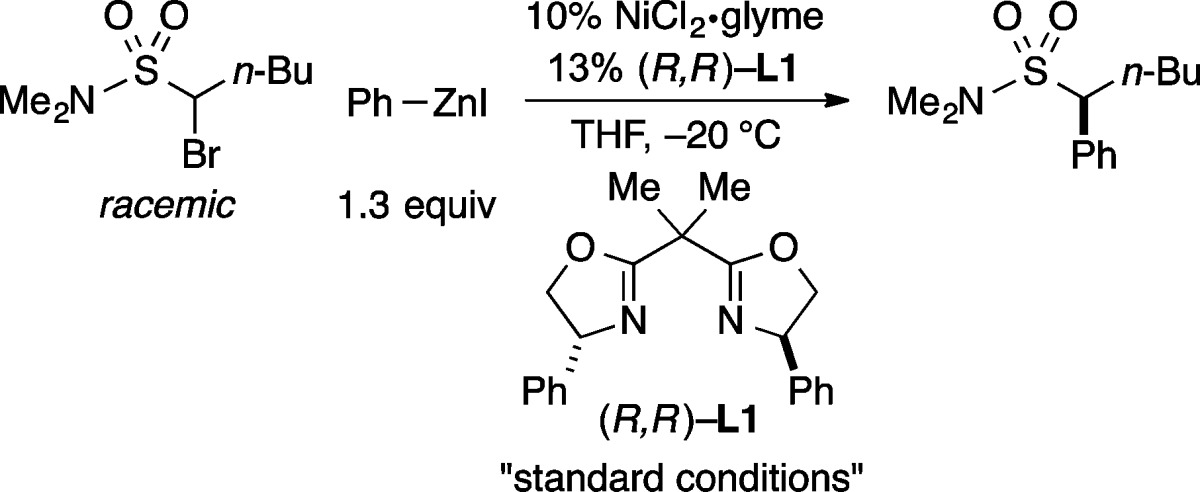
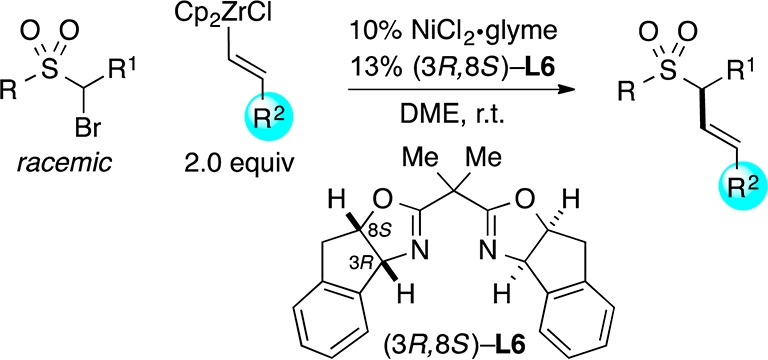
Abstract
The development of efficient methods for the generation of enantioenriched sulfonamides and sulfones is an important objective for fields such as organic synthesis and medicinal chemistry; however, there have been relatively few reports of direct catalytic asymmetric approaches to controlling the stereochemistry of the sulfur-bearing carbon of such targets. In this report, we describe nickel-catalyzed stereoconvergent Negishi arylations and alkenylations of racemic α-bromosulfonamides and -sulfones that furnish the desired cross-coupling product in very good ee and yield for an array of reaction partners. Mechanistic studies are consistent with the generation of a radical intermediate that has a sufficient lifetime to diffuse out of the solvent cage and to cyclize onto a pendant olefin.
Introduction
Sulfonamides and sulfones serve both as useful intermediates in organic synthesis1 and as important target molecules in their own right.2,3 For example, enantioenriched secondary benzylic sulfonamides and sulfones display a range of biological activity (e.g., protein tyrosine phosphatase inhibitor,4 antisepsis agent,5 and γ-secretase inhibitor6).7 However, to the best of our knowledge, there are no methods for the direct catalytic asymmetric synthesis of such sulfonamides, and just a few for such sulfones.8
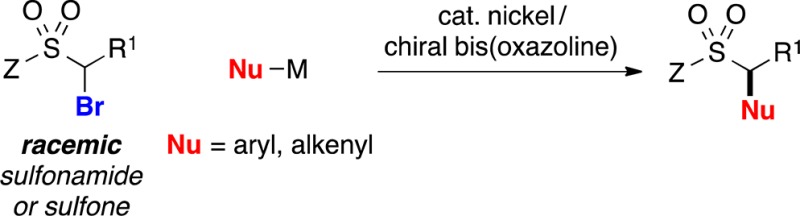
One potential route to these compounds is the stereoconvergent coupling of a racemic α-halosulfonamide/sulfone with an appropriate nucleophile (eq 1). During the past several years, we have described an array of nickel-catalyzed enantioselective cross-couplings of secondary alkyl electrophiles. Whereas couplings of activated electrophiles (a leaving group α to a carbonyl, aryl, alkenyl, alkynyl, or cyano group) proceed in good ee with a variety of nucleophiles (alkyl-, aryl-, and alkenylmetal),9,10 reactions of unactivated electrophiles have been limited, with three exceptions,11 to alkylmetals, specifically alkyl-(9-BBN) reagents.12
