Abstract
Osteosarcoma (OS) is the most common primary malignant bone tumor that has poor prognosis. Molecular mechanisms underlying disease progression remain largely unknown. Sox9, one of the Sox family transcription factors, is closely associated with the development of a variety of malignant tumors. This study investigates the expression of Sox9, Wnt1 and Fzd1 in human osteosarcoma tissues and cells and the role of Sox9 in the proliferation of human osteosarcoma cells. Immunohistochemical analyses for Sox9, Wnt1, Fzd1, and Ki-67 proteins were performed in human primary osteosarcoma tissues from 48 patients. The small interfering RNA (siRNA) of Sox9 was transfected into human osteosarcoma MG63 cells. At 24 and 48 h after transfection with Sox9 siRNA, the expression of Wnt1 and Fzd1 was analyzed by RT-qPCR, Western blot, and immunofluorescence techniques. Cell proliferation was assayed by CCK-8 method, and Ki-67 protein expression was analyzed by Western blot. Results showed that the expressions of Sox9, Wnt1, Fzd1, and Ki-67 proteins in human osteosarcoma tissues were higher than those in the adjacent non-cancerous tissues. Hyperexpressions of Sox9, Wnt1, Fzd1, and Ki-67 proteins occurred more frequently in human osteosarcoma tissues with an advanced clinical stage (IIb/III). Sox9 siRNA reduced both mRNA and protein expression levels of Wnt1 and Fzd1, which result in the distinct inhibition of MG63 cell proliferation. Our study suggests that Sox9 siRNA inhibits the proliferation capability of human osteosarcoma cells by down-regulating the expression of Wnt1 and its receptor Fzd1, which may provide new gene targets for the clinical treatment of osteosarcoma.
Keywords: Osteosarcoma, MG63 cells, Sox9, Wnt1, Fzd1, proliferation
Introduction
Osteosarcoma (OS), the most common primary malignant bone tumor, predominantly occurs in children and adolescents and accounts for 8.9% of cancer-related deaths, carrying an overall five-year survival rate of 60%-70% [1-3]. OS has a high tendency for local invasion and early metastasis. Even with early diagnosis and aggressive treatment, the prognosis for OS remains poor. Molecular mechanisms underlying disease progression remain poorly understood [4,5]. Therefore, a better understanding of the underlying mechanisms of human osteosarcoma development and progression, as well as identifying molecular targets, is necessary to find effective therapeutic interventions.
The Wnt/β-catenin pathway serves important functions in multiple biological processes, including regulation of cell proliferation, differentiation, and migration [6-8]. The Wnt/β-catenin signaling pathway is activated by the binding of Wnt ligands to Frizzled (Fzd) receptors and low-density lipoprotein receptor-related proteins-5/6 (LRP5/6) coreceptors. As a result, β-catenin accumulates in the cytoplasm and subsequently translocates to the nucleus, where it regulates the transcription of target genes. Aberrations in the Wnt/β-catenin pathway are associated with numerous human diseases, including cancer, degenerative diseases, and diseases of the bone [7-9].
The sex determining region Y (SRY) box (Sox) gene family belongs to the high mobility group (HMG) superfamily. Increasing evidence shows that Sox9, one of Sox transcription factors, serves a key function in the progression of several kinds of tumors, such as pancreatic cancer, breast cancer, gastric cancer, and ovarian cancer [10-13]. Wang et al. [13] reported that Sox9-mediated Wnt/β-catenin activation is identified as one of the molecular mechanisms in the development and progression of breast cancer. Zhu et al [14] reported that Sox9 is upregulated in aggressive osteosarcoma tissues indicating that Sox9 may participate in the osteosarcoma progression. In this study, we investigate the potential role and mechanism of Sox9 in the development and progression of human osteosarcoma and clarify the regulatory role of Sox9 on Wnt1 and its receptor Fzd1 in vitro and in vivo. Our study may provide new gene targets for the clinical treatment of osteosarcoma.
Materials and methods
Clinical samples and data
Forty-eight consecutive cases of human primary osteosarcoma samples and adjacent non-cancerous tissue samples from the same specimens were collected in the pathology department of the Affiliated Hospital of Weifang Medical University from 2007 to 2012. No patients had received radio therapy or chemotherapy before surgery. Of the 48 patients, 29 were female, and 19 were male. The patient ages ranged from 10 to 52 years, and the mean age was 26.5 years. All tissues were formalin-fixed and paraffin-embedded. The clinical stages of these osteosarcoma patients were classified according to the Enneking staging system. Patient consent and the approval from the Medical Ethics Committee of Weifang Medical University were obtained prior to the use of clinical materials for research purposes.
