Significance
Females are subjected to a biological clock that dictates the end of the reproductive lifespan, on average, at 50 y of age, whereas fecundity sharply decreases after 30 y of age. Over the past decade, a current trend of postponing childbearing into advanced age has led to a corresponding upward trend in the number of in vitro fertilization (IVF) treatments. Inflammation was reported to affect both IVF outcomes and the ovarian reserve adversely. Identifying a possible culprit, such as IL-1, may offer new insight into the mechanisms responsible for oocyte loss as well as practical interventions, such as IL-1 blockade, which aims to slow down the rate at which oocytes are eliminated and improve IVF outcomes.
Abstract
Oocyte endowment dwindles away during prepubertal and adult life until menopause occurs, and apoptosis has been identified as a central mechanism responsible for oocyte elimination. A few recent reports suggest that uncontrolled inflammation may adversely affect ovarian reserve. We tested the possible role of the proinflammatory cytokine IL-1 in the age-related exhaustion of ovarian reserve using IL-1α and IL-1β–KO mice. IL-1α–KO mice showed a substantially higher pregnancy rate and litter size compared with WT mice at advanced age. The number of secondary and antral follicles was significantly higher in 2.5-mo-old IL-1α–KO ovaries compared with WT ovaries. Serum anti-Müllerian hormone, a putative marker of ovarian reserve, was markedly higher in IL-1α–KO mice from 2.5 mo onward, along with a greater ovarian response to gonadotropins. IL-1β–KO mice displayed a comparable but more subtle prolongation of ovarian lifespan compared with IL-1α–KO mice. The protein and mRNA of both IL-1α and IL-1β mice were localized within the developing follicles (oocytes and granulosa cells), and their ovarian mRNA levels increased with age. Molecular analysis revealed decreased apoptotic signaling [higher B-cell lymphoma 2 (BCL-2) and lower BCL-2–associated X protein levels], along with a marked attenuation in the expression of genes coding for the proinflammatory cytokines IL-1β, IL-6, and TNF-α in ovaries of IL-1α–KO mice compared with WT mice. Taken together, IL-1 emerges as an important participant in the age-related exhaustion of ovarian reserve in mice, possibly by enhancing the expression of inflammatory genes and promoting apoptotic pathways.
Female mammals are born with a finite number of oocytes that gradually decreases during prepubertal development and adult life (1, 2). Each oocyte is encircled by somatic granulosa cells (GCs) to form the basic functioning unit of the ovary, the follicle. An intact nuclear membrane termed the “germinal vesicle” (GV) surrounds the oocyte’s chromosomes (3). Ovarian follicles are subdivided according to their size and developmental stage into four main categories: primordial follicles (PMFs), primary, secondary, and antral follicles. PMFs are in a state of growth arrest and are referred to as the ovarian reserve (4). All other follicles are known as “growing follicles.”
The ovarian functional lifespan is determined by the size of the oocytes’ “stockpile” provided at birth, as well as by the rate at which this endowment is depleted (5). Programmed cell death (apoptosis) has been identified as a central mechanism responsible for the age-related exhaustion of oocytes, whereas a delicate balance between prosurvival and proapoptotic molecules determines the final destiny of the follicle (5–7). The sphingolipid ceramide has emerged as an essential second messenger in the cascade that promotes age-related apoptosis (5, 8), and lower ceramide levels, observed in acid sphingomyelinase-deficient mice, resulted in a larger postnatal pool of oocytes compared with their WT counterparts (5). Furthermore, studies using B-cell lymphoma 2 (BCL-2)–associated X protein (Bax)-null female mice provided evidence that the ovarian lifespan can be extended (9). Despite these reports, the molecular pathways that regulate follicular apoptosis and the consequent reproductive aging remain poorly understood.
The IL-1 family members are among the most potent molecules of the innate immune system (10). The two major prototypic agonist cytokines in this family, IL-1α and IL-1β, induce the expression of a variety of proinflammatory genes upon binding to IL-1 receptor type 1 (IL-1R1) (11–13). Both IL-1α and IL-1β are synthesized as precursors (31 kDa) and are processed to mature forms (17 kDa) via specific cellular proteases (11, 14). IL-1β is generated upon inflammatory signals and is only active as a mature secreted protein after cleavage by caspase-1. In contrast, IL-1α exerts its effects both in the mature and precursor forms when binding to IL-1R1 (11, 14, 15). It is constitutively expressed in the cytosol of epithelial cells, keratinocytes, and fibroblasts, and it is up-regulated during inflammation (10, 12, 14). IL-1α belongs to a newly recognized group of dual-function cytokines that play a role in the nucleus, where it affects transcription, apart from its extracellular, receptor-mediated effects (16, 17).
The mammalian ovary was previously shown to express IL-1, and there is considerable evidence that it plays a role in ovarian physiology (18). Nonetheless, there are conflicting reports regarding the cellular compartments and the time point during the hormonal cycle at which IL-1α and IL-1β are expressed (18–22). It is well recognized that inflammation is a hallmark of many processes in reproductive physiology, including ovulation, menstruation, and implantation (23). However, uncontrolled inflammation can negatively affect hormone production and ovulation (24), and, consequently, fertility (25). In the male, high levels of IL-1 in the testis, as observed in IL-1 receptor antagonist-deficient mice, impair sperm quality and its ability to fertilize oocytes (26). Recent studies suggest that inflammatory conditions may affect ovarian reserve in women (27, 28). However, to the best of our knowledge, the possible involvement of inflammatory pathways and the role of IL-1 in the age-related exhaustion of ovarian reserve have never been directly approached. Our objective was to investigate the possible role of IL-1 and its subordinate genes in female reproductive aging. Because IL-1α is constitutively expressed in the cytosol of living cells and has an intracellular function apart from its receptor-mediated effect, we decided to use IL-1α–KO mice in this study. Our working hypothesis is that IL-1α deficiency renders ovarian follicles less susceptible to apoptosis.
Results
IL-1α Deficiency Increases Pregnancy Rate and Mean Litter Size in Advanced Chronological Age.
Our first observation was that the pregnancy rate is markedly greater in aged (10- to 12-mo-old) IL-1α–KO mice compared with WT mice [36 of 41 (88%) and 23 of 38 (60%) mice, respectively; P = 0.009]. A subanalysis according to age revealed a trend toward a higher pregnancy rate in IL-1α–KO mice at the age of 12 mo and a significantly higher pregnancy rate at 10 mo of age compared with WT mice (Table 1). Importantly, although mean litter size was similar at 10 mo of age, there was a significantly larger litter size in 12-mo-old IL-1α–KO mice compared with WT mice. In addition, we assessed the effect of IL-1β, the second major IL-1 prototypic agonist, on reproduction lifespan by comparing the pregnancy rate of 12-mo-old WT (n = 17) and IL-1β–KO (n = 18) mice. The pregnancy rate in IL-1β–KO mice was not statistically different compared with WT mice [13 of 18 (72%) and 9 of 17 (53%) mice, respectively; P = 0.2]. However, the mean litter size was significantly larger (3.4 ± 0.7 and 1.0 ± 0.5 mice, respectively; P = 0.017), similar to that observed in IL-1α–KO mice.