 |
1 |
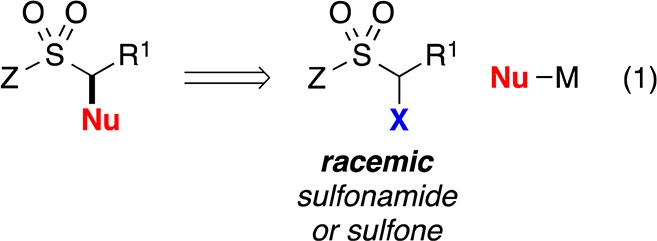
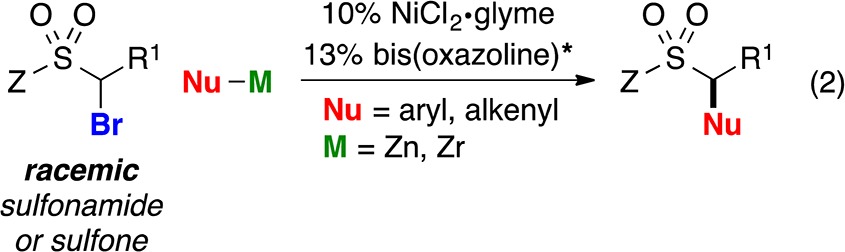
Because α-halosulfonyl compounds are generally poor substrates for SN2 reactions, and the sulfonyl group does not effectively stabilize an adjacent radical (bond dissociation energy for a C–H bond of dimethylsulfone: 99 kcal/mol),13 we view them as unactivated alkyl electrophiles. Herein, we report the first examples of the stereoconvergent cross-coupling of unactivated alkyl electrophiles with nucleophiles other than organoboranes, as well as the first general method for their coupling with aryl- or alkenylmetal reagents; in particular, we describe nickel/bis(oxazoline)-catalyzed cross-couplings of racemic α-bromosulfonamides and -sulfones with organozinc and organozirconium reagents, thereby furnishing secondary benzylic and allylic sulfonamides and sulfones in good enantiomeric excess (eq 2).14
 |
2 |
Results and Discussion
Upon investigating a variety of reaction parameters, we determined that a nickel/bis(oxazoline)15 catalyst can achieve the cross-coupling of an α-bromosulfonamide with an arylzinc reagent in very good ee and yield at −20 °C (Table 1, entry 1); NiCl2·glyme and bis(oxazoline) L1 ((R,R) and (S,S)) are commercially available. All previous reports of stereoconvergent Negishi reactions of racemic electrophiles have employed activated coupling partners.9a−9c,9e−9g
Table 1. Stereoconvergent Negishi Phenylation of a Racemic α-Bromosulfonamide: Effect of Reaction Parametersa.
| entry | change from the “standard conditions” | ee (%) | yield (%)b |
|---|---|---|---|
| 1 | none | 96 | 88 |
| 2 | no NiCl2·glyme | − | <2 |
| 3 | no L1 | − | 16 |
| 4 | r.t., instead of –20 °C | 93 | 39 |
| 5 | L2, instead of L1 | 93 | 84 |
| 6 | L3, instead of L1 | 78 | 28 |
| 7 | L4, instead of L1 | 56 | 6 |
| 8 | L5, instead of L1 | 70 | 44 |
| 9 | PhMgBr, instead of PhZnI | 89 | 44 |
| 10 | 5% NiCl2·glyme, 6.5% L1 | 96 | 72 |
| 11 | under air, rather than under N2 (capped vial) | 96 | 52 |
