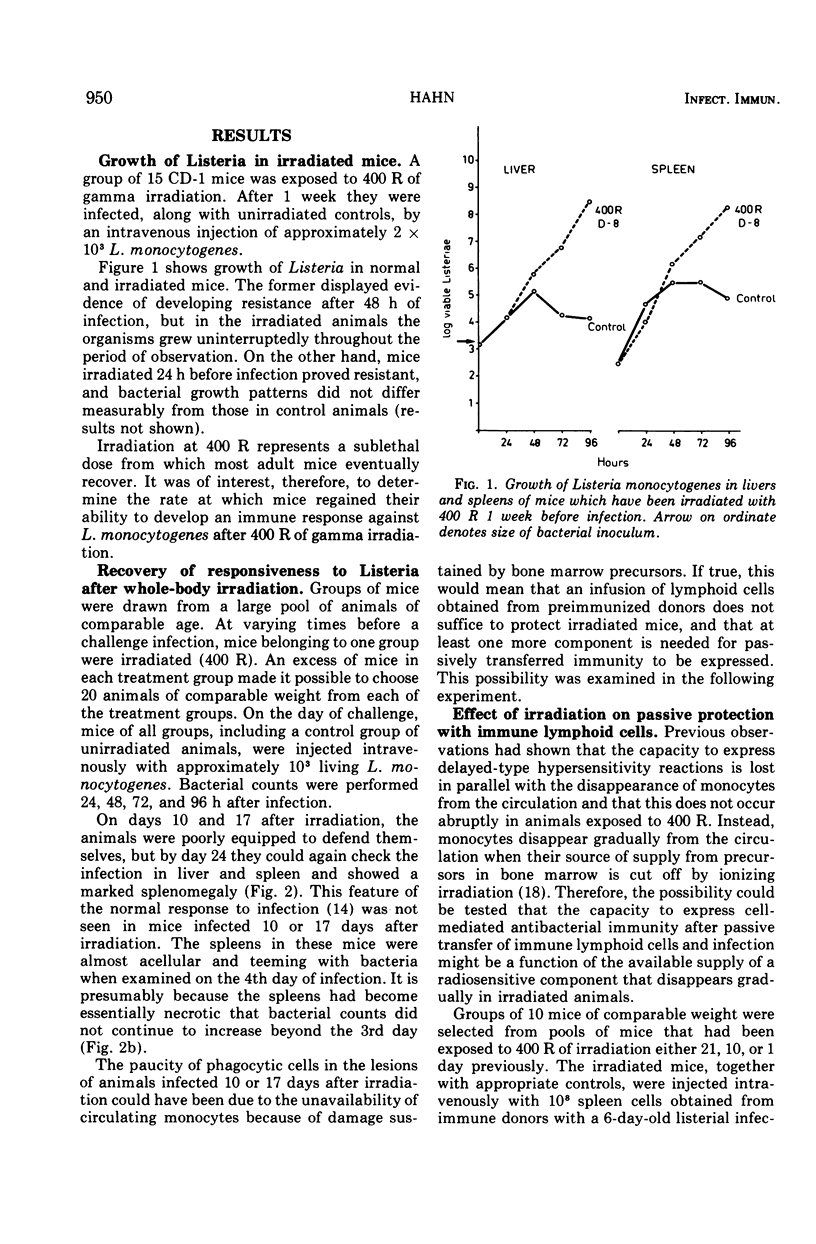
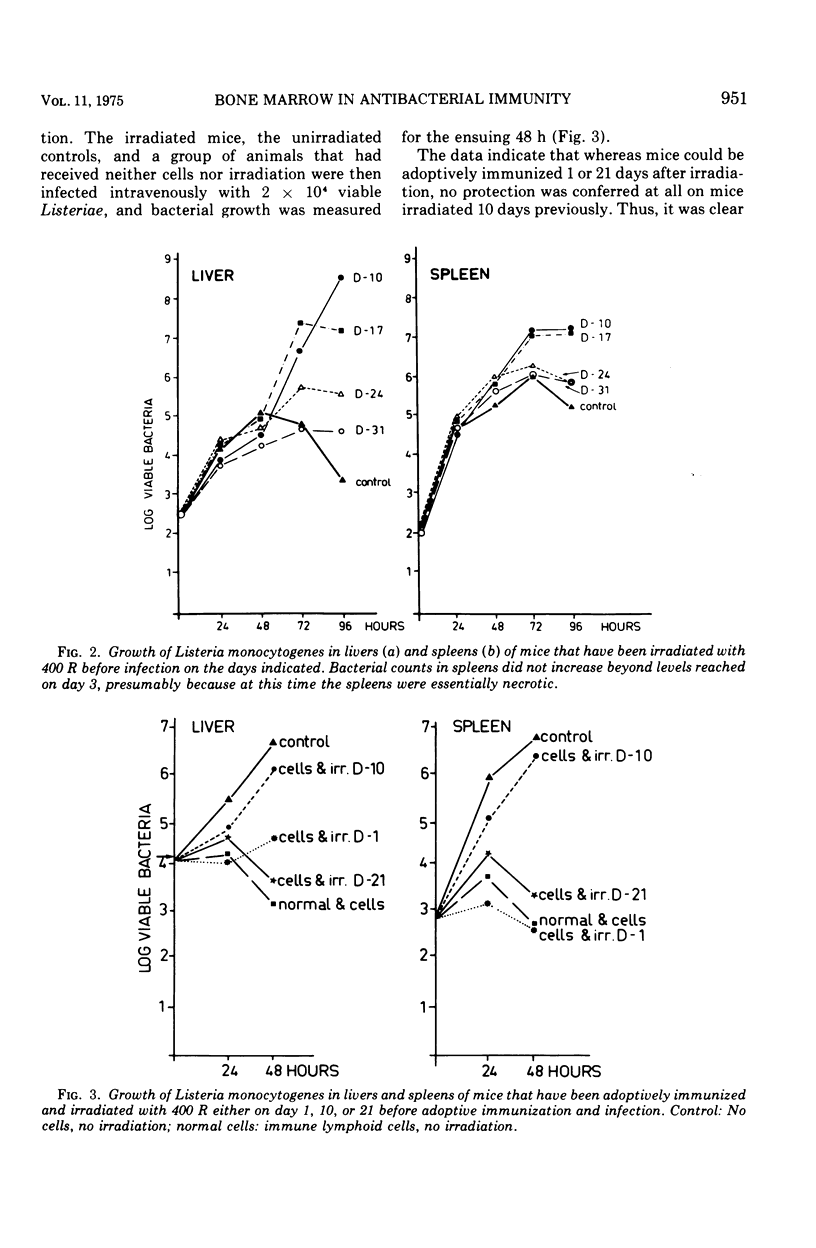
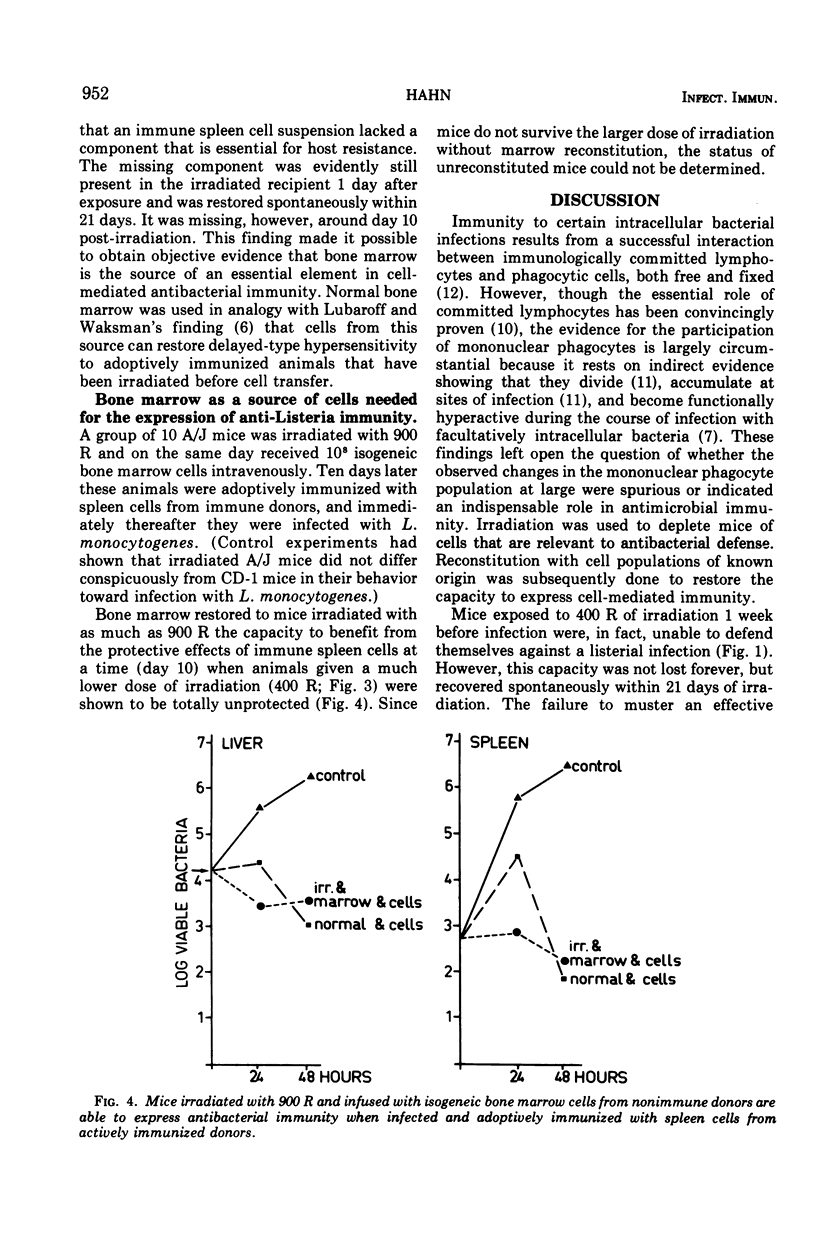
Mice were X irradiated with 400 R and 1 week post-irradiation were found to be unable to develop antilisterial immunity after active or passive immunization with immunologically committed spleen lymphocytes from Listeria-immune donors. This consequence of irradiation disappeared spontaneously within 21 days of exposure to X rays. Mice irradiated with as much as 900 R could be passively protected by immunologically committed lymphoid cells from Listeria-immune donors 10 days after irradiation if they had been given normal bone marrow cells on the day or irradiation. It is concluded that, in addition to immunologically committed lymphocytes, a second cellular component is needed for the expression of antibacterial immunity. This second component is bone marrow derived.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Campbell P. A., Martens B. L., Cooper H. R., McClatchy J. K. Requirement for bone marrow-derived cells in resistance to Listeria. J Immunol. 1974 Apr;112(4):1407–1414. [PubMed] [Google Scholar]
- Feldman J. D., Unanue E. R. Role of macrophages in delayed hypersensitivity. II. Effects of anti-macrophage antibody. Cell Immunol. 1971 Jun;2(3):275–282. doi: 10.1016/0008-8749(71)90047-5. [DOI] [PubMed] [Google Scholar]