Table 1.
Pregnancy rate and mean litter size in aged WT and IL-1α–KO mice
| Parameter tested | 10 mo | 12 mo | ||
| WT | IL-1α–KO | WT | IL-1α–KO | |
| Pregnancy rate | 67% (n = 21) | 95% (n = 20) | 53% (n = 17) | 81% (n = 21) |
| P value | 0.045 | 0.087 | ||
| Mean litter size | 4.9 ± 0.7 | 4.8 ± 0.8 | 1.0 ± 0.5 | 3.6 ± 0.6 |
| P value | 0.808 | 0.009 | ||
IL-1α deficiency increases pregnancy rates and mean litter size in advanced chronological age. Female mice of both genotypes at the indicated age were individually caged with a WT male of proven fertility for a mating period of 1 mo. Pregnancy rates and mean litter size were recorded at days 15–18 postconception. The number of mice per group is indicated. Data are presented as mean ± SE. Statistical significance was determined using Fisher’s exact test and the Mann–Whitney test.
Reproductive Advantage of IL-1α–KO Mice Is First Manifested at 2.5 Mo of Age.
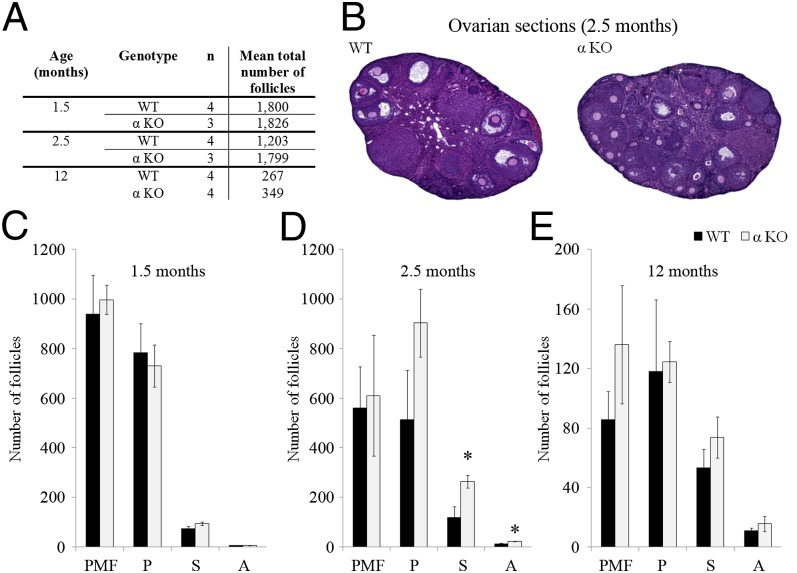
To elucidate the cause underlying the reproductive advantage of IL-1α–KO mice in advanced age, we compared the total follicle number in ovarian serial sections from 1.5-, 2.5-, and 12-mo-old WT and IL-1α–KO mice, and found that the number in both genotypes was not statistically different at these time points (Fig. 1A). We further performed a subanalysis of follicle number according to developmental stage. Although no difference was found in the number of PMFs, primary, secondary and antral follicles in 1.5- and 12-mo-old IL-1α–KO and WT ovaries (Fig. 1 C and E), the number of secondary and antral follicles was significantly higher in 2.5-mo-old IL-1α–KO ovaries compared with WT ovaries (Fig. 1D), along with a trend toward a higher number of primary follicles (P = 0.15). Representative images of 2.5-mo-old WT and IL-1α–KO ovarian sections are shown in Fig. 1B.
Fig. 1.
Ovarian follicular pool in young and aged WT and IL-1α–KO (αKO) mice. (A) Mean total number of follicles in 1.5-, 2.5-, and 12-mo-old WT and αKO ovaries. (B) Representative micrographs of 2.5-mo-old WT and αKO ovarian sections. The number of ovarian follicles at developmental stages [PMF, P (primary follicle), S (secondary follicle), and A (antral follicle)] was assessed in every fifth serial section of 1.5-mo-old mice (C) and 2.5-mo-old mice (D), and in every second serial section of ovaries from 12-mo-old (E) WT and αKO mice. Data are presented as mean ± SE. The number of mice per group is indicated in A. Statistical significance was determined using the Student’s t test. *P < 0.05 compared with WT.
IL-1α Deficiency Results in a Better Ovarian Response to Gonadotropins Throughout the Reproductive Lifespan in Mice.
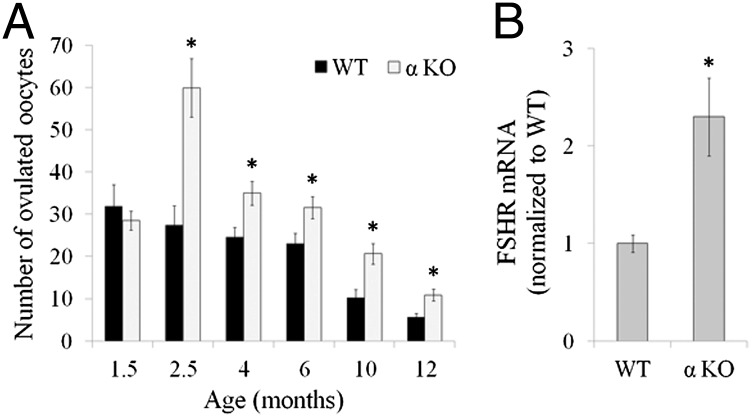
We assessed the ovarian response to gonadotropins in WT and IL-1α–KO mice as an indicator of the growing follicular pool. At 1.5 mo, the number of oocytes retrieved from the oviducts was similar in both genotypes. However, at 2.5 mo, the oocyte number was markedly higher in IL-1α–KO mice compared with WT mice, and this advantage continued throughout the reproductive lifespan until 1 y (Fig. 2A). In light of the striking number of oocytes recovered from 2.5-mo-old IL-1α–KO mice, we also assessed the response to gonadotropins in IL-1β–KO and IL-1R1–KO mice at that age. The response in IL-1β–KO mice was similar to that observed in IL-1α–KO mice, and was significantly higher compared with WT mice (52.8 ± 5.5, n = 11 and 27.4 ± 4.6, n = 8, respectively; P < 0.005). However, the number of oocytes retrieved from IL-1R1–KO mice was similar to that retrieved from WT mice (31.7 ± 3.8, n = 7 and 27.4 ± 4.6, n = 8, respectively). We further analyzed ovarian gene expression of follicle stimulating hormone (FSH) receptor (FSHR) 24 h after injection of pregnant mare serum gonadotropin (PMSG, an FSH analog). FSHR is expressed by GCs of growing follicles and mediates follicular growth following engagement with FSH. FSHR levels were significantly higher in ovaries of IL-1α–KO mice compared with WT mice (Fig. 2B).