| 12 | 0.1 equiv of water added | 97 | 84 |
All data are the average of two experiments.
The yield was
determined through
GC analysis with the aid of a calibrated internal standard.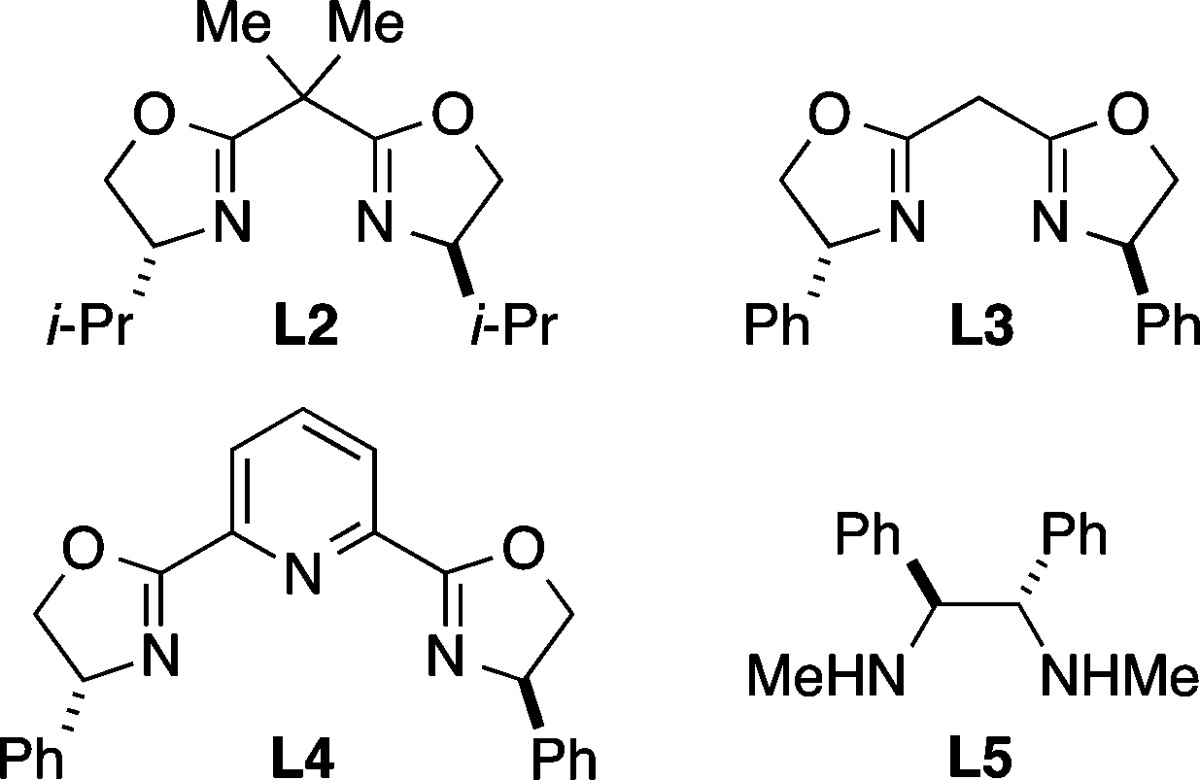
Essentially no cross-coupling is observed in the absence of NiCl2·glyme (Table 1, entry 2), and a low yield of the desired product is obtained if ligand L1 is omitted (entry 3). At room temperature, the coupling proceeds in good ee, but hydrodebromination predominates over C–C bond formation. The corresponding valine-derived bis(oxazoline) (L2) is nearly as effective as phenylglycine-derived L1 (entries 1 and 5), whereas removal of the gem-dimethyl substituents on the linker between the oxazolines of L1 leads to a substantial drop in efficiency (entry 6). Although pybox ligands are often the ligand of choice for nickel-catalyzed stereoconvergent Negishi reactions,9a−9c,9e pybox L4 is not effective for this particular cross-coupling, nor is a 1,2-diamine ligand9d,12 (entries 7 and 8). The use of PhMgBr in place of PhZnI leads to a small loss in ee and a substantial loss in yield (entry 9). When the catalyst loading is reduced in half, no erosion in enantioselectivity is observed, although the yield falls by a modest amount (entry 10). The process is somewhat oxygen-sensitive (entry 11; 66% conversion) but not particularly moisture-sensitive (entry 12).
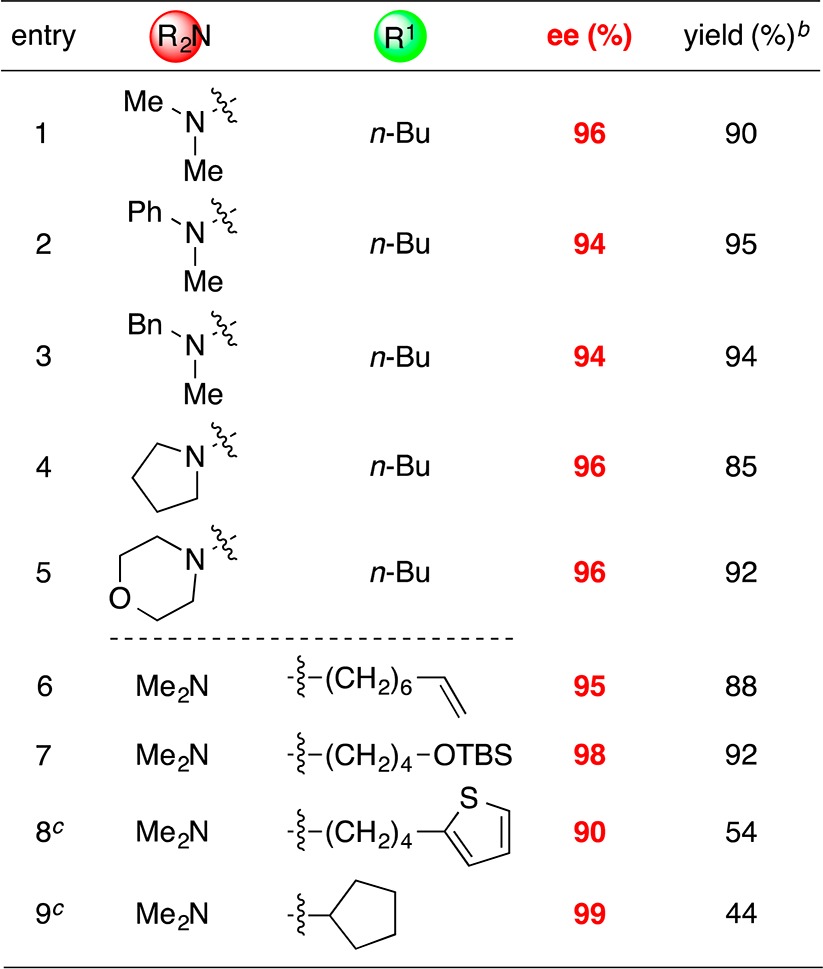
Under these conditions, we can achieve stereoconvergent Negishi cross-couplings of a variety of racemic α-bromosulfonamides with PhZnI to generate highly enantioenriched benzylic sulfonamides (Table 2).16 The nitrogen of the sulfonamide can bear either an alkyl or an aryl substituent, with little impact on ee or yield (entries 1–5). Furthermore, the R1 side chain can include functional groups and can be either primary (entries 6–8) or secondary (entry 9), although in the case of the latter a diminished yield is observed. On a gram scale, the cross-coupling illustrated in entry 2 proceeds in 92% ee and 98% yield.
