Abstract
Innate immunity confers an immediate nonspecific mechanism of microbial recognition through germ line-encoded pattern recognition receptors (PRRs). Of these, Toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs) have shaped our current understanding of innate regulation of adaptive immunity. It is now recognized that PRRs are paramount in instructing an appropriate adaptive immune response. Their ligands have been the focus of adjuvant research with the goal of generating modern vaccine combinations tailored to specific pathogens. In this review we will highlight the recent findings in the field of adjuvant research with a particular focus on the potential of TLR and NLR ligands as adjuvants and their influence on adaptive immune responses.
Keywords: NOD1, NOD2, NLRP3, TLR4 alum
The term “adjuvant” originates from the Latin word adjuvare meaning “to help,” and is assigned to compounds that enhance the host’s humoral and/or cellular immune response toward a coadministered antigen. Historically, traditional vaccination strategies have relied on live attenuated pathogens, whole inactivated organisms, or inactivated bacterial toxins. Due to their design, traditional vaccines possess inherent adjuvant activity as they contain bacterial components and impurities, which correlate with high immunogenicity. However, attenuated and killed vaccines have also been associated with occasional undesired reactogenicity (1). To avoid nonspecific immunogenicity and toxicity, modern vaccine development has turned toward the application of distinct, purified, subunit, and synthetic antigens as vaccines. These vaccines are typically poor immunogens and require the assistance of adjuvants to generate a robust and persistent immune response.
Adjuvants have the additional benefit of reducing the amount of antigen or injections required for protective immunity, subsequently reducing the production cost of vaccines. They are currently used for both traditional and modern vaccines. To date, aluminum adjuvants, including aluminum hydroxide and aluminum phosphate, and squalene-based oil-in-water emulsions are the most widely used adjuvants for human vaccines. However, the mechanisms of action of these compounds remain largely unknown and controversial, often referred to as the “immunologist’s dirty little secret” (ref. 2, p 4480).
Recent progress in our understanding of innate immunity and its potential in shaping adaptive responses have influenced the search for new generation adjuvants. It is now widely believed that pattern recognition receptors (PRRs) of the innate immune system, particularly Toll-like receptors (TLRs) and nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs), can modulate and control the generation of humoral and cellular immune responses. Bacterial components, including TLR and NLR ligands, are known to be strong immunostimulants and have received much attention in recent years. This review will examine the current understanding of the mechanism of action behind empirical adjuvants used in licensed vaccines and will explore the potential of TLR and NLR ligands as adjuvants toward new-generation vaccines.
Orchestrating an Immune Response
Vertebrates have evolved two interrelated methods of resistance to protect the host from infections: the innate and adaptive immune systems. Innate immunity represents the first line of defense by providing nonspecific means of bacterial detection. Specifically, germ line-encoded PRRs recognize conserved pathogen-associated molecular patterns (PAMPs) and danger-associated molecular patterns (DAMPs) (2–5).
Several PRRs have been identified in mammals, of which TLRs and NLRs are the most studied and characterized. TLRs are transmembrane receptors, whereas NLRs are cytoplasmic receptors. Together, they can survey the extracellular environment, cytoplasm, and endosomal compartments for PAMPs and DAMPs. Phagocytic cells, particularly dendritic cells (DCs) and macrophages, represent the main professional antigen-presenting cells (APCs) and are responsible for innate-adaptive cross-talk. Upon recognition of infection, PRRs not only initiate cellular defense mechanisms resulting in the production of innate protective molecules and proinflammatory cytokines, but also differentially activate APCs to specifically modulate the adaptive immune response toward the targeted pathogen (5). One of the goals of modern vaccinology is to harness the ability of TLRs and NLRs to drive adaptive immunity to ensure optimal pathogen clearance.
Innate immune activation through TLR and NLR stimulation leads to a number of pivotal events, ultimately leading to lymphocyte activation. For example, TLRs play an important role in maturing DCs leading to antigen presentation to lymphocytes (6). The codelivery of antigen and TLR ligands promotes the MHC class II pathway of antigen presentation to CD4 T cells (7). TLRs can also promote cross-presentation of antigen on major histocompatibility complex (MHC) class I molecules to activate cytotoxic CD8+ T cells. NLRs are also associated with antigen presentation; NLR stimulation of DCs up-regulates MHC class II (8) and through autophagy, NOD2 was shown to up-regulate MHC class II CD4+ T-cell responses in DCs (9).
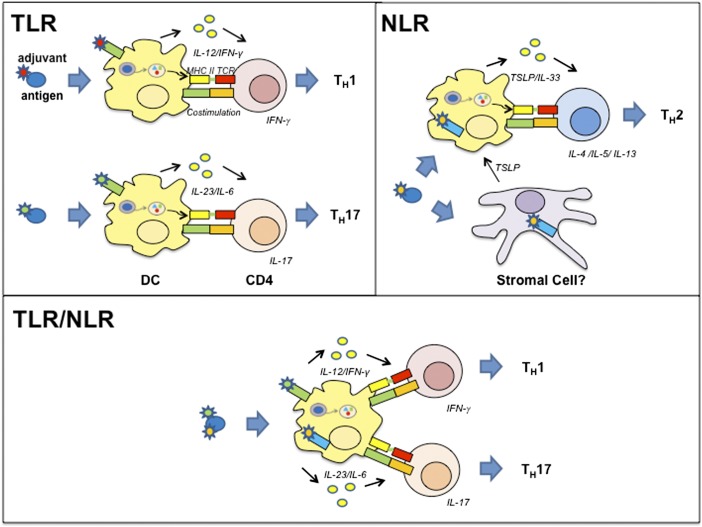
The innate immune system can also modulate the adaptive immune response by providing key signals to prime naïve CD4+ T cells toward specific T-helper (TH) profiles (Fig. 1). TH cells can be subdivided in TH1, TH2, TH17, and Treg subsets according to their production of signature cytokines and are associated with protection against certain classes of pathogens (10–12). Several studies have demonstrated that NLR and TLR stimulation can modulate the adaptive immune response toward the different TH profiles (Fig. 1) (8, 13–16). Understanding the role of TLR/NLR agonists in selectively directing the TH profile (TH1, TH17, or TH2) during immunization will allow for the development of adjuvants specifically tailored for the desired immune outcome.