Fig. 2.
IL-1α deficiency results in an augmented response to gonadotropins. (A) At the indicated age, WT and αKO mice (n = 7–11 in each group) were primed with 10 international units (IU) of human chorionic gonadotropin (hCGs), a luteinizing hormone analog, 48 h after PMSG (an FSH analog) administration (7 IU). The number of oocytes collected from the oviducts was recorded 16 h after hCG administration. (B) RNA was extracted from the ovaries of 2.5-mo-old WT (n = 4) and αKO (n = 5) mice 24 h after PMSG administration and subjected to quantitative PCR (qPCR) analysis for the detection of FSHR. Data are presented as mean ± SE. Statistical significance was determined using the Student’s t test. *P < 0.05 compared with WT.
Anti-Müllerian Hormone Levels Are Higher in IL-1α–KO Mice Compared with WT Mice.
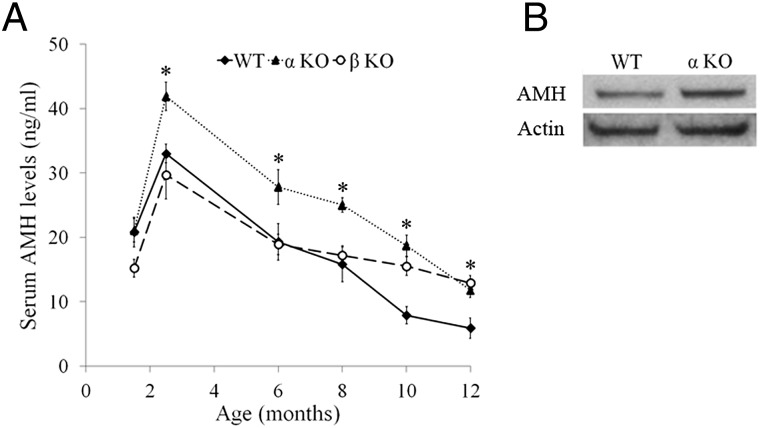
We used ELISA to measure serum anti-Müllerian hormone (AMH) levels as a putative marker of the ovarian reserve (29–32). Serum AMH levels were similar in 1.5-mo-old WT and IL-1α–KO mice, and they rose thereafter in both strains, reaching a peak at 2.5 mo after birth (Fig. 3A). However, serum AMH levels at this time point were markedly higher in IL-1α–KO mice compared with WT mice, and this advantage was maintained throughout the reproductive lifespan (Fig. 3A). Interestingly, in advanced chronological age, serum AMH levels were also higher in IL-1β–KO mice compared with WT mice (Fig. 3A). In accordance with serum level, ovarian AMH protein level was higher in 2.5-mo-old IL-1α–KO mice compared with WT mice as determined by Western blotting (Fig. 3B).
Fig. 3.
αKO mice have higher serum AMH levels compared with WT mice. (A) Mean serum AMH levels in WT, αKO, and IL-1β–KO (βKO) mice (n = 9–11 in each group). At the indicated age, blood was drawn from the retroorbital sinus and measurement of serum AMH was performed using a Beckman Coulter ELISA. (B) Protein lysates of 2.5-mo-old ovaries from WT and αKO mice (n = 5 for each group) were pooled and subjected to Western blot analysis for the detection of AMH levels. Data are presented as mean ± SE. Statistical significance was determined using the Student’s t test. *P < 0.05 compared with WT.
Expression of IL-1α, IL-1β, and IL-1R1 Within the Ovary of WT Mice.
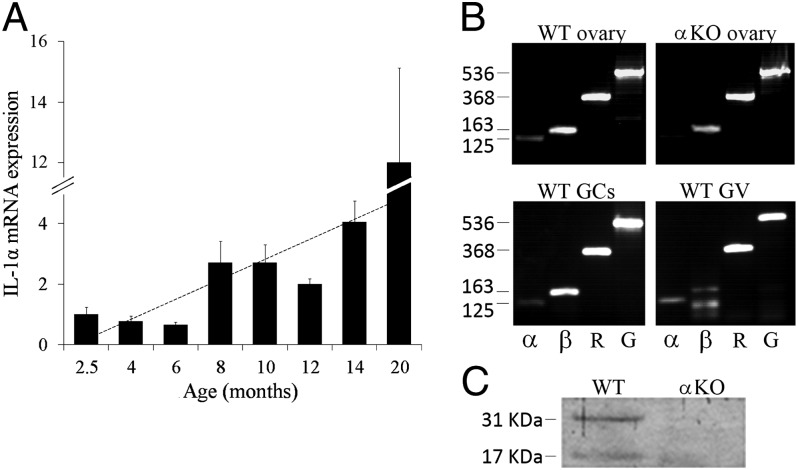
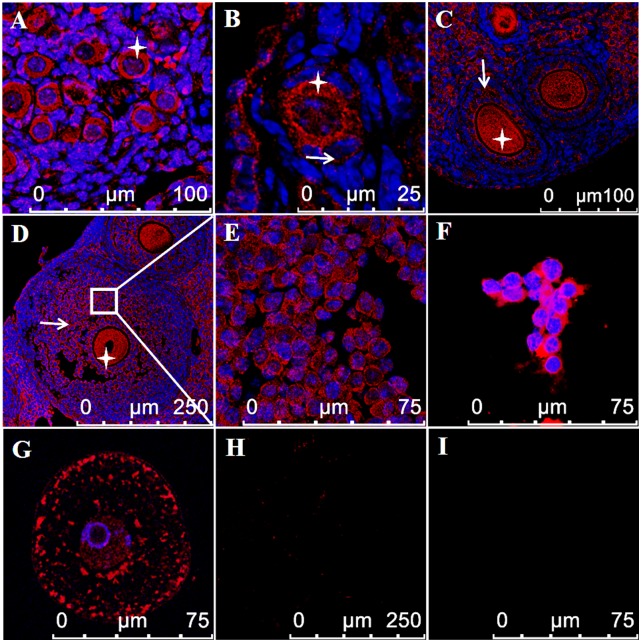
Transcripts levels of IL-1α, as measured by quantitative PCR, were positively correlated with age (Pearson correlation = 0.66, P < 0.001; Fig. 4A). IL-1α, IL-1β, and IL-1R1 transcripts were present in whole ovary, in GCs and GV oocytes from WT mice (Fig. 4B). As expected, IL-1α mRNA was absent in ovaries from IL-1α–KO mice (negative control). The presence of IL-1α protein within the ovary was validated using Western blot analysis (Fig. 4C), and its localization in GCs and oocytes was demonstrated using immunofluorescence. Although GCs from PMFs are difficult to discern, IL-1α protein is clearly seen in the cytoplasm of GCs from primary follicles, and it is expressed throughout follicular development up to the late antral stage (Fig. 5). The presence of IL-1α protein within the oocyte is prominent during all stages of follicular development. It is mainly localized in the cytoplasm and, to a lesser extent, in the GV of the oocyte (Fig. 5).