- Kambara T., Chandrasekhar S., Dannenberg A. M., Jr, Meyer O. T. Radiation, infection, and macrophage function. I. Effects of whole body radiation on dermal tuberculous lesions in rabbits: development, histology, and histochemistry. J Reticuloendothel Soc. 1970 Jan;7(1):53–78. [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane F. C., Unanue E. R. Requirement of thymus (T) lymphocytes for resistance to listeriosis. J Exp Med. 1972 May 1;135(5):1104–1112. doi: 10.1084/jem.135.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubaroff D. M., Waksman B. H. Bone marrow as a source of cells in reactions of cellular hypersensitivity. I. Passive transfer of tuberculin sensitivity in syngeneic systems. J Exp Med. 1968 Dec 1;128(6):1425–1435. doi: 10.1084/jem.128.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B., Hill W. C. The effect of anti-lymphocyte globulin on cell-mediated reistance to infection. J Exp Med. 1969 May 1;129(5):993–1012. doi: 10.1084/jem.129.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackaness G. B. The monocyte in cellular immunity. Semin Hematol. 1970 Apr;7(2):172–184. [PubMed] [Google Scholar]
- North R. J. Cellular mediators of anti-Listeria immunity as an enlarged population of short lived, replicating T cells. Kinetics of their production. J Exp Med. 1973 Aug 1;138(2):342–355. doi: 10.1084/jem.138.2.342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. Importance of thymus-derived lymphocytes in cell-mediated immunity to infection. Cell Immunol. 1973 Apr;7(1):166–176. doi: 10.1016/0008-8749(73)90193-7. [DOI] [PubMed] [Google Scholar]