Fig. 1.
Using PRR agonists to modulate the CD4 T-cell response. APCs expressing PRRs, such as TLRs and NLRs, can take up the antigen and provide key cytokines to prime naïve CD4 T cells toward specific TH phenotypes characterized by their cytokine profile. TH1 cells are induced by IL-12, and produce IFN-γ against viral, intracellular bacterial, or parasitic infections. TH2 cells are induced by TSLP-expressing and IL-33–producing DCs and are characterized by the production of IL-4, IL-5, IL-6, IL-10, and IL-13 against extracellular pathogens (10–12). Finally, TH17 cells producing IL-17 are induced in the presence of IL-23 and IL-6. TLR stimulation mediates TH1 and TH17 responses (14), whereas NOD1 and NOD2 stimulation within the nonhematopoietic compartment induces a predominant TH2 polarized response in vivo. Interestingly, costimulation with TLRs and NLRs synergizes to elicit TH1 and TH17 immune responses (8, 13, 16).
TLRs
TLRs are key pathogen sensors that modulate the host immune system and play a fundamental role in response to microbial infection in many species. The link between Toll and pathogen sensing was first established in Drosophila in 1996 (17), and then extended to humans where nine TLRs have been extensively characterized (18). TLRs are transmembrane receptors and can be individually split into two families according to their cellular localization. TLRs 1, 2, and 4–6 are expressed on the cell surface and sense bacterial, fungal, and protozoal products, whereas TLRs 3 and 7–9 are expressed in endosomes and sense viral nucleic acids.
All of the TLRs, with exception of TLR3, signal via the recruitment of MyD88 to their Toll/IL-1 receptor (TIR) domain, resulting in the phosphorylation of NF-κB and mitogen-activated protein (MAP) kinases (MAPKs). Conversely, TLR3 signals via TIR-domain-containing adapter-inducing IFN-β (TRIF) to NF-κB and IRF1 and IRF3 transcription factors, whereas TLR4 can also signal via the TRIF-related adaptor molecule (TRAM) adaptor pair (for a review, see ref. 19). The two families of TLRs respond to a wide range of stimuli and can modulate the adaptive immune response by providing key cytokines that shift the TH profiles. More precisely, TLRs located on the cell surface are associated with the production of IL-6, IL-12, TNF, and the chemokine IL-8, whereas endosomal TLRs produce type I IFNs.
TLR Agonist Adjuvants Used in Licensed Vaccines
Many preclinical and clinical studies using purified TLR agonists demonstrated that they could be exploited as adjuvants to enhance adaptive responses during vaccination (20). Despite extensive research in the field, monophosphoryl lipid A (MPL), a TLR4 agonist purified from Salmonella minnesota lipopolysaccharide (LPS), remains the only adjuvant used in human licensed vaccines preventing human papillomavirus (HPV) and hepatitis B virus (HBV) infections (Table 1). Clinical studies have shown that HPV vaccination with the MPL/alum adjuvant combination, commonly known as AS04, enhances both humoral and memory B-cell immunity compared with alum alone. The use of AS04 resulted in a faster increase in antibody titers, higher neutralizing antibody titers, and enhanced seroprotection rates while requiring less vaccine doses (21, 22). In vitro studies have shown that AS04 activates human DCs, resulting in the production of IL-12 and increased expression of MHC class II and costimulatory molecules. Finally, experiments conducted in mice have demonstrated that AS04 increases ovalbumin (OVA)-specific CD4+ T-cell priming and TH1 adaptive responses (23).
Table 1.
Clinically tested human vaccine adjuvants
| Name | Components | Receptor/pathway | Disease target |
| Alum* | Aluminum salts (aluminum hydroxide, aluminum phosphate) | NLRP3 uric acid, DNA | Diphteria, tetanus, pneumococcus, HAV, HBV, anthrax, tick-borne encephalitis, MenC, MenB, HPV |
| MF59*, AS03*, AF03, SE | Oil-in-water emulsion squalene oil plus surfactants | MyD88, ASC ATP | Seasonal and pandemic influenza |
| Virosomes* | Liposomes plus influenza HA | Unknown | HAV |
| AS04* | Alum, MPL | TLR4 | HBV, HPV |
| RC-529* | Alum, TLR4 agonist | TLR4 | HBV |
| Imiquimod | Small molecule Imidazoqinoline | TLR7 | Cancer |
| CpG | Synthetic DNA alone or formulated with Alum | TLR9 | HBV, malaria, influenza, anthrax, cancer |
| Poly ICLC | Synthetic double strand RNA | TLR3, MDA5 | Cancer, HIV |
| Flagellin | Linked to HA | TLR5 | Influenza |
| AS01 | Liposomes, MPL, QS21 | TLR4 | Malaria |
| AS02 | Oil-in-water emulsions, MPL, QS21 | TLR4 | Malaria, TB, cancer |
| AS15 | Liposomes, MPL, CpG, QS21 | TLR4 and TLR9 | Cancer |
| Iscomatrix | Saponins, cholesterol | Unknown | HCV, influenza, HPV, cancer |
| IC31 | DNA, peptides | TLR9 agonist | TB |
| CAF01 | Trehalose-dibehenate, cationic liposomes | C-type lectins Mincle and MCL | TB |
| GLA-SE | Oil-in-water emulsion, synthetic MPL | TLR4 | Influenza |
| Montanide (ISA51, ISA720) IFA | Water-in-oil emulsion mineral oil, surfactants | Unknown | Malaria, HIV, cancer, influenza |
| CT, LT, LTK63 | Bacterial toxins | GM1 | Influenza (intranasal), ETEC (patch), cholera (oral) |
ETEC, enterotoxigenic Escherichia coli; HAV, hepatitis A virus; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV, human immunodeficiency virus; HPV, human papillomavirus; MenB, Meningococcal B, MenC, Meningococcal C; Mincle, macrophage inducible Ca2+-dependent (C-type) lectin; TB, tuberculosis.
Adjuvants in licensed vaccines.