Immunohistochemical (IHC) staining
The expressions of Sox9, Wnt1, Fzd1, and Ki-67 proteins were examined in the human primary osteosarcoma samples and the adjacent non-cancerous tissues by IHC. Formalin-fixed, paraffin-embedded tissues were resected to 5 μm and placed on coated glass slides. After heat-induced antigen retrieval, nonspecific staining was blocked by incubation with 10% bovine serum albumin at room temperature for 30 min. The sections for IHC assay were incubated with Sox9 antibody (1:100, Santa Cruz Biotechnology, Inc, USA), Ki-67 (1:100, Santa Cruz Biotechnology, Inc, USA), Wnt1 (1:100, Bioworld, Inc, USA), and Fzd1 antibody (1:50, Santa Cruz Biotechnology, Inc, USA), followed by incubation with the corresponding secondary antibody conjugated with horseradish peroxidase. The protein of interest was visualized using 3,3’-diaminobenzidine (DAB) staining. The degree of immunostaining of the formalin-fixed, paraffin-embedded sections was reviewed and evaluated independently by two observers based on the percentage of positive tumor cells and the intensity of staining. The percentage scoring of immunoreactive tumor cells was as follows: 0 (no positive tumor cells), 1 (< 10% positive tumor cells), 2 (10% to 50% positive tumor cells), and 3 (> 50% positive tumor cells). The intensity of staining was recorded at the following scale: 0 (no staining), 1 (weak staining, light yellow), 2 (moderate staining, yellowish brown), and 3 (strong staining, brown). A final immunoreactivity score (IRS) was obtained for each case by multiplying the percentage and intensity score. The expression levels were further analyzed by classifying IRS values as low (based on an IRS value < 5) and high (based on an IRS value > 5) [15]. We performed staining in the absence of each of the primary antibodies by only adding phosphate buffered saline (PBS) to the sections as negative controls. The sections were photographed by using an optical microscope (Olympus, Tokyo, Japan) and then analyzed using the Image-ProPlus6.0 analytic system (IPP6.0).
Cell culture
MG63 human osteosarcoma cells were purchased from the cell bank of Shanghai Institute of Cell Biology (Shanghai, China) and were maintained in Modified Eagle’s Medium (MEM) with 10% fetal bovine serum (FBS) (Gibco BRL Co. Ltd.), 100 U/mL of penicillin, and 100 g/mL of streptomycin at 37°C in a humidified atmosphere containing 5% CO2.
Small interfering RNA (siRNA) transfection
MG63 cells were seeded in a six-well plate at a density of 4×105 cells per well. Cells were cultured in 6-well plates for 24 h, then transfected with Sox9 siRNA or negative control siRNA with a final concentration of 60 nM using Lipofectamine™2000 transfection reagent (Invitrogen, Carlsbad, CA, USA) following manufacturer’s recommendations. siRNA and negative control were purchased from Guangzhou RiboBio Co., Ltd. The specifications of Sox9 siRNA were as follows: sense was 5’-GCAGCGACGUCAUCUCCAAdTdT-3’ and anti-sense was 5’-dTdTCGUCGCUGCAGUAGAGGUU-3’. Briefly, siRNA and lipofectamine™2000 were diluted in Opti-MEM (Invitrogen) separately and incubated for 5 min at room temperature. The diluted solutions were then mixed and incubated for 20 min at room temperature. Subsequently, the mixtures were added to each well containing the cells and medium. The cell culture plates were then incubated for 6 h at 37°C in a CO2 incubator. The cell medium was then replaced with a complete culture medium. Knockdown efficiency of Sox9 gene expression was then assessed at 24 and 48 h hours after transfection using RT-qPCR and Western blot analysis.
RNA isolation and quantitative real-time polymerase chain reaction (RT-qPCR)
The siRNA transduction efficiency was validated using RT-qPCR analysis after transfection for 24 and 48 h. The total RNA from MG63 cells was extracted using TRIZOL Reagents (Invitrogen, Carlsbad, CA, USA) at 24 and 48 h after transfection, and RNA quality was verified by measuring the OD260/OD280 ratio. Then, 2 μg of total RNA and oligo-dT primer were used for reverse transcription (Promega, Madison, WI, USA) according to the manufacturer’s protocol. The resulting complementary (c) DNA was used for qPCR analysis using SoFast TMEva Green Supermix mix (BIO-RAD, Singapore). RT-qPCR was then performed in triplicate using a Real-time PCR Detection System (BIO-RAD). Target gene expression was normalized to that of the endogenous control β-actin. The reaction system of PCR was as follows: 10 μL of 2× SoFast TMEva Green Supermix mix; 1 μL of each primer, 2 μL of cDNA template, and 6 μL of sterile distilled water. The following primers were used: Sox9: (sense, 5’-TGC TCA AGG GCT ACG ACT G-3’; antisense, 5’-ACG CTT CTC GCT CTC ATT CA-3’); Wnt1: (sense, 5’-CCA CGA GTT TGG ATG TTG TAA A-3’; antisense, 5’-GCA GGG AGA AAG GAG AGA AGA G-3’); Fzd1: (sense, 5’-ACT CCC TTC TCC CAC CTT AGT T3’; antisense, 5’-ATG CTT CTT CCC AAA TCT CAG T-3’); and β-actin: (sense, 5’-TGA CGT GGA CAT CCG CCA AG-3’; antisense, 5’-CTG GAA GGT GGA CAG CGA GG-3’). The initial denaturation step was at 95°C for 5 min, followed by 40 cycles at 95°C for 5 sec, 57°C for 40 s, 72°C for 30 s, and 80°C for 10 s. The relative mRNA expression was measured using the 2-ΔΔCT method [16].