Table 2. Stereoconvergent Negishi Phenylations of Racemic α-Bromosulfonamides: Scope with Respect to the Sulfonamidea.
All data are the average of two experiments.
Yield of purified product.
Amount of PhZnI: 1.5 equiv.
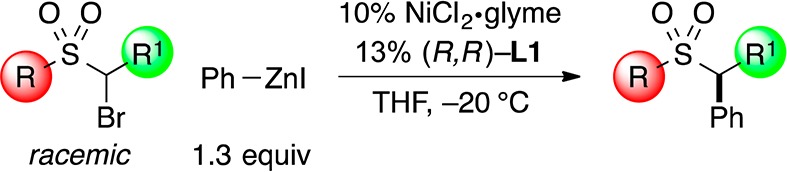
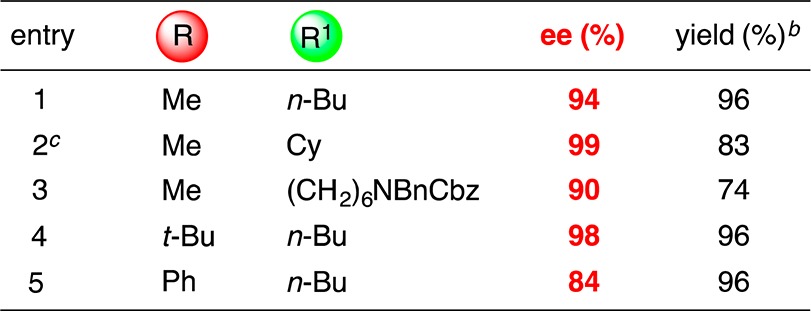
The conditions that we developed for stereoconvergent Negishi reactions of α-bromosulfonamides (Table 2) can be applied without modification to the corresponding sulfones (Table 3).17 The scope with respect to the sulfone is broad. Thus, the R substituent can range in steric demand from methyl to t-butyl (entries 1–4), and it can be aromatic (entry 5). With respect to the R1 group, it may be linear or branched (entries 1–5).
Table 3. Stereoconvergent Negishi Phenylations of Racemic α-Bromosulfones: Scope with Respect to the Sulfonea.
All data are the average of two experiments.
Yield of purified product.
Amount of PhZnI: 1.5 equiv.
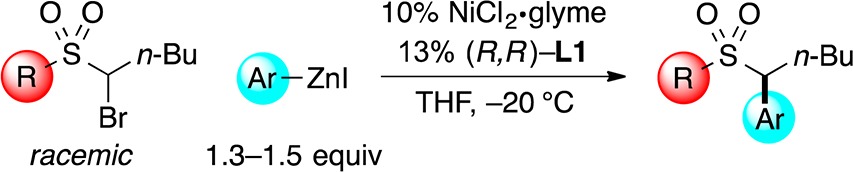
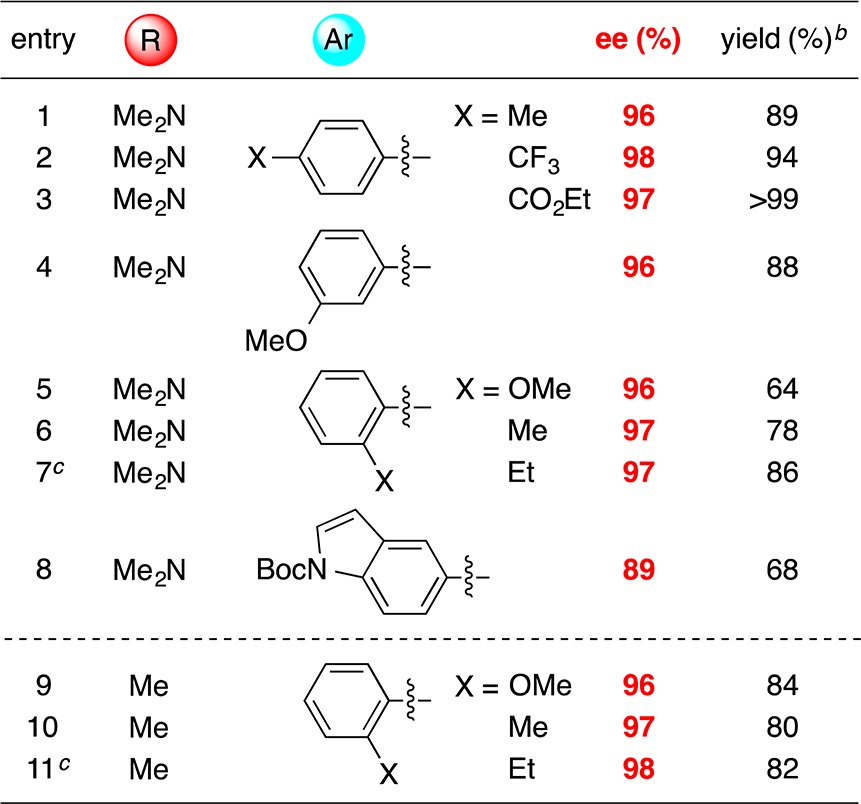
We have examined the scope of arylzinc nucleophiles that participate in this method for the catalytic asymmetric synthesis of benzylic sulfonamides and sulfones (Table 4). Electron-rich and electron-poor arylzinc reagents are suitable partners, as is a heteroarylzinc reagent, furnishing the desired product in very good ee. Especially noteworthy is our observation that o-substituted phenylzinc reagents can be employed with both sulfonamide- and sulfone-based electrophiles (entries 5–7 and 9–11); in our previous studies of nickel-catalyzed enantioselective arylations, o-substituted nucleophiles have generally been poor coupling partners.18,19
Table 4. Stereoconvergent Negishi Arylations of α-Bromosulfonamides and -Sulfones: Scope with Respect to the Arylzinc Reagenta.