Several studies have explored the potential of other TLR agonists as adjuvants and are currently being tested for human vaccines (Table 1). For example, synthetic TLR4 agonists have been used for HBV and influenza vaccines adsorbed to alum (RC529) or incorporated in emulsions (GLA) and the TLR5 agonist, flagellin, has been fused to influenza HA. The agonists of TLR7/8, imiquimod and resiquimod, have thus far been used as topical adjuncts to vaccination in several phase 1 studies, including influenza and cancer antigenic formulations. CPG 7909, a TLR9 agonist, has been extensively tested as a therapeutic molecule for chronic viral infections and cancer. The combination of CPG 7909 with alum and nanoemulsions has recently been proposed as a candidate adjuvant for malaria, anthrax, and CMV vaccines (20). Moreover, CPG 7909 was able to boost the proportion of high responders in HIV-infected individuals when added to seven-valent pneumococcal conjugate and HBV vaccines (24, 25). Continued research into using TLR ligands as adjuvants, either individually or in combination, will help discover new strategies for protection against unsolved diseases as well as the development of mucosal and therapeutic vaccines.
NLRs
NLRs are a family of 22 cytoplasmic innate immune sensors characterized by a tripartite structure, with a C-terminal leucine-rich repeat domain, a central NOD, or NAIP, CIITA, HET-E, TP1 (NACHT) domain, and an N-terminal effector motif, responsible for downstream signal transduction (26–28). The NLRC/X subfamily of NLRs, including NOD1 and NOD2, contains an N-terminal caspase recruitment domain involved in the induction of NF-κB and MAPK signaling (for a review, see ref. 29). NOD1 detects diaminopimelatic acid (DAP)-containing muropeptide found primarily in Gram-negative bacteria (30, 31). Specifically, d-Glu-meso-DAP dipeptide (iE-DAP) was identified as the minimal structural motif required for NOD1 activation (32, 33). Conversely, NOD2 recognizes muramyl dipeptide (MDP) moieties universal to all bacterial peptidoglycan (34, 35). Together, NOD1 and NOD2 can detect an extensive array of bacterial invasion and initiate an innate inflammatory response to prevent systemic infection.
Another NLR subfamily includes pyrin domain-containing proteins (NLRPs), of which NLRP3 is the best-characterized member. NLRP3 can respond to an impressive range of compounds, including certain bacterial infections (36), ATP (36), MDP (37), uric acid crystals (38), silica (39), chitosan, and Quil-A (40). Because it is improbable that a single receptor can detect so many diverse stimuli, it is thought that NLRP3 rather responds to a secondary danger-associated signal produced by these compounds (41), possibly potassium efflux (42) or reactive oxygen species generation (43, 44). Following activation, NLRP3, together with its adaptor ASC, forms the caspase 1-dependent inflammasome. The inflammasome is a multimolecular complex involved in the cleavage of pro–IL-1β and pro–IL-18 into their active and secretable forms (29).
The Contribution of NOD Signaling to Adjuvanticity
Bacterial cell wall peptidoglycans represent a major source of adjuvants given their strong immunostimulatory potential. In fact, MDP was recognized in 1974 as the minimal mycobacterial component responsible for the potent adjuvanticity of complete Freund’s adjuvant (CFA) (45, 46). More than 30 y later, the adjuvanticity of MDP was revealed to require NOD2 to optimally mount humoral and cellular immune responses. The immunization of NOD2-deficient mice using the standard immunopotentiator CFA resulted in a severely altered antigen-specific TH1- and TH2-mediated immunity and a reduced production of IgG1 and IgG2c antibodies. Moreover, immunization with antigen and MDP alone was shown to elicit a predominant TH2 immune response characterized by elevated expression of IL-6 and keratinocyte-derived chemokine, and of MCP-1, a TH2-promoting cytokine (13). These results suggest that NOD2 plays a critical role in priming CD4+ T cells toward distinct TH profiles and subsequently shaping systemic adaptive immunity. Nonetheless, the use of MDP as an adjuvant has been restricted to veterinary vaccines as it was deemed too pyrogenic for human use (47, 48). This motivated the field of adjuvant research to design MDP derivatives that are less pyrogenic, but also maintain their adjuvanticity and immunomodulatory potential.
Several studies have illustrated that the chemical structure of MDP can be modulated to enhance its adjuvant activity while limiting its pyrogenic side effects. For example, the acylation of MDP-derived compounds is believed to facilitate cellular entry via improved endocytosis, resulting in strong cellular immunity (49, 50). In accordance with this, lipohilic derivatives and lipohilic carrier systems, such as oil-in-water emulsions or liposomes, used to deliver MDP have strikingly increased adjuvant activity. Indeed, the synthetic lipophilic glycoprotein, muramyl-tripeptide-phosphatidylethanolamine (MTP-PE), in conjunction with the oil-in-water emulsion MF-59 (MTP-PE/MF-59) received much interest in the late 1990s as a promising adjuvant toward an HIV-1 vaccine (51). Murabutide has also been suggested for HIV-1 immunotherapy. The ester derivate of MDP is apyrogenic and was shown to possess immunomodulatory potential in individuals infected with HIV-1 (52). Activation of HIV-infected APCs by murabutide treatment, lead to the production of HIV-suppressive chemokines that prevented viral replication within macrophages and DCs (53).
Recently, Rubino et al. reexplored the adjuvant activity of several synthetic MDP derivatives, shedding light on their structural/chemical requirements for NOD2 activation. Out of a library of 36 MDP-derived compounds, those with enhanced NOD2 stimulatory capacity typically bared modifications at the first or second amino acid of MDP. One particular peptide, MDP(d-Glu2)-OCH3, revealed higher immunomodulatory activity than MDP itself. Conversely, MDP derivatives modified at the d-lactoyl group of MurNAc, a sugar residue required for NOD2 activation, exhibited impaired stimulatory activity (54). Supporting this notion, MDP(d-Val1) was unable to induce an innate and adaptive immune response in mice, regardless of its previously reported adjuvant potential. Nonetheless, two MDP-derivatives identified stood out, namely MDP(l-Val1) and MDP(l-Ser1). These compounds were able to activate NOD2 to a lesser extent than MDP while maintaining their adjuvanticity (55). It was suggested that these compounds could potentially maintain their adjuvant activity while minimizing the pyrogenicity of MDP. Further studies are required to determine whether MDP(l-Val1) and MDP(l-Ser1) can function as adjuvants in human vaccines.
