Abstract
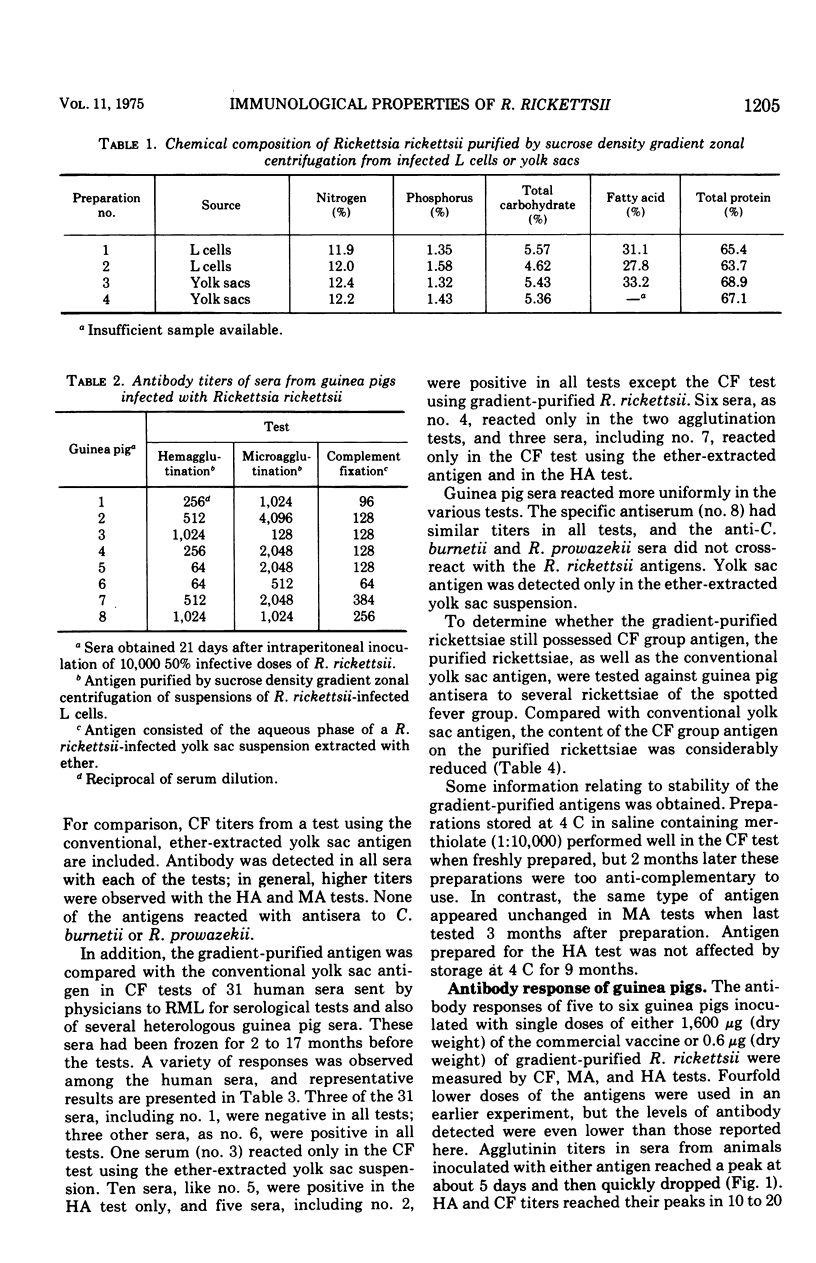
The properties of Rickettsia rickettsii purified from infected chicken yolk sacs or mouse L cell cultures by sucrose density gradient centrifugation in a zonal rotor were examined in various ways. Rickettsiae derived from both L cells and yolk sacs had similar compositions: about 12% nitrogen, 1.5% phosphorus, 5% carbohydrate, and 30% fatty acids. On a dry-weight basis, purified rickettsiae were at least 2,000 times as effective as a commercial spotted fever vaccine in protecting guinea pigs against infection with spotted fever rickettsiae and mice against death from toxin of R. rickettsii. Gradient-purified rickettsiae (0.6 μg) induced a serological response in guinea pigs of the same magnitude as that stimulated by 1,600 μg of the commercial vaccine. Gradient-purified rickettsiae had little group reactivity in complement fixation tests but became anti-complementary upon storage. Microagglutination and hemagglutination tests with the purified antigen gave promise of usefulness in diagnosis of spotted fever. These results suggest that zonal centrifugation may be a valuable procedure for the preparation of R. rickettsii vaccine and diagnostic reagent.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANACKER R. L., HASKINS W. T., LACKMAN D. B., RIBI E., PICKENS E. G. CONVERSION OF THE PHASE I ANTIGEN OF COXIELLA BURNETII TO HAPTEN BY PHENOL TREATMENT. J Bacteriol. 1963 May;85:1165–1170. doi: 10.1128/jb.85.5.1165-1170.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anacker R. L., Gerloff R. K., Thomas L. A., Mann R. E., Brown W. R., Bickel W. D. Purification of Rickettsia rickettsi by density-gradient zonal centrifugation. Can J Microbiol. 1974 Nov;20(11):1523–1527. doi: 10.1139/m74-238. [DOI] [PubMed] [Google Scholar]
- BELL E. J., PICKENS E. G. A toxic substance associated with the rickettsias of the spotted fever group. J Immunol. 1953 May;70(5):461–472. [PubMed] [Google Scholar]
- BELL E. J., STOENNER H. G. Immunologic relationships among the spotted fever group of rickettsias determined by toxin neutralization tests in mice with convalescent animal serums. J Immunol. 1960 Feb;84:171–182. [PubMed] [Google Scholar]
- BELL E. J., STOENNER H. G. Spotted fever vaccine; potency assay by direct challenge of vaccinated mice with toxin of Rickettsia rickettsii. J Immunol. 1961 Dec;87:737–746. [PubMed] [Google Scholar]