Western blot analysis
After transfection with siRNA Sox9 or negative control for 24 and 48 h, MG63 cells were washed twice with ice-cold PBS. The cells were then suspended in a RIPA buffer containing protease inhibitor cocktail (Sigma, St. Louis, MO, USA) for 30 min on ice. Suspensions were centrifuged at 12,000 rpm for 15 min at 4°C, and the supernatant was collected for experiments. After the protein concentration was determined using a BCA protein assay kit (Pierce, South Logan, UT, USA), the total protein (60 μg of total protein per well) was separated on a 12% polyacrylamide gel by electrophoresis and transferred to a polyvinylidene difluoride membrane. The membranes were blocked with 5% non-fat milk for 1 h at room temperature and then probed overnight at 4°C with primary rabbit polyclonal antibodies against Sox9 (1:1000, Santa Cruz), Wnt1 (1:800, Bioworld), Fzd1 (1:1000, Santa Cruz), Ki-67 (1:800, Santa Cruz), and mouse monoclonal antibody against GAPDH (1:2500, Proteintech Group. Inc, USA) diluted in 3% BSA in PBS. After washing with PBS, the membranes were incubated with the appropriate horseradish peroxidase-linked secondary antibody (1:20000, Jackson ImmunoResearch, West Grove, PA, USA) diluted in 5% non-fat milk for 2 h at room temperature. The protein bands were visualized using an enhanced chemiluminescence assay instrument (Thermo, Rockford, IL, USA) [17]. The house-keeping protein GAPDH was used as an internal control. The intensity of each band was measured by IPP6.0.
Immunofluorescence
Cells were plated onto coverslips in MEM medium with 10% FBS for 24 h. The cells were then transfected with siRNA Sox9 or negative control. At 24 and 48 h after transfection, the cells were fixed with 4% paraformaldehyde for 20 min, incubated in 0.3% Triton X-100-PBS for 10 min at room temperature, followed by blocking with 5% goat serum at 37°C for 30 min. The cells were then incubated with the following primary antibodies at 4°C overnight: rabbit anti-Sox9 IgG (1:100, Santa Cruz), rabbit anti-Wnt1 IgG (1:100, Bioworld), and rabbit anti-Fzd1 IgG (1:100, Santa Cruz). The samples were soaked in goat anti-rabbit IgG conjugated to Cy3 (1:400; Jackson ImmunoResearch) at 37°C for 1 h. Subsequently, the nuclei were counter stained with Hoechst33258 (1:1000; Sigma-Aldrich, Inc., MO, USA). Images were obtained using an inverted fluorescence microscope (Olympus). The primary antibody was replaced with PBS as the negative control.
Cell counting Kit-8 (CCK-8) assay
MG63 cells were seeded in 96-well plates at a density of 5×103 cells/well. After 24 h of incubation, 60 nM siRNA-sox9 or negative control was added to each well. The plates were then incubated for 6 h at 37°C, and the cell medium was replaced with 100 μL of culture medium. Cell proliferation was measured using a CCK-8 assay. At 24 h intervals, 10 μL of CCK-8 reagent (Roche Biochemicals, Mannheim, Germany) was added to each well, and the cells were further incubated for 2 h at 37°C. The optical absorbance (OD) was then measured at 450 nm using a 96-well microplate reader. All experiments were performed in triplicate.
Statistical analysis
Statistical analyses were performed using SPSS 13.0 software. All the data were expressed as mean ± standard deviation (SD). Comparison between two groups was carried out using a Student’s t test. The Chi-square test was used to analyze the relationship among Sox9, Wnt1, Fzd1, Ki-67 expression and clinicopathologic characteristics. A p value < 0.05 was considered statistically significant.
Results
Expressions of Sox9, Wnt1, Fzd1, and Ki-67 proteins in human osteosarcoma tissues
The expression and localization of Sox9 were examined by using IHC staining. Sox9 proteins were expressed mainly in the nucleus of human osteosarcoma cells. Results show that the expression level of Sox9 in osteosarcoma tissues was distinctly higher than the adjacent non-cancerous tissues (Figure 1). Among 48 osteosarcoma specimens, 32 (66.7%) cases were identified as high-level Sox9 expression (IRS value > 5) and 16 (33.3%) as low-level Sox9 expression (IRS value < 5). We then analyzed the associations of Sox9 expression with various clinicopathological parameters of osteosarcoma tissues. As shown in Table 1, the rates of high Sox9 expression in stages IIa and IIb/III of osteosarcoma tissues were 40.9% (9/22) and 88.5% (23/26), respectively (Table 1). This finding suggests that the level of Sox9 expression is strongly correlated to tumor stages. No significant difference was observed between Sox9 expression and the patient age or gender.
Figure 1.