All data are the average of two experiments.
Yield of purified product.
Amount of ArZnI: 2.0 equiv; amount of catalyst: 20% NiCl2·glyme, 26% (R,R)-L1.
Next, we sought to expand the scope of stereoconvergent cross-couplings of α-bromosulfonamides and -sulfones to include a second family of nucleophiles, specifically, alkenylmetal reagents. We determined that, whereas reactions of alkenylzinc reagents proceed in poor yield using the method described above, alkenylzirconium reagents serve as suitable nucleophiles under modified conditions (Table 5).20,21 Thus, an array of allylic sulfonamides and sulfones22 can be synthesized with good enantioselectivity in the presence of functional groups such as a thiophene and a primary alkyl chloride.
Table 5. Stereoconvergent Negishi Cross-Couplings of α-Bromosulfonamides and -Sulfones: Alkenylzirconium Reagents as Nucleophilesa.
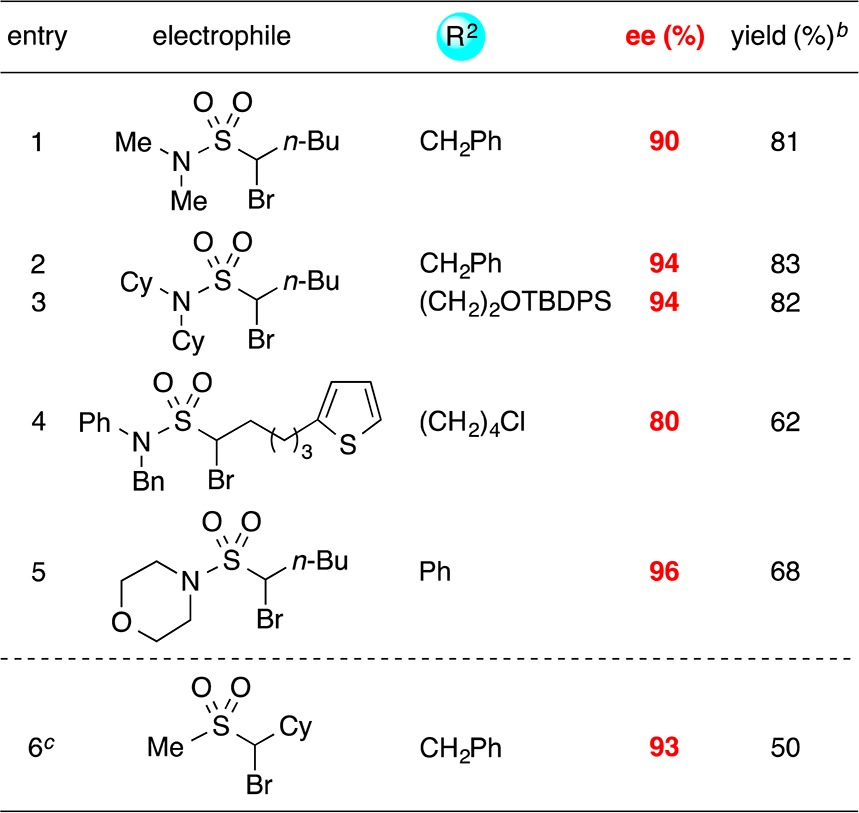
All data are the average of two experiments.
Yield of purified product.
Ligand used: (R,R)-L1.
We have suggested that, for at least some nickel-catalyzed cross-couplings of alkyl electrophiles, an alkyl radical may be generated during the oxidative-addition step of the catalytic cycle.23,24 In the case of electrophiles that bear an appropriately positioned pendant olefin, we have observed complete cyclization of the putative radical onto the olefin in some instances,25 and no cyclization in another.9f
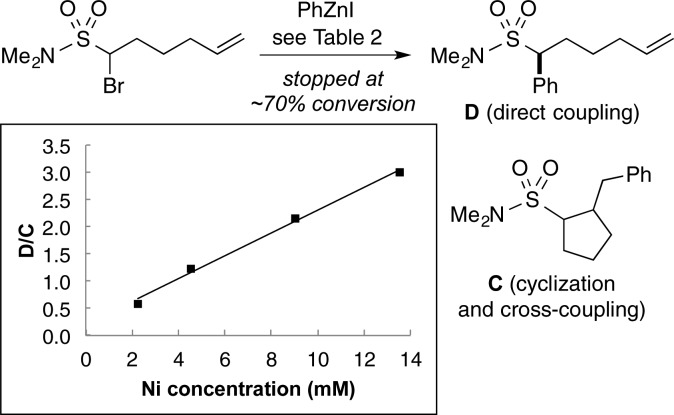
In order to gain insight into the mechanism of the stereoconvergent arylations described herein, we investigated the Negishi reaction of an α-bromosulfonamide bearing a pendant olefin (eq 3).26 At 70% conversion of the electrophile, we observe a 38% yield of the direct (uncyclized) cross-coupling product (8) and a 17% combined yield of cyclized cross-coupling products (9).