- CHANG S. M. A serologically-active erythrocyte-sensitizing substance from typhus rickettsiae. I. Isolation and titration. J Immunol. 1953 Mar;70(3):212–214. [PubMed] [Google Scholar]
- Cox H. R. CULTIVATION OF RICKETTSIAE OF THE ROCKY MOUNTAIN SPOTTED FEVER, TYPHUS AND Q FEVER GROUPS IN THE EMBRYONIC TISSUES OF DEVELOPING CHICKS. Science. 1941 Oct 31;94(2444):399–403. doi: 10.1126/science.94.2444.399. [DOI] [PubMed] [Google Scholar]
- DISCHE Z. New color reactions for determination of sugars in polysaccharides. Methods Biochem Anal. 1955;2:313–358. doi: 10.1002/9780470110188.ch11. [DOI] [PubMed] [Google Scholar]
- DRYER R. L., TAMMES A. R., ROUTH J. I. The determination of phosphorus and phosphatase with N-phenyl-p-phenylenediamine. J Biol Chem. 1957 Mar;225(1):177–183. [PubMed] [Google Scholar]
- DuPont H. L., Hornick R. B., Dawkins A. T., Heiner G. G., Fabrikant I. B., Wisseman C. L., Jr, Woodward T. E. Rocky Mountain spotted fever: a comparative study of the active immunity induced by inactivated and viable pathogenic Rickettsia rickettsii. J Infect Dis. 1973 Sep;128(3):340–344. doi: 10.1093/infdis/128.3.340. [DOI] [PubMed] [Google Scholar]
- Fiset P., Ormsbee R. A., Silberman R., Peacock M., Spielman S. H. A microagglutination technique for detection and measurement of rickettsial antibodies. Acta Virol. 1969 Jan;13(1):60–66. [PubMed] [Google Scholar]
- Kenyon R. H., Acree W. M., Wright G. G., Melchior F. W., Jr Preparation of vaccines for Rocky Mountain spotted fever from rickettsiae propagated in cell culture. J Infect Dis. 1972 Feb;125(2):146–152. doi: 10.1093/infdis/125.2.146. [DOI] [PubMed] [Google Scholar]
- Peacock M., Munoz J., Tallent G. L., Ormsbee R. A. Passive cutaneous anaphylaxis with antigens from Coxiella burneti. J Bacteriol. 1968 May;95(5):1580–1586. doi: 10.1128/jb.95.5.1580-1586.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- STOENNER H. G., LACKMAN D. B., BELL E. J. Factors affecting the growth of rickettsias of the spotted fever group in fertile hens' eggs. J Infect Dis. 1962 Mar-Apr;110:121–128. doi: 10.1093/infdis/110.2.121. [DOI] [PubMed] [Google Scholar]
- Wood W. H., Jr, Wisseman C. L., Jr The cell wall of Rickettsia mooseri. I. Morphology and chemical composition. J Bacteriol. 1967 Mar;93(3):1113–1118. doi: 10.1128/jb.93.3.1113-1118.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]