Fig. 4.
IL-1α, IL-1β, and IL-1R1 mRNA and IL-1α protein are expressed in the ovary of WT mice. (A) RNA was extracted from ovaries of WT mice at various ages from 2.5–20 mo of age. The expression of IL-1α mRNA was analyzed using qPCR and normalized to the expression level measured at 2.5 mo. Pearson correlation = 0.66. The dotted line represents the trend of IL-1α expression. (B) RNA extracted from 2.5-mo-old ovaries of WT and αKO mice and from GCs and GV oocytes of WT mice was subjected to PCR analysis for detection of IL-1α (α, 125 bp), IL-1β (β, 163 bp), and IL-1R1 (R, 368 bp) mRNA transcripts. GAPDH (G, 536 bp) is indicated as an internal control. (C) Protein lysates of 2.5-mo-old ovaries from WT and αKO mice (n = 5 for each group) were pooled and subjected to Western blot analysis for detection of IL-1α.
Fig. 5.
IL-1α protein is expressed in GCs and oocytes of WT ovaries. Paraffin-embedded ovarian sections from 6-d-old (A) and 2.5-mo-old (B–E) WT mice and isolated primary GCs (F) and GV oocytes (G) from 2.5-mo-old WT mice were stained with anti–IL-1α antibody (red) and Hoechst (blue) for DNA labeling. IL-1α is detected in the cytoplasm of GCs (arrows) and oocytes (star) throughout follicular development. A negative control using secondary antibody alone is demonstrated in micrographs of a paraffin-embedded ovarian section (H) and isolated oocyte (I). The scale bars are indicated in each micrograph.
Apoptotic Proteins and mRNA of Inflammation-Related Genes Are Lower in Ovaries from IL-1α–KO Mice Compared with WT Mice.
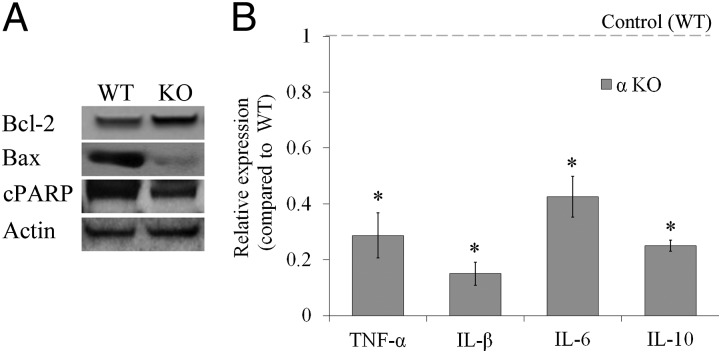
We assessed whether IL-1α deficiency renders ovaries less susceptible to apoptosis during follicular development. The expression of the proapoptotic proteins Bax and the cleaved form of poly (ADP-ribose) polymerase (cPARP) were substantially lower, whereas the antiapoptotic protein BCL-2 was markedly higher in IL-1α–KO ovaries compared with WT ovaries (Fig. 6A). Furthermore, the expression of TNF-α, as well as IL-1β, IL-6, and IL-10, was markedly lower in ovaries from IL-1α–KO mice compared with WT mice (Fig. 6B).
Fig. 6.
Expression of apoptotic proteins and inflammation-related mRNA levels is lower in ovaries from αKO mice compared with WT mice. (A) Protein lysates of ovaries from 2.5-mo-old WT and αKO (KO) mice (n = 5 for each group) were pooled and subjected to Western blot analysis for the detection of Bcl-2, Bax, and the cleaved form of PARP (cPARP). (B) RNA was extracted from ovaries of 2.5-mo-old WT and αKO mice and subjected to qPCR analysis for the detection of TNF-α, IL-1β, IL-6, and IL-10. Raw data are normalized to GAPDH expression, and fold change was calculated relative to control (WT) (n = 3–9 in each group). Data are presented as mean ± SE. Statistical significance was determined using the Student’s t test. *P < 0.05.
Discussion
The present study demonstrates that gene deletion of IL-1α results in a higher pregnancy rate and higher litter size in aged mice. Importantly, the reproductive advantage of IL-1α–KO mice compared with WT mice is first manifested at 2.5 mo of age and lasts up to advanced age. In our study, the similar ovarian follicular pool found 1.5 mo after birth confirms the report by Morita et al. (33) that IL-1α–KO and WT mice are endowed with a comparable number of germ cells. However, our results show that 2.5 mo after birth, ovaries from IL-1α–KO mice contain a significantly higher number of growing follicles compared with WT mice. The dramatically increased response to gonadotropins at 2.5 mo and up to 1 y also supports a higher number of growing follicles in IL-1α–KO mice compared with WT mice.
AMH is expressed in GCs of growing follicles; therefore, its serum levels serve as a direct measure for the size of the growing follicular pool (34). In both strains, we observed a postpubertal ascent in serum AMH, which was previously reported in the literature (35). However, the AMH peak at 2.5 mo was markedly higher in IL-1α–KO mice compared with WT mice, and this advantage was maintained throughout the reproductive lifespan. Furthermore, mRNA levels of FSHR, which, similar to AMH, is expressed by growing follicles (36), were higher in IL-1α–KO ovaries compared with WT ovaries 24 h after PMSG administration. Taken together, these findings indicate that from 2.5 mo onward, IL-1α–KO mice have a larger population of viable growing follicles both at the basal state and after PMSG priming. The morphometric analysis did not reveal a significantly higher number of follicles in IL-1α–KO mice at 12 mo. This finding can be explained by the small sample size. In aged mice, serum AMH levels reflect the size of the resting follicular pool (34). Therefore, the higher AMH levels in aged IL-1α–KO mice suggest a larger pool of resting follicles compared with WT mice. AMH is known to inhibit the recruitment of the nongrowing follicles (37, 38); therefore, higher serum AMH levels from 2.5 mo onward could possibly explain the larger nongrowing follicular pool suggested in IL-1α–KO mice.
We next sought to study the expression pattern of IL-1 within the developing follicle. To date, the reports related to the localization of IL-1 within the follicle are somewhat contradictory or species-specific (18–22, 24). IL-1R1 expression in mice was localized within oocytes and GCs throughout follicular development (22). IL-1α mRNA was reported to be expressed in cow and bovine GCs in response to LPS (24), and IL-1β mRNA was reported in equine and rat GCs (18, 21). Nonetheless, IL-1α or IL-1β mRNA and protein were not previously shown to be expressed within oocytes and/or GCs during follicular development in mice. It is only after follicular rupture that these proteins were observed in these cells (22). Herein, we demonstrate the expression of both IL-1α and IL-1β mRNA in oocytes and GCs of developing follicles. Moreover, we show the localization of IL-1α protein within oocytes and GCs throughout follicular development.