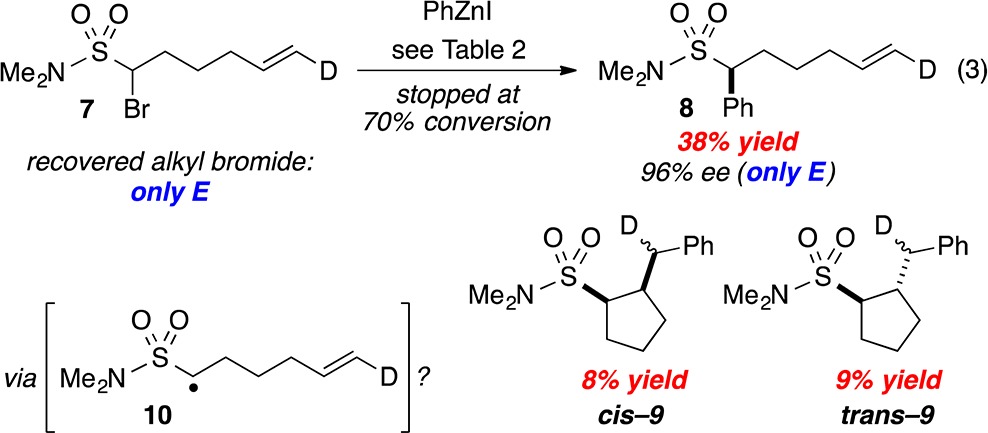 |
3 |
Cyclopentane derivatives cis-9 and trans-9 are each an ∼1:1 mixture of diastereomers (differing in the relative stereochemistry at the deuterium-bearing carbon), consistent with the expectation for cyclization of radical intermediate 10 (but not for a simple organometallic pathway involving only β-migratory insertion and then reductive elimination). Our observation that uncyclized cross-coupling product 8 has deuterium only in the trans position suggests that, when intermediate 10 does cyclize, the process is irreversible.
Simple 5-hexenyl radical cyclizations proceed with first-order rate constants of ∼105 s–1.27 Although, to the best of our knowledge, the rate of cyclization of sulfonamide-substituted radical 10 has not been determined, it is very likely to be significantly slower than diffusion (∼109 s–1).13a Our observation of cyclized, cross-coupled products (9) therefore represents evidence that a nickel-catalyzed asymmetric cross-coupling process may include a noncage radical pathway. Consistent with this suggestion, the ratio of uncyclized (D)/cyclized (C) product changes as the concentration of catalyst changes (Figure 1).28
Figure 1.
Dependence of the ratio of uncyclized (D)/cyclized (C) product on the concentration of nickel.
Conclusions
We have developed methods for the enantioselective synthesis of secondary sulfonamides and sulfones, specifically, nickel-catalyzed Negishi arylations and alkenylations of racemic α-bromosulfonamides and -sulfones with readily available organozinc and organozirconium reagents; with regard to stereoconvergent couplings of unactivated alkyl electrophiles, previous examples had been limited to organoboron nucleophiles and, with the exception of three isolated examples, to alkylation reactions. In terms of mechanism, a cyclization/stereochemical probe has provided evidence for a radical intermediate that has a sufficient lifetime to escape the solvent cage and cyclize irreversibly under the coupling conditions. Additional studies directed at elucidating the mechanisms of cross-coupling reactions of alkyl electrophiles, as well as expanding the scope of such processes, are underway.
Acknowledgments
Support has been provided by the National Institutes of Health (National Institute of General Medical Sciences: R01-GM62871), the Kwanjeong Educational Foundation (fellowship for J.C.), and MICINN (fellowship for P.M.-G.). We thank Dr. Ashraf Wilsily, Dr. Nathan D. Schley, Dr. David VanderVelde (Caltech NMR Facility), and Dr. Scott C. Virgil (Caltech Center for Catalysis and Chemical Synthesis, supported by the Gordon and Betty Moore Foundation) for assistance and for helpful discussions.