The use of NOD2 ligands as adjuvants has been studied in greater depth compared with NOD1 ligands. Nonetheless, peptidoglycans containing DAP motifs have also been shown to have adjuvant activity and to contribute to CFA’s adjuvanticity (56–58). Indeed, NOD1-deficient mice immunized with OVA and CFA were more recently reported to mount compromised T- and B-cell immunity toward OVA. Specifically, NOD1 deficiency resulted in significantly less TH1-dependent IgG2b, IgG2c, and IgG3 antibody production. Moreover, NOD1 stimulation in combination with TLR agonists was shown to drive an adaptive immune response with a TH1 and TH17 profile, whereas NOD1 stimulation with the synthetic peptide FK-156 alone was sufficient to prime a TH2 response. Surprisingly, the NOD1-dependent induction of TH2 immunity required key early signals from the nonhematopoietic lineage (16). The authors further demonstrated that NOD1 and NOD2 stimulation within the stromal compartment alone was sufficient to induce TH2 immunity, whereas CD11c+ cells were required for optimal immunity (8). Together, these observations suggest that, in the setting of NLR signaling, TH cell polarization does not necessarily occur through DCs (Fig. 1). Trans-activation is gaining attention in the field of mucosal immunity, where fibroblasts and epithelial and stromal cells are believed to produce key cytokines that can influence the immune response. It remains unclear, however, how NLR activation in different cell populations modulates the adaptive immune response. This is a particular challenge when studying the immunomodulator potential of NLRs, as is the case with the NLRP3 inflammasome (see next section).
Empirical Adjuvants and the Controversial Role of NLRP3 in Their Mode of Action
With the exception of MPL, which is known to target TLR4, all other vaccine adjuvants used in licensed human vaccines have been empirically identified and act on unknown molecular targets. In recent years, extensive work has been performed to elucidate the mechanism of action of two classes of adjuvants: aluminum salts and oil-in-water emulsions.
Numerous subunit vaccines in use today are adsorbed to aluminum hydroxide or aluminum phosphate (Table 1). These adjuvants are thought to create an antigen depot at the inoculation site leading to the gradual release of antigen and increased antigen delivery to immune cells. In vitro studies have indeed demonstrated that alum adsorption increased antigen uptake by DCs (59). Additionally, alum injection recruits monocytes at the site of injection, which then migrate to the draining lymph nodes to differentiate into inflammatory DCs capable of priming T cells (60). Collectively, these findings suggest that alum’s adjuvant activity is twofold: promoting antigen uptake and stimulation of innate immunity at the injection site. However, it remains unclear how alum activates innate immunity and which innate immune receptors are responsible for its adjuvanticity. There is evidence that alum internalization by immune cells leads to phagosomal destabilization resulting in the activation of NLR protein NLRP3 (61). Nonetheless, it remains controversial how NLRP3 contributes to alum adjuvanticity in vivo. Although some studies conducted in NLRP3-deficient mice have shown a requirement for NLRP3 activation for the adjuvant activity of alum (60, 62), other studies using different immunization models have challenged this notion (63–65). Adding to the confusion on the role played by NLRP3 on alum’s mechanism of action in vivo, Kool et al. suggested that aluminum hydroxide mediates its adjuvant activity by inducing the production of endogenous uric acid, which in turn activates NLRP3 within APCs (39). It has also been proposed that alum activates innate immunity by inducing the release of host DNA from dying cells. DNA can act as a danger signal with the potential to activate several innate immune cells including APCs. Recent studies have demonstrated that DNA-mediated activation of STING and IFN regulatory factor 3 (IRF3) is involved, at least partially, in alum adjuvanticity in mice (66, 67).
Oil-in-water emulsions are liquid dispersions of oil droplets stabilized by surfactants. The first oil-in-water emulsion to be approved for human use in a seasonal influenza vaccine was MF59, composed of squalene oil emulsified with two surfactants (Tween80 and Span85). More recently, a second squalene-based oil-in-water emulsion called AS03, composed of squalene, Tween80, and a-tocopherol, has been approved for use in a licensed pandemic influenza vaccine (Table 1). Similarly to alum, MF59 acts as an antigen delivery system, enhancing antigen uptake by DCs in mouse muscle (68) and in vitro in human peripheral blood mononuclear cells (69). Moreover, the intramuscular injection of fluorescently labeled MF59 and antigen revealed that muscle APCs internalize antigen and MF59 and migrate to the draining lymph node where they can prime an adaptive response (70). Nonetheless, the innate immune pathways activated by oil-in-water emulsions remain poorly characterized. Unlike alum, MF59 does not activate NLRP3 in vitro and its adjuvant activity is not dependent on NLRP3 or caspase-1 in mouse immunization studies (71). Rather, MF59 adjuvanticity was shown to require the inflammasome adaptor protein ASC, but did not depend on the other inflammasome components. The exact role of ASC in the mechanism of action of MF59 is still unknown. Adding to the complexity, MF59’s adjuvanticity was shown to require an additional signal transduction adaptor protein, namely MyD88. However, MF59 does not have any TLR agonist activity and the MyD88-dependent pathway required for its adjuvanticity remains unidentified (72). A recent study revealed that injection of MF59 results in the local, transient release of extracellular ATP at the site of injection, which acts as a danger signal and contributes to the adjuvant activity of MF59 (73). In summary, empirical particulate adjuvants share common features in their ability to promote antigen uptake and to induce the activation of innate immunity, at least in part, through the release of DAMPs: endogenous DNA and/or uric acid in the case of alum and extracellular ATP in the case of MF59. Importantly, these findings suggest that the adjuvant activity of alum and MF59 is mediated in response to a secondary danger-associated signal, highlighting a role for innate PRRs and/or transduction proteins downstream of their signaling pathways. Further investigation is required to fully understand these pathways for the more rational design of vaccine adjuvants.
Adjuvant Combinations
As the mechanisms behind NLR and TLR activation continue to be elucidated, the interaction between the two families of innate PRRs have received great attention and respect over the years. It is now widely supported that NLR-mediated peptidoglycan recognition synergizes with TLRs to elicit an optimal and successful adaptive immune response. NOD1-deficent mice immunized with CFA, an adjuvant preparation containing both NLR and TLR ligands, have a severely reduced adaptive response due to weakened NOD1–TLR cross-talk (16). Additionally, several adjuvants, believed to function through TLR-dependent mechanisms, were shown to maintain diminished adjuvanticity in TLR-signaling-deficient mice (74, 75). These results support the idea that NLRs and TLRs exhibit complementary functions in modulating adaptive immunity (13).