We further investigated whether IL-1α deficiency affects apoptosis-related pathways in the ovary. Very few papers report on the role of IL-1α in apoptosis, and its involvement in apoptosis within the ovary has not yet been approached. In the mammalian ovary, more than 99% of follicles are destined to atretic degeneration (an apoptotic process) (39). Follicular atresia is mediated via the engagement of death ligands (e.g., TNF-α, Fas) to plasma membrane death receptors, or through the mitochondrial pathway within the cell in which the Bcl-2 family members play an important role (6). Nevertheless, the molecular mechanisms responsible for the delicate balance to determine life and death of cells in the ovary are not well understood. We found that IL-1α deficiency results in higher expression of the antiapoptotic Bcl-2 protein and lower levels of the proapoptotic proteins Bax and PARP within the ovary. The Bcl-2 family members Bcl-2 and Bax are found mainly in developing and atretic follicles, respectively (6). We show that BAX is expressed in GCs and oocytes of the growing follicles (Fig. S1). Our findings are in accordance with former studies showing that BAX is expressed both in GCs and oocytes (9). Likewise, the antiapoptotic protein Bcl-2 is expressed in GCs at all follicular stages, but not in theca cells (40). Therefore, we can determine with a great deal of confidence that the detected pro- and antiapoptotic proteins examined in our study are follicle-derived. Furthermore, mRNA levels of the cell death-inducing cytokine TNF-α (41), which was previously shown to induce apoptosis within the ovary (42), were 3.5-fold lower in IL-1α–KO ovaries compared with WT ovaries. Taken together, these findings suggest that IL-1α promotes apoptotic signaling within the ovarian follicles by both the extracellular TNF-α pathway and the intracellular mitochondrial pathway. Importantly, IL-1α deficiency resulted in reduced ovarian expression of proinflammatory cytokines other than TNF-α, such as IL-1β and IL-6. The lower mRNA levels of the anti-inflammatory cytokine IL-10 can be attributed to a decreased necessity to counteract the inflammatory milieu that is attenuated in IL-1α–KO ovaries. Inflammatory conditions are related to poor in vitro fertilization (IVF) outcomes. Endometriosis, a chronic inflammatory condition, is associated with a poor ovarian response to gonadotropins and with lower fertilization and pregnancy rates compared with those in unaffected patients undergoing IVF (27, 43). Elevated levels of TNF-α in the follicular fluid were correlated with poor oocyte quality (44), and Winger et al. (45) showed that TNF-α blockage improves IVF outcomes in young infertile women. Recent studies show that patients with endometriosis and Crohn disease have significantly lower AMH levels compared with healthy controls, supporting the hypothesis that uncontrolled inflammation adversely affects the ovarian reserve (27, 28). Previous studies suggested that IL-1 hinders hormonal regulation within the ovary (46, 47). However, at present, no direct solid connection links specific inflammation-related genes, including IL-1, with ovarian longevity. The extended reproductive lifespan observed in mice lacking IL-1 strengthens the possible relevance of an attenuated inflammatory milieu to maintenance of the ovarian reserve. We found that IL-1α transcripts in the ovary increase with age. Aging was previously characterized by an overall increase in organ IL-1 expression (48). The age-related increase in systemic inflammation is responsible for several age-related pathologies in which IL-1 is thought to play an important role (49). The age-related increase in IL-1α expression was also shown in endometrial stromal fibroblasts (50), human endothelial cells (51), and fibroblasts (52). Moreover, IL-1α accelerates the increase in other age-related transcripts (50). Taken together, the observation that IL-1α expression increases with age in normal ovarian tissue supports its involvement in ovarian senescence as well.
Interestingly, mRNA levels of IL-1β are lower in ovaries from IL-1α–KO mice. The mutual induction of IL-1α and IL-1β has been previously documented (14, 53, 54). We recently showed that IL-1α deficiency results in reduced expression of IL-1β in macrophages (55, 56) and in the liver (57). Importantly, IL-1β–KO mice revealed a more subtle but a similar phenotype of reproductive advantage as seen in IL-1α–KO mice. These findings in IL-1β–KO mice suggest that lower expression levels of IL-1β contributed, at least in part, to the prolonged ovarian lifespan observed in IL-1α–KO mice. In contrast to IL-1α–KO mice, the ovarian response to gonadotropins in IL-1R1–KO mice was similar to that in WT mice at 2.5 mo of age. Among other reasons, the difference in the results can be attributed to the fact that IL-1α is a dual-function cytokine that plays a role in the nucleus apart from its extracellular, receptor-mediated effects. It is important to note that this finding does not mean that IL-1R1–KO mice at an older age would not show a phenotype similar to IL-1–KO mice, namely, of a prolonged fertility lifespan. Further work is required to support the involvement of IL-1 in ovarian senescence, including the use of IL-1R1–KO or IL-1Ra–KO mice.
The present study unequivocally highlights the reproductive preponderance of IL-1–KO mice over WT mice. We propose that this proinflammatory cytokine plays a critical role in the age-related exhaustion of the ovarian reserve in mice by affecting pro- and antiapoptotic signaling pathways in the ovary and pointing to a possible important linkage between inflammation and female reproductive aging.
Materials and Methods
Mice.
The generation of IL-1α–ΚΟ, IL-1β–ΚΟ, and IL-1R1–KO mice on a C57BL/6 genetic background has been described previously (53, 58), and these mice were generously given to us by Ron N. Apte (Ben-Gurion University of the Negev, Beer-Sheva, Israel). WT C57BL/6 mice were purchased from Harlan Laboratories. Mice were maintained on a 12-h light/12-h dark cycle in the animal facility of the Sackler Faculty of Medicine at Tel-Aviv University. Animal care was provided in accordance with institutional guidelines and was approved by the Institutional Animal Care and Use Committee of Tel-Aviv University. Both IL-1α–KO and IL-1β–KO mice exhibit normal development and health, without any observed differences compared with their WT counterparts. These mice do not exhibit evidence of spontaneous carcinogenesis, and their lifespan appears normal (14).
Breeding.
WT and IL-1α–KO female mice were individually caged with a WT male of proven fertility for a mating period of 1 mo. Female mice were weighed at the beginning of the mating period and 14 d after mating, followed by daily weighing. Female mice that gained weight (at least 3 g) were considered pregnant and killed close to delivery. Pregnancy was defined as having at least one fetus at laparotomy. The number of fetuses per female was recorded.
Superovulation.
WT and IL-1α–KO female mice at various ages were primed with gonadotropins as previously described (59). Ovulated cumulus–oocyte complexes were isolated from the oviductal ampullae into M2 medium (Sigma) 16–18 h after human chorionic gonadotropin administration. Oocytes were denuded by a brief exposure to 400 IU/mL hyaluronidase (Sigma) and counted.
Serum AMH Measurement.
Blood samples were drawn via the retroorbital sinus, and sera samples were extracted and kept at −20 °C until use. Measurements were performed using ELISA according to the manufacturer’s instructions (Beckman Coulter). The calculated interassay variation (n = 5) was 8%, and the calculated intraassay variation (n = 37) was 3.2%.