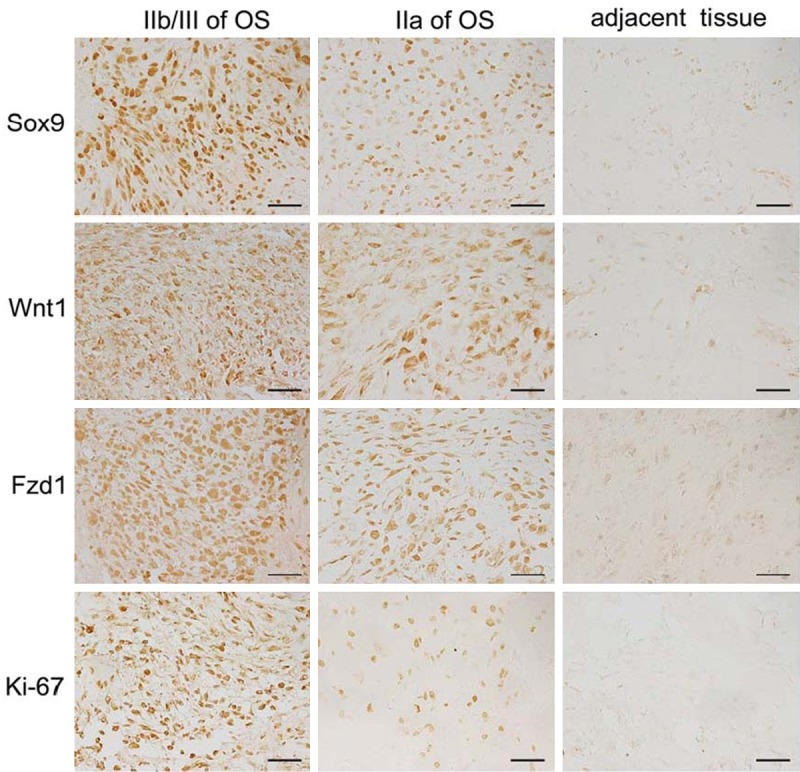
Immunohistochemistry of Sox9, Wnt1, Fzd1, and Ki-67 protein expression in osteosarcoma tissues and the adjacent non-cancerous tissue, bar = 50 μm.
Table 1.
Associations of the expressions of Sox9, Wnt1, Fzd1 and Ki-67 with tumor clinical stages
| IIa stage (n = 22) | IIb/III stage (n = 26) | P-value | |
|---|---|---|---|
| Sox9 expression | |||
| High (n, %) | 9 (40.9) | 23 (88.5) | P < 0.001* |
| Low (n, %) | 13 (59.1) | 3 (11.5) | |
| Wnt1 expression | |||
| High (n, %) | 11 (50.0) | 24 (92.3) | P = 0.001* |
| Low (n, %) | 11 (50.0) | 2 (7.7) | |
| Fzd1 expression | |||
| High (n, %) | 6 (27.2) | 21 (80.8) | P < 0.001* |
| Low (n, %) | 16 (72.8) | 5 (19.2) | |
| Ki-67 expression | |||
| High (n, %) | 7 (31.8) | 19 (73.1) | P = 0.004* |
| Low (n, %) | 15 (68.2) | 7 (26.9) |
With significant difference.
Our data shows that the staining of Wnt1 and Fzd1 in the tissues of osteosarcoma was obviously stronger than that in the adjacent non-cancerous tissues (Figure 1), which is consistent with the expression of Sox9. We also found that the expression of Wnt1 and Fzd1 is correlated to the tumor clinical stage in human osteosarcoma patients. The rates of high-level Wnt1 expression in stages IIa and IIb/III OS were 50.0% (11/22) and 92.3% (24/26), respectively (Table 1). The rates of high-level Fzd1 expression in stages IIa and IIb/III OS were 27.2% (6/22) and 80.8% (21/26) (Table 1). Additionally, hyper-expressions of Wnt1 and Fzd1 more frequently occurred in osteosarcoma tissues with advanced clinical stages.
We also found that a high level of Ki-67, a cellular marker for proliferation, expression is strongly correlated to the osteosarcoma clinical stage of the tissue specimens (Figure 1). The rate of high-level Ki-67 expression in stages IIb/III of OS (73.1%) was higher than that in stage IIa of OS (31.8%) (Table 1).
Sox9 siRNA effectively inhibited the expression of Sox9 mRNA and protein of human osteosarcoma cells
To examine the potential function of Sox9 in osteosarcoma progression, we targeted Sox9 expression in MG63 cells using siRNA against Sox9. At 24 and 48 h after transfection, RT-qPCR and Western blot analysis were used to determine the effect of siRNA on endogenous Sox9 expression. As shown in Figure 2, both the mRNA and protein levels of Sox9 were significantly decreased compared with those of the negative control (P < 0.05). This result suggests that Sox9 siRNA effectively inhibited the expressions of Sox9 mRNA and protein in human osteosarcoma cells.
Figure 2.
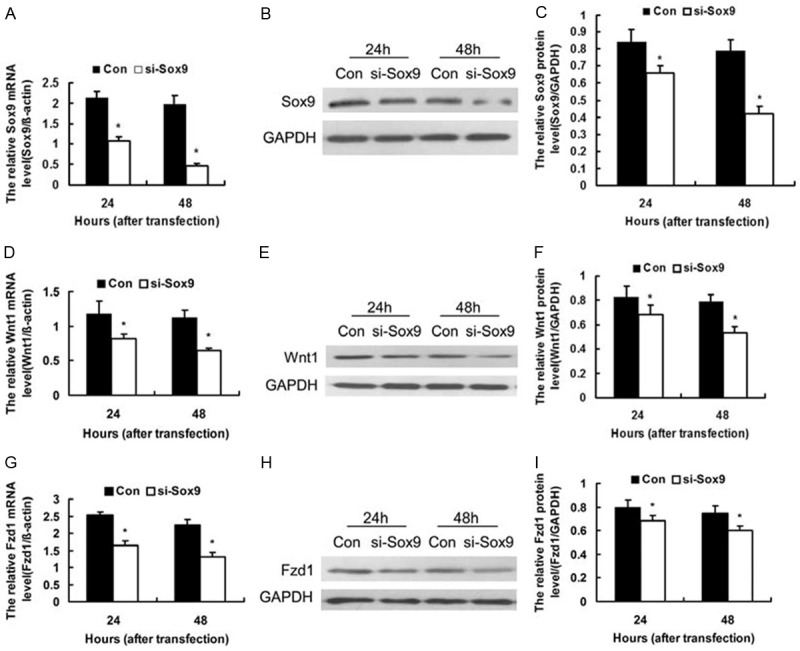
Knockdown of Sox9 down-regulated the expression of Wnt1 and its receptor Fzd1 in MG63 cells at 24 and 48 h after transfection with Sox9 siRNA (si-Sox9) and the negative control siRNA (Con). A: Bar chart showing knockdown efficiency of Sox9 mRNA expression by RT-qPCR (n = 3). B: Representative Western blot of Sox9 protein. GAPDH was used for normalization. C: Knockdown efficiency of Sox9 protein expression analyzed by Western blot (n = 4). D: Bar chart showing results of RT-qPCR analysis of Wnt1 mRNA level, with β-actin used for normalization (n = 3). E: Representative Western blot of Wnt1 protein. F: Bar chart showing the relative intensity of Wnt1 protein as analyzed by Western blot (n = 4) with GAPDH used for normalization. G: Bar chart showing results of RT-qPCR analysis of Fzd1 mRNA level with β-actin used for normalization (n = 3). H: Representative Western blot of Fzd1 protein. I: Bar chart showing the relative intensity of Fzd1 protein level as analyzed by Western blot (n = 4) with GAPDH used for normalization. *P < 0.05, vs. Con.