Researchers have recently explored the possibility of combining different immunostimulatory ligands to target specific receptors to generate new adjuvants with tailored TH1 and TH2 activity (76). This is of particular relevance for designing vaccines that require a predominant cellular TH1 response to target viruses (77). However, combining ligands can be challenging as some adjuvants can annul each other and/or result in anergy (78). An optimal adjuvant–vaccine formulation should combine synergistic immunostimulants such that their potency and adjuvanticity is enhanced while limiting their pyrogenicity. The copresentation of antigen and adjuvant has also been shown to be crucial in shaping the adaptive immune response (7). For instance, the adjuvanticity of MDP was shown to depend on the administration context. MDP primarily induces a humoral response when delivered in saline solutions, but evokes a potent cellular response when delivered with lipophilic carrier systems (49). As such, the antigen has to be strategically coformulated with an appropriate adjuvant to obtain a vaccine with a desired TH profile. One category of vaccines being developed involves combining adjuvants with carrier systems, such as liposomes, oil-in-water emulsions, lipophilic derivatives and the novel biodegradable polymer microspheres. A recent paper has highlighted the use of biodegradable polymer microspheres to optimize adjuvant/antigen delivery to APCs on mucosal surfaces. The authors used poly (lactic acid) nanoparticles carrying Gag p24 HIV-1 antigen to encapsulate NOD ligands and efficiently target DCs. The coinjection of encapsulated NOD ligands with the antigen resulted in a mark increased antibody response compared with the common adjuvant alum (79).
There are several examples of adjuvant combinations including TLR agonists tested in humans. AS01 and AS02 are combinations of MPL, the saponin QS21 and liposomes or oil-in-water emulsions, respectively, and have been tested in phase 2 and 3 clinical trials with malaria and tuberculosis vaccines (Table 1). More recently phase 2 clinical studies revealed that a vaccine based on the tumor antigen MAGE-3 formulated with the adjuvant AS15 (a combination of MPL, QS21, CpG, and liposomes) can improve disease-free intervals in a subset of melanoma patients better than the same antigen formulated with AS02 (80). These results support the theory that synergy between different TLR agonists can be exploited to improve vaccine efficacy. At least in this case, a vaccine formulated with a mix of TLR4 and TLR9 agonists was more efficient than a vaccine targeting TLR4 alone (81).
Conclusions
Currently, the adjuvants that are used for human vaccines are relatively few and have a number of drawbacks. For example, aluminum-based compounds enhance TH2 humoral immune responses, but fail to stimulate cellular TH1 immune responses (82). Oil-in-water emulsions induce a more balanced immune response, but remain weak inducers of TH1 immunity. In addition, all adjuvants used in licensed products fail to induce CD8 responses in humans. Consequently, there is a growing need for new, safe, and nontoxic adjuvants that are more effective in inducing long-lasting protective responses to vaccination and CD8 cellular immunity (Box 1). Our increasing understanding of the innate immune system, particularly of TLRs and NLRs, has shed light on the mechanisms of current adjuvants and has unveiled potential new targets for adjuvant development. The large number of successful clinical studies conducted with new adjuvants suggests that a panel of novel immune modulators, targeting different arms of the innate immune systems, will be available for human vaccine formulations in a near future. The availability of these adjuvants in various combinations will greatly help the rational design of vaccines adapted to a specific disease. The same tools may also be useful to develop therapeutic vaccines against chronic infections and cancer.
Box 1.
Impact of adjuvants on vaccines
| • Increase functional antibody titers |
| • Increase frequencies of effector T cells |
| • Induce protective responses more rapidly |
| • Reduce the number of injections |
| • Enhance memory/persistence (B and T cells) |
| • Increase breadth of response: heterologous protection |
| • Overcome limited response in the immune compromised |
| • Reduce antigen dose |
Supplementary Material
Footnotes
Conflict of interest statement: S.B. and E.D.G. are full-time employees of Novartis Vaccines and Diagnostics.
This article is a PNAS Direct Submission.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century.
References
- 1.Gupta RK, et al. Adjuvants—a balance between toxicity and adjuvanticity. Vaccine. 1993;11(3):293–306. doi: 10.1016/0264-410x(93)90190-9. [DOI] [PubMed] [Google Scholar]
- 2.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb Symp Quant Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 3.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 4.Kumar H, Kawai T, Akira S. Pathogen recognition by the innate immune system. Int Rev Immunol. 2011;30(1):16–34. doi: 10.3109/08830185.2010.529976. [DOI] [PubMed] [Google Scholar]
- 5.Medzhitov R. Recognition of microorganisms and activation of the immune response. Nature. 2007;449(7164):819–826. doi: 10.1038/nature06246. [DOI] [PubMed] [Google Scholar]
- 6.Pasare C, Medzhitov R. Toll-like receptors and acquired immunity. Semin Immunol. 2004;16(1):23–26. doi: 10.1016/j.smim.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 7.Blander JM, Medzhitov R. Toll-dependent selection of microbial antigens for presentation by dendritic cells. Nature. 2006;440(7085):808–812. doi: 10.1038/nature04596. [DOI] [PubMed] [Google Scholar]