Western Blot Analysis.
Ovaries were subjected to Western blot analysis as previously described (60). Primary antibodies included rabbit anti–IL-1α (sc-7929; Santa Cruz Biotechnology), goat anti–IL-1α (AF-400-NA; R&D Systems), rabbit anti-AMH (sc-28912; Santa Cruz Biotechnology), mouse anti-Bax (sc-7480; Santa Cruz Biotechnology), anti–Bcl-2 (sc-783; Santa Cruz Biotechnology), anti-PARP (sc-7150; Santa Cruz Biotechnology), and goat antiactin (sc-1615; Santa Cruz Biotechnology). Secondary antibodies included Cy3 anti-goat and HRP-conjugated monoclonal and polyclonal antibodies (Jackson Immunoresearch).
RNA Isolation, RT-PCR, and Quantitative Real-Time PCR.
Total RNA was isolated from mouse ovaries using TRIzol reagent (Invitrogen), according to the manufacturer’s instructions, and was quantified with a Nano-Drop spectrophotometer (ND-1000; Thermo Scientific). First-strand cDNA was created by RT (Applied Biosystems) from a total of 1 μg of RNA using 1 μL of reverse transcriptase. cDNA was amplified (35 cycles) with 0.4 μM gene-specific primers using either ReadyMix mixture (Sigma–Aldrich) or TaKaRaEx Taq (Takara Biotechnology). The PCR products were separated by 2% (wt/vol) agarose gel electrophoresis and visualized by ethidium bromide staining. Changes in the level of mRNA expression were detected using gene-specific primers and Fast SYBR Green Master Mix reagent (Applied Biosystems) or FastStart Universal Probe Master (Roche), along with the appropriate Universal ProbeLibrary (UPL) probe and 20–50 ng of cDNA on a StepOnePlus Real-Time PCR System (Life Technologies). The thermal cycling conditions for the Fast SYBR Green reaction were 20 s at 95 °C, followed by 40 cycles of 3 s at 95 °C and 30 s at 60 °C. The thermal cycling conditions for FastStart Universal Probe Master were 10 min at 95 °C, followed by 40 cycles of 10 s at 95 °C and 30 s at 60 °C. PCR primers for probe library assays were designed with the Probe Library Assay Design Center (www.roche-applied-science.com/sis/rtpcr/upl/adc.jsp). We used UPL no. 29 for amplification of IL-1α and GAPDH, UPL no. 48 for IL-10 and FSHR, UPL no. 78 for IL-1β, UPL no. 69 for Bax and HPRT1, UPL no. 2 for BCL-2, and UPL no. 49 for TNF-α. The specific primers used are shown in Table S1.
Morphometric Analysis.
Ovaries were collected from WT and IL-1α–KO female mice at the indicated ages and fixed in Bouin’s solution. Paraffin-embedded ovaries were serially sectioned (8 μm) and stained with H&E. In every fifth ovarian section, the number of PMLs and primary, secondary, and antral follicles was counted. Follicles were classified as previously described (61). In aged mice (12 mo), analysis was done in every second ovarian section and only follicles containing an oocyte with a visible nucleus were counted to avoid double-counting. The results are reported as the number of counted follicles per ovary.
Immunofluorescence.
Ovaries were collected from WT and IL-1α–KO mice at the indicated age, fixed in 4% (wt/vol) paraformaldehyde (PFA), and embedded in paraffin. Ovarian sections (6 μm) were stained as previously described (62). Primary GCs were extracted according to the method of Orly et al. (63) and plated on 13-mm round glass coverslips in Dulbecco's modified Eagle's medium-F12 medium supplemented with 10% (vol/vol) FBS. After 24 h, cells were fixed for 30 min in 4% PFA and permeabilized for 1 min with 0.02% Triton X-100 in PBS. Cells were subsequently incubated at room temperature with goat anti–IL-1α for 1.5 h; washed three times in PBS; and incubated with the appropriate secondary antibodies, together with Hoechst 33342 for DNA labeling, for an additional 45 min. GV oocytes were isolated into M2 medium (Sigma) supplemented with 1 μM Milrinon (Sigma) to avoid oocyte maturation. Cumulus cells were removed mechanically, and oocytes were stained as previously described (64). All experimental samples were visualized and photographed using a laser confocal microscope (SP5; Leica) that was calibrated to a secondary-only control.
Statistical Analyses.
The values reported are the mean ± SE. The Student t test was used as applicable. The Mann–Whitney test was used to assess differences between means when the variable tested was not normally distributed. Fisher’s exact test or the χ2 test was used as appropriate. The strength of the association between IL-1α or IL-1β and age, used as continuous variables, was tested by the Pearson correlation test. P < 0.05 is accepted as statistically significant. All experiments were repeated at least three times, with similar results.
Supplementary Material
Acknowledgments
We thank Prof. R. N. Apte (Ben Gurion University) for IL-1–KO mice and Hanna Levkowitz and Zehava Shabtay for technical assistance. This study was supported, in part, by March of Dimes Grant 21-FY07-610 (to D.H.), the Talpiot Medical Leadership Program Award, the Sheba Medical Center, and a “Sheba R&D” Grant (to Y.K.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323955111/-/DCSupplemental.