Supporting Information Available
Experimental procedures and compound characterization data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For leading references, see:; a Ashfaq M.; Shah S. S. A.; Najjam T.; Shaheen S.; Rivera G. Mini-Rev. Org. Chem. 2013, 10, 160–170. [Google Scholar]; b Wilden J. D. J. Chem. Res. 2010, 34, 541–548. [Google Scholar]; c Organosulfur Chemistry in Asymmetric Synthesis; Toru T., Bolm C., Eds.; Wiley–VCH: Weinheim, 2008. [Google Scholar]; d Simpkins N. S.Sulfones in Organic Synthesis; Pergamon: Oxford, 1993. [Google Scholar]
- For leading references, see:; a Shah S. S. A.; Rivera G.; Ashfaq M. Mini-Rev. Med. Chem. 2013, 13, 70–86. [PubMed] [Google Scholar]; b Scozzafava A.; Carta F.; Supuran C. T. Expert Opin. Ther. Patents 2013, 23, 203–213. [DOI] [PubMed] [Google Scholar]; c Chen X.; Hussain S.; Parveen S.; Zhang S.; Yang Y.; Zhu C. Curr. Med. Chem. 2012, 19, 3578–3604. [DOI] [PubMed] [Google Scholar]; d Kalgutkar A. S.; Jones R.; Sawant A. RSC Drug Discovery Series 2010, 1, 210–274. [Google Scholar]
- Examples of pharmaceuticals: Celebrex (NSAID), Crestor (cardiovascular), and Viagra (erectile dysfunction).
- Cyclic sulfonamides:; a Rawls K. A.; Grundner C.; Ellman J. A. Org. Biomol. Chem. 2010, 8, 4066–4070. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Yue E. W.; Wayland B.; Douty B.; Crawley M. L.; McLaughlin E.; Takvorian A.; Wasserman Z.; Bower M. J.; Wei M.; Li Y.; Ala P. J.; Gonneville L.; Wynn R.; Burn T. C.; Liu P. C. C.; Combs A. P. Bioorg. Med. Chem. 2006, 14, 5833–5849. [DOI] [PubMed] [Google Scholar]
- Yamada M.; Ichikawa T.; Ii M.; Itoh K.; Tamura N.; Kitazaki T. Bioorg. Med. Chem. 2008, 16, 3941–3958. [DOI] [PubMed] [Google Scholar]
- Teall M.; Oakley P.; Harrison T.; Shaw D.; Kay E.; Elliott J.; Gerhard U.; Castro J. L.; Shearman M.; Ball R. G.; Tsou N. N. Bioorg. Med. Chem. Lett. 2005, 15, 2685–2688. [DOI] [PubMed] [Google Scholar]
- For examples of reports by medicinal chemists that describe the synthesis and the utility of benzylic sulfonamides and sulfones, see:; a Grimm J. B.; Katcher M. H.; Witter D. J.; Northrup A. B. J. Org. Chem. 2007, 72, 8135–8138. [DOI] [PubMed] [Google Scholar]; b Zhou G.; Ting P.; Aslanian R.; Piwinski J. J. Org. Lett. 2008, 10, 2517–2520. [DOI] [PubMed] [Google Scholar]; c Zhou G.; Ting P. C.; Aslanian R. G. Tetrahedron Lett. 2010, 51, 939–941. [Google Scholar]
- For example, see:; a Nakamura S.; Hirata N.; Kita T.; Yamada R.; Nakane D.; Shibata N.; Toru T. Angew. Chem., Int. Ed. 2007, 46, 7648–7650. [DOI] [PubMed] [Google Scholar]; b Wu G.; Zhu J.; Ding Z.; Shen Z.; Zhang Y. Tetrahedron Lett. 2009, 50, 427–429. [Google Scholar]; c Ueda M.; Hartwig J. F. Org. Lett. 2010, 12, 92–94. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Jin Z.; Xu J.; Yang S.; Song B.-A.; Chi Y. R. Angew. Chem., Int. Ed. 2013, 52, 12354–12358. [DOI] [PubMed] [Google Scholar]
- Initial reports of various activated electrophiles: α-Halocarbonyl/alkylation:; a Fischer C.; Fu G. C. J. Am. Chem. Soc. 2005, 127, 4594–4595. [DOI] [PubMed] [Google Scholar]; Benzylic halide/alkylation:; b Arp F. O.; Fu G. C. J. Am. Chem. Soc. 2005, 127, 10482–10483. [DOI] [PubMed] [Google Scholar]; Allylic halide/alkylation:; c Son S.; Fu G. C. J. Am. Chem. Soc. 2008, 130, 2756–2757. [DOI] [PubMed] [Google Scholar]; α-Halocarbonyl/arylation and alkenylation: ; d Dai X.; Strotman N. A.; Fu G. C. J. Am. Chem. Soc. 2008, 130, 3302–3303. [DOI] [PubMed] [Google Scholar]; Propargylic halide/arylation: ; e Smith S. W.; Fu G. C. J. Am. Chem. Soc. 2008, 130, 12645–12647. [DOI] [PMC free article] [PubMed] [Google Scholar]; α-Halonitrile/arylation and alkenylation:; f Choi J.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 9102–9105. [DOI] [PMC free article] [PubMed] [Google Scholar]; Benzylic halide/arylation:; g Do H.-Q.; Chandrashekar E. R. R.; Fu G. C. J. Am. Chem. Soc. 2013, 135, 16288–16291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For examples of related work by others, see:; a Caeiro J.; Sestelo J. P.; Sarandeses L. A. Chem.—Eur. J. 2008, 14, 741–746. [DOI] [PubMed] [Google Scholar]; b Schmidt T.; Kirschning A. Angew. Chem., Int. Ed. 2012, 51, 1063–1066. [DOI] [PubMed] [Google Scholar]; c Cherney A. H.; Kadunce N. T.; Reisman S. E. J. Am. Chem. Soc. 2013, 135, 7442–7445. [DOI] [PubMed] [Google Scholar]; d Shields J. D.; Ahneman D. T.; Graham T. J. A.; Doyle A. G. Org. Lett. 2014, 16, 142–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- All are phenylation reactions PhBX2:; a Zultanski S. L.; Fu G. C. J. Am. Chem. Soc. 2011, 133, 15362–15364. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Wilsily A.; Tramutola F.; Owston N. A.; Fu G. C. J. Am. Chem. Soc. 2012, 134, 5794–5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- a Saito B.; Fu G. C. J. Am. Chem. Soc. 2008, 130, 6694–6695. [DOI] [PubMed] [Google Scholar]; b Owston N. A.; Fu G. C. J. Am. Chem. Soc. 2010, 132, 11908–11909. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Lu Z.; Wilsily A.; Fu G. C. J. Am. Chem. Soc. 2011, 133, 8154–8157. [DOI] [PMC free article] [PubMed] [Google Scholar]; d ref (11)
- For example, see:; a Paquette L. A. Synlett 2001, 1–12. [Google Scholar]; b Luo Y.-R.Chemical Bond Energies; CRC Press: Boca Raton, 2007. [Google Scholar]
- For a study of enantioselective alkyl–alkyl Suzuki cross-couplings that includes the use of sulfonamides and sulfones as remote directing groups, see ref (11)b.