- 8.Magalhaes JG, et al. Nucleotide oligomerization domain-containing proteins instruct T cell helper type 2 immunity through stromal activation. Proc Natl Acad Sci USA. 2011;108(36):14896–14901. doi: 10.1073/pnas.1015063108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooney R, et al. NOD2 stimulation induces autophagy in dendritic cells influencing bacterial handling and antigen presentation. Nat Med. 2010;16(1):90–97. doi: 10.1038/nm.2069. [DOI] [PubMed] [Google Scholar]
- 10.Mosmann TR, Cherwinski H, Bond MW, Giedlin MA, Coffman RL. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986;136(7):2348–2357. [PubMed] [Google Scholar]
- 11.Croft M, Carter L, Swain SL, Dutton RW. Generation of polarized antigen-specific CD8 effector populations: Reciprocal action of interleukin (IL)-4 and IL-12 in promoting type 2 versus type 1 cytokine profiles. J Exp Med. 1994;180(5):1715–1728. doi: 10.1084/jem.180.5.1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy KM, Reiner SL. The lineage decisions of helper T cells. Nat Rev Immunol. 2002;2(12):933–944. doi: 10.1038/nri954. [DOI] [PubMed] [Google Scholar]
- 13.Magalhaes JG, et al. Nod2-dependent Th2 polarization of antigen-specific immunity. J Immunol. 2008;181(11):7925–7935. doi: 10.4049/jimmunol.181.11.7925. [DOI] [PubMed] [Google Scholar]
- 14.Agrawal S, et al. Cutting edge: Different Toll-like receptor agonists instruct dendritic cells to induce distinct Th responses via differential modulation of extracellular signal-regulated kinase-mitogen-activated protein kinase and c-Fos. J Immunol. 2003;171(10):4984–4989. doi: 10.4049/jimmunol.171.10.4984. [DOI] [PubMed] [Google Scholar]
- 15.Dillon S, et al. A Toll-like receptor 2 ligand stimulates Th2 responses in vivo, via induction of extracellular signal-regulated kinase mitogen-activated protein kinase and c-Fos in dendritic cells. J Immunol. 2004;172(8):4733–4743. doi: 10.4049/jimmunol.172.8.4733. [DOI] [PubMed] [Google Scholar]
- 16.Fritz JH, et al. Nod1-mediated innate immune recognition of peptidoglycan contributes to the onset of adaptive immunity. Immunity. 2007;26(4):445–459. doi: 10.1016/j.immuni.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 17.Lemaitre B, Nicolas E, Michaut L, Reichhart JM, Hoffmann JA. The dorsoventral regulatory gene cassette spätzle/Toll/cactus controls the potent antifungal response in Drosophila adults. Cell. 1996;86(6):973–983. doi: 10.1016/s0092-8674(00)80172-5. [DOI] [PubMed] [Google Scholar]
- 18.Kawai T, Akira S. Toll-like receptors and their crosstalk with other innate receptors in infection and immunity. Immunity. 2011;34(5):637–650. doi: 10.1016/j.immuni.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 19.Qian C, Cao X. Regulation of Toll-like receptor signaling pathways in innate immune responses. Ann N Y Acad Sci. 2013;1283:67–74. doi: 10.1111/j.1749-6632.2012.06786.x. [DOI] [PubMed] [Google Scholar]
- 20.De Gregorio E, d'Oro U, Bertholet S, Rappuoli R. Fundamental Immunology. 7th Ed. Philadelphia: Lippincott Williams & Wilkins; 2013. Vaccines; pp. 1032–1068. [Google Scholar]
- 21.Giannini SL, et al. Enhanced humoral and memory B cellular immunity using HPV16/18 L1 VLP vaccine formulated with the MPL/aluminium salt combination (AS04) compared to aluminium salt only. Vaccine. 2006;24(33-34):5937–5949. doi: 10.1016/j.vaccine.2006.06.005. [DOI] [PubMed] [Google Scholar]
- 22.Tong NK, et al. Immunogenicity and safety of an adjuvanted hepatitis B vaccine in pre-hemodialysis and hemodialysis patients. Kidney Int. 2005;68(5):2298–2303. doi: 10.1111/j.1523-1755.2005.00689.x. [DOI] [PubMed] [Google Scholar]
- 23.Didierlaurent AM, et al. AS04, an aluminum salt- and TLR4 agonist-based adjuvant system, induces a transient localized innate immune response leading to enhanced adaptive immunity. J Immunol. 2009;183(10):6186–6197. doi: 10.4049/jimmunol.0901474. [DOI] [PubMed] [Google Scholar]
- 24.Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 2008;46(8):1310–1314. doi: 10.1086/533467. [DOI] [PubMed] [Google Scholar]
- 25.Søgaard OS, et al. Improving the immunogenicity of pneumococcal conjugate vaccine in HIV-infected adults with a toll-like receptor 9 agonist adjuvant: A randomized, controlled trial. Clin Infect Dis. 2010;51(1):42–50. doi: 10.1086/653112. [DOI] [PubMed] [Google Scholar]
- 26.Franchi L, et al. Intracellular NOD-like receptors in innate immunity, infection and disease. Cell Microbiol. 2008;10(1):1–8. doi: 10.1111/j.1462-5822.2007.01059.x. [DOI] [PubMed] [Google Scholar]
- 27.Fritz JH, Ferrero RL, Philpott DJ, Girardin SE. Nod-like proteins in immunity, inflammation and disease. Nat Immunol. 2006;7(12):1250–1257. doi: 10.1038/ni1412. [DOI] [PubMed] [Google Scholar]
- 28.Werts C, Girardin SE, Philpott DJ. TIR, CARD and PYRIN: Three domains for an antimicrobial triad. Cell Death Differ. 2006;13(5):798–815. doi: 10.1038/sj.cdd.4401890. [DOI] [PubMed] [Google Scholar]
- 29.Philpott DJ, Sorbara MT, Robertson SJ, Croitoru K, Girardin SE. NOD proteins: Regulators of inflammation in health and disease. Nat Rev Immunol. 2014;14(1):9–23. doi: 10.1038/nri3565. [DOI] [PubMed] [Google Scholar]
- 30.Chamaillard M, et al. An essential role for NOD1 in host recognition of bacterial peptidoglycan containing diaminopimelic acid. Nat Immunol. 2003;4(7):702–707. doi: 10.1038/ni945. [DOI] [PubMed] [Google Scholar]
- 31.Girardin SE, et al. Nod1 detects a unique muropeptide from gram-negative bacterial peptidoglycan. Science. 2003;300(5625):1584–1587. doi: 10.1126/science.1084677. [DOI] [PubMed] [Google Scholar]