References
- 1.Broekmans FJ, Soules MR, Fauser BC. Ovarian aging: Mechanisms and clinical consequences. Endocr Rev. 2009;30(5):465–493. doi: 10.1210/er.2009-0006. [DOI] [PubMed] [Google Scholar]
- 2.Tilly JL. Commuting the death sentence: How oocytes strive to survive. Nat Rev Mol Cell Biol. 2001;2(11):838–848. doi: 10.1038/35099086. [DOI] [PubMed] [Google Scholar]
- 3.Levi M, Maro B, Shalgi R. The involvement of Fyn kinase in resumption of the first meiotic division in mouse oocytes. Cell Cycle. 2010;9(8):1577–1589. doi: 10.4161/cc.9.8.11299. [DOI] [PubMed] [Google Scholar]
- 4.Kerr JB, Myers M, Anderson RA. The dynamics of the primordial follicle reserve. Reproduction. 2013;146(6):R205–R215. doi: 10.1530/REP-13-0181. [DOI] [PubMed] [Google Scholar]
- 5.Morita Y, et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine-1-phosphate therapy. Nat Med. 2000;6(10):1109–1114. doi: 10.1038/80442. [DOI] [PubMed] [Google Scholar]
- 6.Hussein MR. Apoptosis in the ovary: Molecular mechanisms. Hum Reprod Update. 2005;11(2):162–177. doi: 10.1093/humupd/dmi001. [DOI] [PubMed] [Google Scholar]
- 7.Tilly JL. Apoptosis and ovarian function. Rev Reprod. 1996;1(3):162–172. doi: 10.1530/ror.0.0010162. [DOI] [PubMed] [Google Scholar]
- 8.Perez GI, et al. A central role for ceramide in the age-related acceleration of apoptosis in the female germline. FASEB J. 2005;19(7):860–862. doi: 10.1096/fj.04-2903fje. [DOI] [PubMed] [Google Scholar]
- 9.Perez GI, et al. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21(2):200–203. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 10.Sims JE, Smith DE. The IL-1 family: Regulators of immunity. Nat Rev Immunol. 2010;10(2):89–102. doi: 10.1038/nri2691. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood. 2011;117(14):3720–3732. doi: 10.1182/blood-2010-07-273417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamari Y, et al. Differential role and tissue specificity of interleukin-1alpha gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195(1):31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Weber A, Wasiliew P, Kracht M. Interleukin-1 (IL-1) pathway. Sci Signal. 2010;3(105) doi: 10.1126/scisignal.3105cm1. cm1. [DOI] [PubMed] [Google Scholar]
- 14.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 15.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–2147. [PubMed] [Google Scholar]
- 16.Cohen I, et al. Differential release of chromatin-bound IL-1alpha discriminates between necrotic and apoptotic cell death by the ability to induce sterile inflammation. Proc Natl Acad Sci USA. 2010;107(6):2574–2579. doi: 10.1073/pnas.0915018107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luheshi NM, Rothwell NJ, Brough D. Dual functionality of interleukin-1 family cytokines: Implications for anti-interleukin-1 therapy. Br J Pharmacol. 2009;157(8):1318–1329. doi: 10.1111/j.1476-5381.2009.00331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gérard N, Caillaud M, Martoriati A, Goudet G, Lalmanach A-C. The interleukin-1 system and female reproduction. J Endocrinol. 2004;180(2):203–212. doi: 10.1677/joe.0.1800203. [DOI] [PubMed] [Google Scholar]
- 19.Hurwitz A, et al. Human intraovarian interleukin-1 (IL-1) system: Highly compartmentalized and hormonally dependent regulation of the genes encoding IL-1, its receptor, and its receptor antagonist. J Clin Invest. 1992;89(6):1746–1754. doi: 10.1172/JCI115777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de los Santos MJ, Anderson DJ, Racowsky C, Simón C, Hill JA. Expression of interleukin-1 system genes in human gametes. Biol Reprod. 1998;59(6):1419–1424. doi: 10.1095/biolreprod59.6.1419. [DOI] [PubMed] [Google Scholar]
- 21.Martoriati A, Gérard N. Interleukin-1 (IL-1) system gene expression in granulosa cells: Kinetics during terminal preovulatory follicle maturation in the mare. Reprod Biol Endocrinol. 2003;1:42. doi: 10.1186/1477-7827-1-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simón C, Frances A, Piquette G, Polan ML. Immunohistochemical localization of the interleukin-1 system in the mouse ovary during follicular growth, ovulation, and luteinization. Biol Reprod. 1994;50(2):449–457. doi: 10.1095/biolreprod50.2.449. [DOI] [PubMed] [Google Scholar]
- 23.Jabbour HN. Inflammatory pathways in female reproductive health and disease. Reproduction. 2009;138(6):903–919. doi: 10.1530/REP-09-0247. [DOI] [PubMed] [Google Scholar]
- 24.Herath S, et al. Ovarian follicular cells have innate immune capabilities that modulate their endocrine function. Reproduction. 2007;134(5):683–693. doi: 10.1530/REP-07-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weiss G, Goldsmith LT, Taylor RN, Bellet D, Taylor HS. Inflammation in reproductive disorders. Reprod Sci. 2009;16(2):216–229. doi: 10.1177/1933719108330087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ganaiem M, et al. Effect of interleukin-1 receptor antagonist gene deletion on male mouse fertility. Endocrinology. 2009;150(1):295–303. doi: 10.1210/en.2008-0848. [DOI] [PubMed] [Google Scholar]
- 27.Falconer H, et al. IVF outcome in women with endometriosis in relation to tumour necrosis factor and anti-Müllerian hormone. Reprod Biomed Online. 2009;18(4):582–588. doi: 10.1016/s1472-6483(10)60138-1. [DOI] [PubMed] [Google Scholar]
- 28.Fréour T, et al. Ovarian reserve in young women of reproductive age with Crohn’s disease. Inflamm Bowel Dis. 2012;18(8):1515–1522. doi: 10.1002/ibd.21872. [DOI] [PubMed] [Google Scholar]
- 29.Hansen KR, Hodnett GM, Knowlton N, Craig LB. Correlation of ovarian reserve tests with histologically determined primordial follicle number. Fertil Steril. 2011;95(1):170–175. doi: 10.1016/j.fertnstert.2010.04.006. [DOI] [PubMed] [Google Scholar]
- 30.Visser JA, de Jong FH, Laven JS, Themmen AP. Anti-Müllerian hormone: A new marker for ovarian function. Reproduction. 2006;131(1):1–9. doi: 10.1530/rep.1.00529. [DOI] [PubMed] [Google Scholar]
- 31.van Rooij IA, et al. Serum anti-Müllerian hormone levels: A novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071. doi: 10.1093/humrep/17.12.3065. [DOI] [PubMed] [Google Scholar]
- 32.de Vet A, Laven JSE, de Jong FH, Themmen APN, Fauser BCJM. Antimüllerian hormone serum levels: A putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–362. doi: 10.1016/s0015-0282(01)02993-4. [DOI] [PubMed] [Google Scholar]
- 33.Morita Y, et al. Caspase-2 deficiency prevents programmed germ cell death resulting from cytokine insufficiency but not meiotic defects caused by loss of ataxia telangiectasia-mutated (Atm) gene function. Cell Death Differ. 2001;8(6):614–620. doi: 10.1038/sj.cdd.4400845. [DOI] [PubMed] [Google Scholar]
- 34.Kevenaar ME, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147(7):3228–3234. doi: 10.1210/en.2005-1588. [DOI] [PubMed] [Google Scholar]
- 35.Kelsey TW, Anderson RA, Wright P, Nelson SM, Wallace WHB. Data-driven assessment of the human ovarian reserve. Mol Hum Reprod. 2012;18(2):79–87. doi: 10.1093/molehr/gar059. [DOI] [PubMed] [Google Scholar]