Knockdown of Sox9 down-regulated the expression of Wnt1 and its receptor Fzd1 in MG63 cells in vitro
To examine the potential role of Sox9 in regulating Wnt signaling, we detected the expression of Wnt1 and its receptor Fzd1 in MG63 cells with Sox9 siRNA using RT-qPCR and Western blot analysis at 24 and 48 h after transfection. The result showed that compared with those of the negative control, the mRNA and protein levels of Wnt1 and Fzd1 are significantly decreased at 24 and 48 h after transfection (P < 0.05) (Figure 2). Immunofluorescence results showed that Wnt1 and Fzd1 expression at 24 and 48 h are decreased in MG63 cells transfected with siRNA Sox9 compared with negative control (Figure 3).
Figure 3.
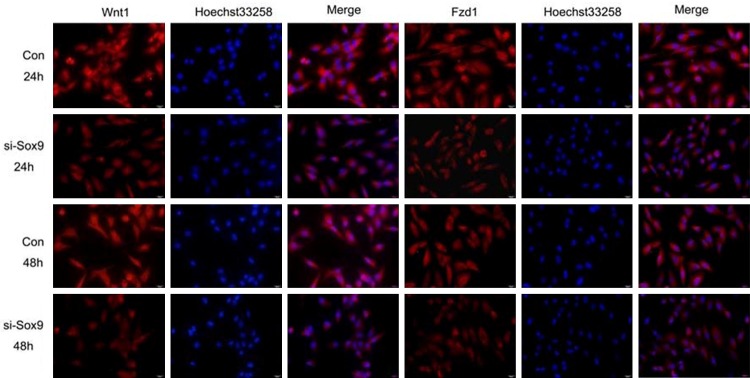
Expression of Wnt1 and its receptor Fzd1 in MG63 cells at 24 and 48 h after transfection with Sox9 siRNA (si-Sox9) and the negative control siRNA (Con) by immunofluorescence staining, bar = 20 μm.
Knockdown of Sox9 inhibited the proliferation of MG63 cells in vitro
To investigate the effects of Sox9 on the proliferation of osteosarcoma cells, CCK-8 assay was performed every 24 h. A proliferation curve was then plotted. Absorbance at 450 nm showed that MG63 cells transfected with Sox9 siRNA are less than those transfected with the negative control (Figure 4A), which suggests that the knockdown of Sox9 inhibited MG63 cell proliferation.
Figure 4.
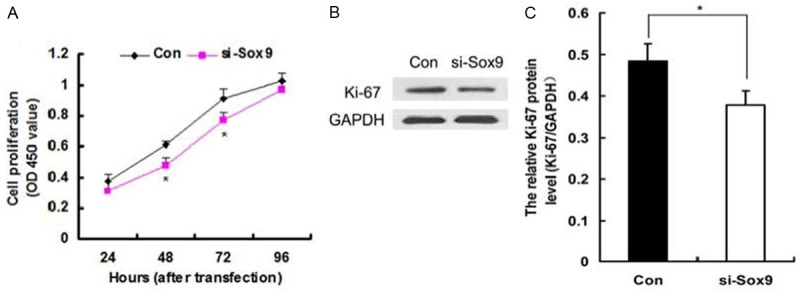
Silencing of Sox9 inhibited cell proliferation of MG63 cells in vitro. A: CCK-8 assay demonstrated that silencing of Sox9 inhibited the cell proliferation capability of MG63 cells on the indicated time points following transfection with Sox9 siRNA (si-Sox9). B: Representative Western blot of Ki-67 protein in MG63 cells at 48 h after transfection with Sox9 siRNA and negative control siRNA (Con). C: Bar chart showing the relative intensity of Ki-67 protein as analyzed by Western blot (n = 3) with GAPDH used for normalization. *P < 0.05 vs. Con.
We also detected the expression of Ki-67 in MG63 cells at 48 h after transfection with Sox9 siRNA using Western blot analysis. The result showed that the knockdown of Sox9 decreases the expression of Ki-67 (Figure 4B, 4C), consistent with the results of the CCK-8 assay, which indicated that proliferation is inhibited by the knockdown of Sox9.