- 32.Girardin SE, et al. Peptidoglycan molecular requirements allowing detection by Nod1 and Nod2. J Biol Chem. 2003;278(43):41702–41708. doi: 10.1074/jbc.M307198200. [DOI] [PubMed] [Google Scholar]
- 33.Girardin SE, et al. Identification of the critical residues involved in peptidoglycan detection by Nod1. J Biol Chem. 2005;280(46):38648–38656. doi: 10.1074/jbc.M509537200. [DOI] [PubMed] [Google Scholar]
- 34.Girardin SE, et al. Nod2 is a general sensor of peptidoglycan through muramyl dipeptide (MDP) detection. J Biol Chem. 2003;278(11):8869–8872. doi: 10.1074/jbc.C200651200. [DOI] [PubMed] [Google Scholar]
- 35.Inohara N, et al. Host recognition of bacterial muramyl dipeptide mediated through NOD2. Implications for Crohn’s disease. J Biol Chem. 2003;278(8):5509–5512. doi: 10.1074/jbc.C200673200. [DOI] [PubMed] [Google Scholar]
- 36.Mariathasan S, et al. Cryopyrin activates the inflammasome in response to toxins and ATP. Nature. 2006;440(7081):228–232. doi: 10.1038/nature04515. [DOI] [PubMed] [Google Scholar]
- 37.Martinon F, Agostini L, Meylan E, Tschopp J. Identification of bacterial muramyl dipeptide as activator of the NALP3/cryopyrin inflammasome. Curr Biol. 2004;14(21):1929–1934. doi: 10.1016/j.cub.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 38.Martinon F, Pétrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature. 2006;440(7081):237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 39.Kool M, et al. Alum adjuvant boosts adaptive immunity by inducing uric acid and activating inflammatory dendritic cells. J Exp Med. 2008;205(4):869–882. doi: 10.1084/jem.20071087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li H, Willingham SB, Ting JP, Re F. Cutting edge: Inflammasome activation by alum and alum’s adjuvant effect are mediated by NLRP3. J Immunol. 2008;181(1):17–21. doi: 10.4049/jimmunol.181.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benko S, Philpott DJ, Girardin SE. The microbial and danger signals that activate Nod-like receptors. Cytokine. 2008;43(3):368–373. doi: 10.1016/j.cyto.2008.07.013. [DOI] [PubMed] [Google Scholar]
- 42.Muñoz-Planillo R, et al. K+ efflux is the common trigger of NLRP3 inflammasome activation by bacterial toxins and particulate matter. Immunity. 2013;38(6):1142–1153. doi: 10.1016/j.immuni.2013.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sorbara MT, Girardin SE. Mitochondrial ROS fuel the inflammasome. Cell Res. 2011;21(4):558–560. doi: 10.1038/cr.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou R, Yazdi AS, Menu P, Tschopp J. A role for mitochondria in NLRP3 inflammasome activation. Nature. 2011;469(7329):221–225. doi: 10.1038/nature09663. [DOI] [PubMed] [Google Scholar]
- 45.Ellouz F, Adam A, Ciorbaru R, Lederer E. Minimal structural requirements for adjuvant activity of bacterial peptidoglycan derivatives. Biochem Biophys Res Commun. 1974;59(4):1317–1325. doi: 10.1016/0006-291x(74)90458-6. [DOI] [PubMed] [Google Scholar]
- 46.Adam A, Ciorbaru R, Ellouz F, Petit JF, Lederer E. Adjuvant activity of monomeric bacterial cell wall peptidoglycans. Biochem Biophys Res Commun. 1974;56(3):561–567. doi: 10.1016/0006-291x(74)90640-8. [DOI] [PubMed] [Google Scholar]
- 47.Lemesre JL, et al. Protection against experimental visceral leishmaniasis infection in dogs immunized with purified excreted secreted antigens of Leishmania infantum promastigotes. Vaccine. 2005;23(22):2825–2840. doi: 10.1016/j.vaccine.2004.11.061. [DOI] [PubMed] [Google Scholar]
- 48.Lemesre JL, et al. Long-lasting protection against canine visceral leishmaniasis using the LiESAp-MDP vaccine in endemic areas of France: Double-blind randomised efficacy field trial. Vaccine. 2007;25(21):4223–4234. doi: 10.1016/j.vaccine.2007.02.083. [DOI] [PubMed] [Google Scholar]
- 49.Parant MA, et al. Immunostimulant activities of a lipophilic muramyl dipeptide derivative and of desmuramyl peptidolipid analogs. Infect Immun. 1980;27(3):826–831. doi: 10.1128/iai.27.3.826-831.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Geddes K, Magalhães JG, Girardin SE. Unleashing the therapeutic potential of NOD-like receptors. Nat Rev Drug Discov. 2009;8(6):465–479. doi: 10.1038/nrd2783. [DOI] [PubMed] [Google Scholar]
- 51.Hancock RE, Nijnik A, Philpott DJ. Modulating immunity as a therapy for bacterial infections. Nat Rev Microbiol. 2012;10(4):243–254. doi: 10.1038/nrmicro2745. [DOI] [PubMed] [Google Scholar]
- 52.Bahr GM. Non-specific immunotherapy of HIV-1 infection: Potential use of the synthetic immunodulator murabutide. J Antimicrob Chemother. 2003;51(1):5–8. doi: 10.1093/jac/dkg063. [DOI] [PubMed] [Google Scholar]
- 53.Darcissac EC, et al. The synthetic immunomodulator murabutide controls human immunodeficiency virus type 1 replication at multiple levels in macrophages and dendritic cells. J Virol. 2000;74(17):7794–7802. doi: 10.1128/jvi.74.17.7794-7802.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rubino SJ, et al. Identification of a synthetic muramyl peptide derivative with enhanced Nod2 stimulatory capacity. Innate Immun. 2013;19(5):493–503. doi: 10.1177/1753425912471691. [DOI] [PubMed] [Google Scholar]
- 55.Chedid L, Audibert F, Lefrancier P, Choay J, Lederer E. Modulation of the immune response by a synthetic adjuvant and analogs. Proc Natl Acad Sci USA. 1976;73(7):2472–2475. doi: 10.1073/pnas.73.7.2472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Migliore-Samour D, et al. 1979. Immunostimulating and adjuvant activities of a low molecular weight lipopeptide. Comptes rendus des seances de l’Academie des sciences. Serie D, Sciences Naturelles 289(5):473–476.