- 36.George JW, Dille EA, Heckert LL. Current concepts of follicle-stimulating hormone receptor gene regulation. Biol Reprod. 2011;84(1):7–17. doi: 10.1095/biolreprod.110.085043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Durlinger ALL, et al. Anti-Müllerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143(3):1076–1084. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 38.Gigli I, Cushman RA, Wahl CM, Fortune JE. Evidence for a role for anti-Mullerian hormone in the suppression of follicle activation in mouse ovaries and bovine ovarian cortex grafted beneath the chick chorioallantoic membrane. Mol Reprod Dev. 2005;71(4):480–488. doi: 10.1002/mrd.20338. [DOI] [PubMed] [Google Scholar]
- 39.Inoue N, Matsuda F, Goto Y, Manabe N. Role of cell-death ligand-receptor system of granulosa cells in selective follicular atresia in porcine ovary. J Reprod Dev. 2011;57(2):169–175. doi: 10.1262/jrd.10-198e. [DOI] [PubMed] [Google Scholar]
- 40.Bas D, Abramovich D, Hernandez F, Tesone M. Altered expression of Bcl-2 and Bax in follicles within dehydroepiandrosterone-induced polycystic ovaries in rats. Cell Biol Int. 2011;35(5):423–429. doi: 10.1042/CBI20100542. [DOI] [PubMed] [Google Scholar]
- 41.Van den Oever IA, Raterman HG, Nurmohamed MT, Simsek S. Endothelial dysfunction, inflammation, and apoptosis in diabetes mellitus. Mediators Inflamm. 2010;2010:792393. doi: 10.1155/2010/792393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morrison LJ, Marcinkiewicz JL. Tumor necrosis factor alpha enhances oocyte/follicle apoptosis in the neonatal rat ovary. Biol Reprod. 2002;66(2):450–457. doi: 10.1095/biolreprod66.2.450. [DOI] [PubMed] [Google Scholar]
- 43.González-Fernández R, et al. Patients with endometriosis and patients with poor ovarian reserve have abnormal follicle-stimulating hormone receptor signaling pathways. Fertil Steril. 2011;95(7):2373–2378. doi: 10.1016/j.fertnstert.2011.03.030. [DOI] [PubMed] [Google Scholar]
- 44.Lee KS, et al. Relationships between concentrations of tumor necrosis factor-alpha and nitric oxide in follicular fluid and oocyte quality. J Assist Reprod Genet. 2000;17(4):222–228. doi: 10.1023/A:1009495913119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Winger EE, et al. Treatment with adalimumab (Humira) and intravenous immunoglobulin improves pregnancy rates in women undergoing IVF. Am J Reprod Immunol. 2009;61(2):113–120. doi: 10.1111/j.1600-0897.2008.00669.x. [DOI] [PubMed] [Google Scholar]
- 46.Gottschall PE, Katsuura G, Dahl RR, Hoffmann ST, Arimura A. Discordance in the effects of interleukin-1 on rat granulosa cell differentiation induced by follicle-stimulating hormone or activators of adenylate cyclase. Biol Reprod. 1988;39(5):1074–1085. doi: 10.1095/biolreprod39.5.1074. [DOI] [PubMed] [Google Scholar]
- 47.Gottschall PE, Katsuura G, Arimura A. Interleukin-1 suppresses follicle-stimulating hormone-induced estradiol secretion from cultured ovarian granulosa cells. J Reprod Immunol. 1989;15(3):281–290. doi: 10.1016/0165-0378(89)90018-1. [DOI] [PubMed] [Google Scholar]
- 48.Hacham M, Argov S, White RM, Segal S, Apte RN. Different patterns of interleukin-1alpha and interleukin-1beta expression in organs of normal young and old mice. Eur Cytokine Netw. 2002;13(1):55–65. [PubMed] [Google Scholar]
- 49.Youm Y-H, et al. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 2013;18(4):519–532. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rinehart CA, Watson JM, Torti VR, Palmieri D. The role of interleukin-1 in interactive senescence and age-related human endometrial cancer. Exp Cell Res. 1999;248(2):599–607. doi: 10.1006/excr.1999.4430. [DOI] [PubMed] [Google Scholar]
- 51.Maier JA, Voulalas P, Roeder D, Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990;249(4976):1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- 52.Kumar S, Millis AJ, Baglioni C. Expression of interleukin 1-inducible genes and production of interleukin 1 by aging human fibroblasts. Proc Natl Acad Sci USA. 1992;89(10):4683–4687. doi: 10.1073/pnas.89.10.4683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Horai R, et al. Production of mice deficient in genes for interleukin (IL)-1alpha, IL-1beta, IL-1alpha/beta, and IL-1 receptor antagonist shows that IL-1beta is crucial in turpentine-induced fever development and glucocorticoid secretion. J Exp Med. 1998;187(9):1463–1475. doi: 10.1084/jem.187.9.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kuida K, et al. Altered cytokine export and apoptosis in mice deficient in interleukin-1 beta converting enzyme. Science. 1995;267(5206):2000–2003. doi: 10.1126/science.7535475. [DOI] [PubMed] [Google Scholar]
- 55.Kamari Y, et al. Reduced atherosclerosis and inflammatory cytokines in apolipoprotein-E-deficient mice lacking bone marrow-derived interleukin-1α. Biochem Biophys Res Commun. 2011;405(2):197–203. doi: 10.1016/j.bbrc.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 56.Shemesh S, et al. Interleukin-1 receptor type-1 in non-hematopoietic cells is the target for the pro-atherogenic effects of interleukin-1 in apoE-deficient mice. Atherosclerosis. 2012;222(2):329–336. doi: 10.1016/j.atherosclerosis.2011.12.010. [DOI] [PubMed] [Google Scholar]
- 57.Kamari Y, et al. Lack of interleukin-1α or interleukin-1β inhibits transformation of steatosis to steatohepatitis and liver fibrosis in hypercholesterolemic mice. J Hepatol. 2011;55(5):1086–1094. doi: 10.1016/j.jhep.2011.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Glaccum MB, et al. Phenotypic and functional characterization of mice that lack the type I receptor for IL-1. J Immunol. 1997;159(7):3364–3371. [PubMed] [Google Scholar]
- 59.Bar-Joseph H, et al. Doxorubicin-induced apoptosis in germinal vesicle (GV) oocytes. Reprod Toxicol. 2010;30(4):566–572. doi: 10.1016/j.reprotox.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 60.Eliyahu E, Park J-H, Shtraizent N, He X, Schuchman EH. Acid ceramidase is a novel factor required for early embryo survival. FASEB J. 2007;21(7):1403–1409. doi: 10.1096/fj.06-7016com. [DOI] [PubMed] [Google Scholar]
- 61.Greenfeld CR, et al. BAX is involved in regulating follicular growth, but is dispensable for follicle atresia in adult mouse ovaries. Reproduction. 2007;133(1):107–116. doi: 10.1530/REP-06-0144. [DOI] [PubMed] [Google Scholar]
- 62.Ben-Aharon I, et al. Doxorubicin-induced ovarian toxicity. Reprod Biol Endocrinol. 2010;8:20. doi: 10.1186/1477-7827-8-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Orly J, Sato G, Erickson GF. Serum suppresses the expression of hormonally induced functions in cultured granulosa cells. Cell. 1980;20(3):817–827. doi: 10.1016/0092-8674(80)90328-1. [DOI] [PubMed] [Google Scholar]
- 64.Levi M, Ghetler Y, Shulman A, Shalgi R. Morphological and molecular markers are correlated with maturation-competence of human oocytes. Hum Reprod. 2013;28(9):2482–2489. doi: 10.1093/humrep/det261. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.