Discussion
Osteosarcomagenesis is a complex process involved in multiple factors and regulated by multiple genes. A disorder of the regulation genes results in uncontrolled cell proliferation and tumorigenesis. Sox gene family belongs to the HMG superfamily. Sox9 serves an important function in embryonic development, cell proliferation, and sex differentiation [18-22]. Recent studies have also implicated the Sox9 gene in the development and progression of different neoplasms [10-13]. Raspaglio et al. [11] reported that Sox9 regulates TUBB3 gene expression and affects ovarian cancer aggressiveness. Additionally, aberrant Sox9 expression by GKN1 inactivation may be involved in the development of sporadic gastric cancers [12]. Our study showed that the expression of Sox9 in osteosarcoma tissues is distinctly higher than that in adjacent non-cancerous tissues. A strong correlation was observed between levels of Sox9 expression and the clinical stage of the tumor. High expression more frequently occurred in osteosarcoma tissues with advanced clinical stages, suggesting that the high expression levels of Sox9 in osteosarcoma may partly be responsible for disease progression. Our results confirmed the data of Zhu et al. that Sox9 is upregulated in aggressive osteosarcoma tissues indicating that Sox9 may participate in the osteosarcoma progression [14].
To investigate the function of Sox9 in osteosarcoma cell proliferation, a CCK-8 assay was performed in MG63 osteosarcoma cells. The result showed that the cell proliferation of MG63 osteosarcoma cell lines is significantly inhibited by Sox9 siRNA in vitro. The rate of high-level Ki-67, a cellular marker for proliferation, expression significantly increased in high-grade osteosarcoma clinical samples and silencing of Sox9 decreased the expression of Ki-67 protein in MG63 cells. All the results demonstrated that the knockdown of Sox9 significantly inhibits the proliferation of MG63 cells, suggested that Sox9 is an essential factor involved in the growth of human osteosarcoma cells. These observations are in agreement with the findings of similar studies on a variety of tumor cells, which further confirms the critical function of Sox9 in the survival and proliferation of tumor cells [10-13]. Thus, an investigation of the underlying molecular mechanism of Sox9 on cell proliferation in OS is required.
The Wnt signaling pathway serves an important function in regulating cell proliferation and differentiation. The deregulation of Wnt signaling pathway has been implicated in many human diseases, ranging from cancers to skeletal disorders. Previous reports have demonstrated that abnormal expressions of a variety of Wnt signaling molecules are closely related to the development of osteosarcoma. Haydon et al. [23] demonstrated increased β-catenin accumulation in 33 out of 47 OS samples. The deregulation of β-catenin signaling is implicated in the pathogenesis of osteosarcoma. Hoang et al. [24] found that the expression of LRP5, one of the coreceptors of Wnt ligands, is a novel marker for the disease progression of high-grade osteosarcoma. Dickkopf3 inhibits the invasion and motility of osteosarcoma cells SAOS-2 by modulating the Wnt/β-catenin signaling pathway [25].
The function of Wnt1 and its receptor Fzd1 in the pathogenesis of OS is not yet fully elucidated. Our result indicated that the expressions of Wnt1 and Fzd1 in osteosarcoma tissues are significantly higher than that in adjacent non-cancerous tissues. A strong correlation was found between levels of Wnt1 and Fzd1 with clinical tumor stage. A consistent change in the hyperexpression of Wnt1 and Fzd1 corresponded with that of Sox9.
Research on the relationship between the Sox gene family and Wnt signaling pathway has increased in recent years [26-29]. Sox9 has emerged as a modulator of the Wnt signaling pathway in diverse development and disease contexts. Sox9 regulates chondrogenesis by promoting efficient β-catenin phosphorylation in the nucleus [30]. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner [31]. Sox9 mediated the activation of Wnt/β-catenin signaling pathway in breast cancer [13].
However, limited information is known about the relationship between Sox9 and Wnt1 or Fzd1 in the OS. To examine the potential function of Sox9 in regulating Wnt signaling, we detected the expressions of Wnt1 and Fzd1 in MG63 cells at 24 and 48 h after transfection with Sox9 siRNA. The result showed that the knockdown of Sox9 down-regulates the expression of Wnt1 and its receptor Fzd1 in MG63 cells in vitro.
This finding suggests that the mechanism of osteosarcoma cell proliferation inhibited by Sox9 knockdown may be related to its abnormalities on Wnt signaling pathway.
In conclusion, the results of this study demonstrated that Sox9, Wnt1, Fzd1, and Ki-67 are highly expressed in high-grade osteosarcoma tissues. Knockdown of Sox9 gene decreases the proliferation capability of MG63 cells that may results by down-regulation of Wnt1 and its receptor Fzd1 expression, thus suggesting that Sox9 serves an important factor in the development and progression of osteosarcoma by regulating Wnt1/Fzd1 signaling pathway. Therefore, Sox9, Wnt1 and Fzd1 might be critical therapeutical targets for osteosarcoma.
Acknowledgements
This work was financially supported by the Shandong Province Natural Science Foundation of China (ZR2012HQ021), the Shandong Provincial Education Department of China (J11LF16, J12LK51, J12LK05), the Science and Technology Development Program of Weifang in China (201301074), the Science and Technology Innovation Foundation of Weifang Medical University (K11QC1005, K11QC1021), Brigham and Women’s Hospital BRI Fund to Sustain Research Excellence, the Muscular Dystrophy Association, the ALS Therapy Alliance, the Bill & Melinda Gates Foundation, and the Shandong Province Taishan Scholar Project.