- 57.Adam A, Ellouz F, Ciorbaru R, Petit JF, Lederer E. Peptidoglycan adjuvants: Minimal structure required for activity. Z Immunitatsforsch Exp Klin Immunol. 1975;149(2-4):341–348. [PubMed] [Google Scholar]
- 58.Kotani S, Watanabe Y, Shimono T, Narita T, Kato K. Immunoadjuvant activities of cell walls, their water-soluble fractions and peptidoglycan subunits, prepared from various gram-positive bacteria, and of synthetic n-acetylmuramyl peptides. Z Immunitatsforsch Exp Klin Immunol. 1975;149(2-4):302–319. [PubMed] [Google Scholar]
- 59.Morefield GL, et al. Role of aluminum-containing adjuvants in antigen internalization by dendritic cells in vitro. Vaccine. 2005;23(13):1588–1595. doi: 10.1016/j.vaccine.2004.07.050. [DOI] [PubMed] [Google Scholar]
- 60.Kool M, et al. Cutting edge: Alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J Immunol. 2008;181(6):3755–3759. doi: 10.4049/jimmunol.181.6.3755. [DOI] [PubMed] [Google Scholar]
- 61.Hornung V, et al. Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat Immunol. 2008;9(8):847–856. doi: 10.1038/ni.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eisenbarth SC, Colegio OR, O’Connor W, Sutterwala FS, Flavell RA. Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature. 2008;453(7198):1122–1126. doi: 10.1038/nature06939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Franchi L, Núñez G. The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1beta secretion but dispensable for adjuvant activity. Eur J Immunol. 2008;38(8):2085–2089. doi: 10.1002/eji.200838549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.McKee AS, et al. Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J Immunol. 2009;183(7):4403–4414. doi: 10.4049/jimmunol.0900164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marrack P, McKee AS, Munks MW. Towards an understanding of the adjuvant action of aluminium. Nat Rev Immunol. 2009;9(4):287–293. doi: 10.1038/nri2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Marichal T, et al. DNA released from dying host cells mediates aluminum adjuvant activity. Nat Med. 2011;17(8):996–1002. doi: 10.1038/nm.2403. [DOI] [PubMed] [Google Scholar]
- 67.McKee AS, et al. Host DNA released in response to aluminum adjuvant enhances MHC class II-mediated antigen presentation and prolongs CD4 T-cell interactions with dendritic cells. Proc Natl Acad Sci USA. 2013;110(12):E1122–E1131. doi: 10.1073/pnas.1300392110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dupuis M, et al. Dendritic cells internalize vaccine adjuvant after intramuscular injection. Cell Immunol. 1998;186(1):18–27. doi: 10.1006/cimm.1998.1283. [DOI] [PubMed] [Google Scholar]
- 69.Seubert A, Monaci E, Pizza M, O’Hagan DT, Wack A. The adjuvants aluminum hydroxide and MF59 induce monocyte and granulocyte chemoattractants and enhance monocyte differentiation toward dendritic cells. J Immunol. 2008;180(8):5402–5412. doi: 10.4049/jimmunol.180.8.5402. [DOI] [PubMed] [Google Scholar]
- 70.Calabro S, et al. Vaccine adjuvants alum and MF59 induce rapid recruitment of neutrophils and monocytes that participate in antigen transport to draining lymph nodes. Vaccine. 2011;29(9):1812–1823. doi: 10.1016/j.vaccine.2010.12.090. [DOI] [PubMed] [Google Scholar]
- 71.Ellebedy AH, et al. Inflammasome-independent role of the apoptosis-associated speck-like protein containing CARD (ASC) in the adjuvant effect of MF59. Proc Natl Acad Sci USA. 2011;108(7):2927–2932. doi: 10.1073/pnas.1012455108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Seubert A, et al. Adjuvanticity of the oil-in-water emulsion MF59 is independent of Nlrp3 inflammasome but requires the adaptor protein MyD88. Proc Natl Acad Sci USA. 2011;108(27):11169–11174. doi: 10.1073/pnas.1107941108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vono M, et al. The adjuvant MF59 induces ATP release from muscle that potentiates response to vaccination. Proc Natl Acad Sci USA. 2013;110(52):21095–21100. doi: 10.1073/pnas.1319784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gavin AL, et al. Adjuvant-enhanced antibody responses in the absence of toll-like receptor signaling. Science. 2006;314(5807):1936–1938. doi: 10.1126/science.1135299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yoder A, et al. Tripalmitoyl-S-glyceryl-cysteine-dependent OspA vaccination of toll-like receptor 2-deficient mice results in effective protection from Borrelia burgdorferi challenge. Infect Immun. 2003;71(7):3894–3900. doi: 10.1128/IAI.71.7.3894-3900.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kornbluth RS, Stone GW. Immunostimulatory combinations: Designing the next generation of vaccine adjuvants. J Leukoc Biol. 2006;80(5):1084–1102. doi: 10.1189/jlb.0306147. [DOI] [PubMed] [Google Scholar]
- 77.Moingeon P, Haensler J, Lindberg A. Towards the rational design of Th1 adjuvants. Vaccine. 2001;19(31):4363–4372. doi: 10.1016/s0264-410x(01)00193-1. [DOI] [PubMed] [Google Scholar]
- 78.Bagchi A, et al. MyD88-dependent and MyD88-independent pathways in synergy, priming, and tolerance between TLR agonists. J Immunol. 2007;178(2):1164–1171. doi: 10.4049/jimmunol.178.2.1164. [DOI] [PubMed] [Google Scholar]
- 79.Pavot V, et al. Encapsulation of Nod1 and Nod2 receptor ligands into poly(lactic acid) nanoparticles potentiates their immune properties. J Control Release. 2013;167(1):60–67. doi: 10.1016/j.jconrel.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 80.Kruit WH, et al. Selection of immunostimulant AS15 for active immunization with MAGE-A3 protein: Results of a randomized phase II study of the European Organisation for Research and Treatment of Cancer Melanoma Group in Metastatic Melanoma. J Clin Oncol. 2013;31(19):2413–2420. doi: 10.1200/JCO.2012.43.7111. [DOI] [PubMed] [Google Scholar]
- 81.Ulloa-Montoya F, et al. Predictive gene signature in MAGE-A3 antigen-specific cancer immunotherapy. J Clin Oncol. 2013;31(19):2388–2395. doi: 10.1200/JCO.2012.44.3762. [DOI] [PubMed] [Google Scholar]
- 82.Petrovsky N, Aguilar JC. Vaccine adjuvants: Current state and future trends. Immunol Cell Biol. 2004;82(5):488–496. doi: 10.1111/j.0818-9641.2004.01272.x. [DOI] [PubMed] [Google Scholar]