- For previous examples of the use of bis(oxazoline) ligands in nickel-catalyzed stereoconvergent cross-couplings, see:; a Lou S.; Fu G. C. J. Am. Chem. Soc. 2010, 132, 1264–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]; b Lou S.; Fu G. C. J. Am. Chem. Soc. 2010, 132, 5010–5011. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Refs (9f), (9g), and (10c); d Liang Y.; Fu G. C. J. Am. Chem. Soc. 2014, 136, 5520–5524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Under our standard conditions: (a) During the course of a cross-coupling, the ee of the product was constant. (b) A modest kinetic resolution of an α-bromosulfonamide was observed (33% ee at 75% conversion). (c) In preliminary studies, an α-chlorosulfonamide, an alkylzinc halide, and a 2-thienylzinc halide were not suitable coupling partners.
- Under our standard conditions, an α-chlorosulfone was not a suitable cross-coupling partner.
- For recent examples, see refs (9g) and (15d).
- Under our standard conditions, doubly ortho-substituted arylzinc reagents are not suitable coupling partners.
- For a report of the use of alkenylzirconium reagents in nickel-catalyzed stereoconvergent cross-couplings of α-haloketones, see ref (15b).
- Under our standard conditions, an alkenylzirconium reagent derived from the hydrozirconation of an internal alkyne was not a suitable cross-coupling partner.
- For leading references to the synthesis and utility of these compounds, see:; a Gais H.-J. In Asymmetric Synthesis with Chemical and Biological Methods; Enders D., Jäger K.-E., Eds.; Wiley–VCH: Weinheim, 2007; pp 215–250. [Google Scholar]; b Wu X.-S.; Chen Y.; Li M.-B.; Zhou M.-G.; Tian S.-K. J. Am. Chem. Soc. 2012, 134, 14694–14697. [DOI] [PubMed] [Google Scholar]
- For an early suggestion, see:; a Zhou J.; Fu G. C. J. Am. Chem. Soc. 2004, 126, 1340–1341. [DOI] [PubMed] [Google Scholar]; For a recent discussion and leading references, see:; b Zultanski S. L.; Fu G. C. J. Am. Chem. Soc. 2013, 135, 624–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For early mechanistic studies by others of nickel-catalyzed cross-couplings of unactivated alkyl electrophiles, see:; a Jones G. D.; Martin J. L.; McFarland C.; Allen O. R.; Hall R. E.; Haley A. D.; Brandon R. J.; Konovalova T.; Desrochers P. J.; Pulay P.; Vicic D. A. J. Am. Chem. Soc. 2006, 128, 13175–13183. [DOI] [PubMed] [Google Scholar]; b Phapale V. B.; Buñuel E.; García-Iglesias M.; Caŕdenas D. J. Angew. Chem., Int. Ed. 2007, 46, 8790–8795. [DOI] [PubMed] [Google Scholar]; c Lin X.; Phillips D. L. J. Org. Chem. 2008, 73, 3680–3688. [DOI] [PubMed] [Google Scholar]
- For early examples, see: Stille arylations:; a Powell D. A.; Maki T.; Fu G. C. J. Am. Chem. Soc. 2005, 127, 510–511. [DOI] [PubMed] [Google Scholar]; Suzuki arylations:; b González-Bobes F.; Fu G. C. J. Am. Chem. Soc. 2006, 128, 5360–5361. [DOI] [PubMed] [Google Scholar]
- During the later stages of the cross-coupling, side reactions, such as olefin isomerization, intervene.
- Newcomb M. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu C., Studer A., Eds.; John Wiley & Sons: Chichester, 2012; Vol. 1, pp 107–124. [Google Scholar]
- For a recent related study of a primary alkyl iodide, see:Biswas S.; Weix D. J. J. Am. Chem. Soc. 2013, 135, 16192–16197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.