Disclosure of conflict of interest
None.
References
- 1.Posthumadeboer J, Piersma SR, Pham TV, van Egmond PW, Knol JC, Cleton-Jansen AM, van Geer MA, van Beusechem VW, Kaspers GJ, van Royen BJ, Jiménez CR, Helder MN. Surface proteomic analysis of osteosarcoma identifies EPHA2 as receptor for targeted drug delivery. Br J Cancer. 2013;109:2142–2154. doi: 10.1038/bjc.2013.578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen X, Luther G, Zhang W, Nan G, Wagner ER, Liao Z, Wu N, Zhang H, Wang N, Wen S, He Y, Deng F, Zhang J, Wu D, Zhang B, Haydon RC, Zhou L, Luu HH, He TC. The E-F hand calcium-binding protein S100A4 regulates the proliferation, survival and differentiation potential of human osteosarcoma cells. Cell Physiol Biochem. 2013;32:1083–1096. doi: 10.1159/000354508. [DOI] [PubMed] [Google Scholar]
- 3.Yan K, Gao J, Yang T, Ma Q, Qiu X, Fan Q, Ma B. MicroRNA-34a inhibits the proliferation and metastasis of osteosarcoma cells both in vitro and in vivo. PLoS One. 2012;7:e33778. doi: 10.1371/journal.pone.0033778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McQueen P, Ghaffar S, Guo Y, Rubin EM, Zi X, Hoang BH. The Wnt signaling pathway: implications for therapy in osteosarcoma. Expert Rev Anticancer Ther. 2011;11:1223–1232. doi: 10.1586/era.11.94. [DOI] [PubMed] [Google Scholar]
- 5.Wu CL, Tsai HC, Chen ZW, Wu CM, Li TM, Fong YC, Tang CH. Ras activation mediates WISP-1-induced increases in cell motility and matrix metalloproteinase expression in human osteosarcoma. Cell Signal. 2013;25:2812–2822. doi: 10.1016/j.cellsig.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Moon RT, Bowerman B, Boutros M, Perrimon N. The promise and perils of Wnt signaling through beta-catenin. Science. 2002;296:1644–1646. doi: 10.1126/science.1071549. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Guan Y, Liu H, Wu X, Yu L, Wang S, Zhao C, Du H, Wang X. Activation of the Wnt/β-catenin signaling pathway is associated with glial proliferation in the adult spinal cord of ALS transgenic mice. Biochem Biophys Res Commun. 2012;420:397–403. doi: 10.1016/j.bbrc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Schmitz Y, Rateitschak K, Wolkenhauer O. Analysing the impact of nucleo-cytoplasmic shuttling of β-catenin and its antagonists APC, Axin and GSK3 on Wnt/β-catenin signalling. Cell Signal. 2013;25:2210–2221. doi: 10.1016/j.cellsig.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 9.Cai Y, Cai T, Chen Y. Wnt pathway in osteosarcoma, from oncogenic to therapeutic. J Cell Biochem. 2014;115:625–631. doi: 10.1002/jcb.24708. [DOI] [PubMed] [Google Scholar]
- 10.Shroff S, Rashid A, Wang H, Katz MH, Abbruzzese JL, Fleming JB, Wang H. SOX9: a useful marker for pancreatic ductal lineage of pancreatic neoplasms. Hum Pathol. 2014;45:456–463. doi: 10.1016/j.humpath.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raspaglio G, Petrillo M, Martinelli E, Li Puma DD, Mariani M, De Donato M, Filippetti F, Mozzetti S, Prislei S, Zannoni GF, Scambia G, Ferlini C. Sox9 and Hif-2α regulate TUBB3 gene expression and affect ovarian cancer aggressiveness. Gene. 2014;542:173–181. doi: 10.1016/j.gene.2014.03.037. [DOI] [PubMed] [Google Scholar]
- 12.Choi YJ, Song JH, Yoon JH, Choi WS, Nam SW, Lee JY, Park WS. Aberrant expression of SOX9 is associated with gastrokine 1 inactivation in gastric cancers. Gastric Cancer. 2014;17:247–254. doi: 10.1007/s10120-013-0277-3. [DOI] [PubMed] [Google Scholar]
- 13.Wang H, He L, Ma F, Regan MM, Balk SP, Richardson AL, Yuan X. SOX9 regulates low density lipoprotein receptor-related protein 6 (LRP6) and T-cell factor 4 (TCF4) expression and Wnt/β-catenin activation in breast cancer. J Biol Chem. 2013;288:6478–6487. doi: 10.1074/jbc.M112.419184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu H, Tang J, Tang M, Cai H. Upregulation of SOX9 in osteosarcoma and its association with tumor progression and patients’ prognosis. Diagn Pathol. 2013;8:183. doi: 10.1186/1746-1596-8-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, Lin H, Mo X, Chen G, Lin L. Synergistic relationship between dipeptidyl peptidase IV and neutral endopeptidase expression and the combined prognostic significance in osteosarcoma patients. Med Oncol. 2013;30:608. doi: 10.1007/s12032-013-0608-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Guan Y, Chen Y, Zhang C, Yu L, Gao H, Du H, Liu B, Wang X. miRNA-9 expression is upregulated in the spinal cord of G93A-SOD1 transgenic mice. Int J Clin Exp Pathol. 2013;6:1826–1838. [PMC free article] [PubMed] [Google Scholar]