- North R. J. Methyl green-pyronin for staining autoradiographs of hydroxyethyl methacrylate-embedded lymphoid tissue. Stain Technol. 1971 Mar;46(2):59–62. doi: 10.3109/10520297109067823. [DOI] [PubMed] [Google Scholar]
- North R. J. The mitotic potential of fixed phagocytes in the liver as revealed during the development of cellular immunity. J Exp Med. 1969 Aug 1;130(2):315–326. doi: 10.1084/jem.130.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. J. The relative importance of blood monocytes and fixed macrophages to the expression of cell-mediated immunity to infection. J Exp Med. 1970 Sep 1;132(3):521–534. doi: 10.1084/jem.132.3.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the passive transfer of cell-mediated immunity. J Exp Med. 1969 Jul 1;130(1):17–30. doi: 10.1084/jem.130.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathy S. P., Mackaness G. B. The effect of cytotoxic agents on the primary immune response to Listeria monocytogenes. J Exp Med. 1969 Jul 1;130(1):1–16. doi: 10.1084/jem.130.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A., Collins F. M. Recovery of delayed-type hypersensitivity in mice following suppressive doses of X-radiation. J Immunol. 1968 Nov;101(5):846–859. [PubMed] [Google Scholar]
- Volkman A., Collins F. M. The restorative effect of peritoneal macrophages on delayed hypersensitivity following ionizing radiation. Cell Immunol. 1971 Dec;2(6):552–566. doi: 10.1016/0008-8749(71)90004-9. [DOI] [PubMed] [Google Scholar]