- 17.Chen Y, Guan Y, Zhang Z, Liu H, Wang S, Yu L, Wu X, Wang X. Wnt signaling pathway is involved in the pathogenesis of amyotrophic lateral sclerosis in adult transgenic mice. Neurol Res. 2012;34:390–399. doi: 10.1179/1743132812Y.0000000027. [DOI] [PubMed] [Google Scholar]
- 18.Schepers GE, Teasdale RD, Koopman P. Twenty pairs of sox: extent, homology, and nomenclature of the mouse and human sox transcription factor gene families. Dev Cell. 2002;3:167–170. doi: 10.1016/s1534-5807(02)00223-x. [DOI] [PubMed] [Google Scholar]
- 19.Foster JW, Dominguez-Steglich MA, Guioli S, Kwok C, Weller PA, Stevanović M, Weissenbach J, Mansour S, Young ID, Goodfellow PN, et al. Campomelic dysplasia and autosomal sex reversal caused by mutations in an SRY-related gene. Nature. 1994;372:525–530. doi: 10.1038/372525a0. [DOI] [PubMed] [Google Scholar]
- 20.Jakob S, Lovell-Badge R. Sex determination and the control of Sox9 expression in mammals. FEBS J. 2011;278:1002–1009. doi: 10.1111/j.1742-4658.2011.08029.x. [DOI] [PubMed] [Google Scholar]
- 21.Seymour PA, Freude KK, Tran MN, Mayes EE, Jensen J, Kist R, Scherer G, Sander M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc Natl Acad Sci U S A. 2007;104:1865–1870. doi: 10.1073/pnas.0609217104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Szénási T, Kénesi E, Nagy A, Molnár A, Bálint BL, Zvara Á, Csabai Z, Deák F, Boros Oláh B, Mátés L, Nagy L, Puskás LG, Kiss I. Hmgb1 can facilitate activation of the matrilin-1 gene promoter by Sox9 and L-Sox5/Sox6 in early steps of chondrogenesis. Biochim Biophys Acta. 2013;1829:1075–1091. doi: 10.1016/j.bbagrm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 23.Haydon RC, Deyrup A, Ishikawa A, Heck R, Jiang W, Zhou L, Feng T, King D, Cheng H, Breyer B, Peabody T, Simon MA, Montag AG, He TC. Cytoplasmic and/or nuclear accumulation of the beta-catenin protein is a frequent event in human osteosarcoma. Int J Cancer. 2002;102:338–342. doi: 10.1002/ijc.10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoang BH, Kubo T, Healey JH, Sowers R, Mazza B, Yang R, Huvos AG, Meyers PA, Gorlick R. Expression of LDL receptor-related protein 5 (LRP5) as a novel marker for disease progression in high-grade osteosarcoma. Int J Cancer. 2004;109:106–111. doi: 10.1002/ijc.11677. [DOI] [PubMed] [Google Scholar]
- 25.Hoang BH, Kubo T, Healey JH, Yang R, Nathan SS, Kolb EA, Mazza B, Meyers PA, Gorlick R. Dickkopf 3 inhibits invasion and motility of Saos-2 osteosarcoma cells by modulating the Wnt-beta-catenin pathway. Cancer Res. 2004;64:2734–2739. doi: 10.1158/0008-5472.can-03-1952. [DOI] [PubMed] [Google Scholar]
- 26.Kormish JD, Sinner D, Zorn AM. Interactions between SOX factors and Wnt/beta-catenin signaling in development and disease. Dev Dyn. 2010;239:56–68. doi: 10.1002/dvdy.22046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bernard P, Harley VR. Acquisition of SOX transcription factor specificity through protein-protein interaction, modulation of Wnt signalling and post-translational modification. Int J Biochem Cell Biol. 2010;42:400–410. doi: 10.1016/j.biocel.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 28.Chan DW, Mak CS, Leung TH, Chan KK, Ngan HY. Down-regulation of Sox7 is associated with aberrant activation of Wnt/β-catenin signaling in endometrial cancer. Oncotarget. 2012;3:1546–1556. doi: 10.18632/oncotarget.667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sinner D, Kordich JJ, Spence JR, Opoka R, Rankin S, Lin SC, Jonatan D, Zorn AM, Wells JM. Sox17 and Sox4 differentially regulate beta-catenin/T-cell factor activity and proliferation of colon carcinoma cells. Mol Cell Biol. 2007;27:7802–7815. doi: 10.1128/MCB.02179-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Topol L, Chen W, Song H, Day TF, Yang Y. Sox9 inhibits Wnt signaling by promoting beta-catenin phosphorylation in the nucleus. J Biol Chem. 2009;284:3323–3333. doi: 10.1074/jbc.M808048200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yano F, Kugimiya F, Ohba S, Ikeda T, Chikuda H, Ogasawara T, Ogata N, Takato T, Nakamura K, Kawaguchi H, Chung UI. The canonical Wnt signaling pathway promotes chondrocyte differentiation in a Sox9-dependent manner. Biochem Biophys Res Commun. 2005;333:1300–1308. doi: 10.1016/j.bbrc.2005.06.041. [DOI] [PubMed] [Google Scholar]


