Abstract

The heterogeneous reactions of ambient particulate matter (PM)-bound polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs (NPAHs) with NO3/N2O5, OH radicals, and O3 were studied in a laboratory photochemical chamber. Ambient PM2.5 and PM10 samples were collected from Beijing, China, and Riverside, California, and exposed under simulated atmospheric long-range transport conditions for O3 and OH and NO3 radicals. Changes in the masses of 23 PAHs and 20 NPAHs, as well as the direct and indirect-acting mutagenicity of the PM (determined using the Salmonella mutagenicity assay with TA98 strain), were measured prior to and after exposure to NO3/N2O5, OH radicals, and O3. In general, O3 exposure resulted in the highest relative degradation of PM-bound PAHs with more than four rings (benzo[a]pyrene was degraded equally well by O3 and NO3/N2O5). However, NPAHs were most effectively formed during the Beijing PM exposure to NO3/N2O5. In ambient air, 2-nitrofluoranthene (2-NF) is formed from the gas-phase NO3 radical- and OH radical-initiated reactions of fluoranthene, and 2-nitropyrene (2-NP) is formed from the gas-phase OH radical-initiated reaction of pyrene. There was no formation of 2-NF or 2-NP in any of the heterogeneous exposures, suggesting that gas-phase formation of NPAHs did not play an important role during chamber exposures. Exposure of Beijing PM to NO3/N2O5 resulted in an increase in direct-acting mutagenic activity which was associated with the formation of mutagenic NPAHs. No NPAH formation was observed in any of the exposures of the Riverside PM. This was likely due to the accumulation of atmospheric degradation products from gas-phase reactions of volatile species onto the surface of PM collected in Riverside prior to exposure in the chamber, thus decreasing the availability of PAHs for reaction.
Introduction
The long-range atmospheric transport of particulate matter (PM)-bound polycyclic aromatic hydrocarbons (PAHs) and nitro-PAHs (NPAHs) to remote sites, including mountains in France,1 Norway,2 Sweden,2 Czech Republic,3 and the Canadian Arctic,4 as well as trans-Pacific transport to the Olympic Peninsula of Washington5,6 and to Oregon6,7 has been documented. Once emitted from combustion sources, some PAHs undergo reaction with OH radicals, NO3 radicals, N2O5, and O3, converting the parent PAHs into more polar species, including NPAHs. The transformation of PAHs presumably can occur locally (i.e., near emission sources) and/or enroute to downwind receptor sites. Since some PAH derivatives exhibit higher direct acting mutagenicity than their parent PAHs,8,9 human exposure to PAH derivatives, including NPAHs, is of interest and these PAH derivatives have been detected at many sites throughout the world.10−14 Some NPAHs are classified as “probable or possible human carcinogens”15 and have been identified as major contributors to the overall direct-acting mutagenicity of ambient PM despite being present at much lower concentrations than those of their parent PAHs.16
The reactivity of PM-bound PAHs varies, to some extent, with the composition and the microenvironment of the particles.17−21 The mineral content, organic and black carbon concentrations, water content, physical state of the organic layer surrounding the core of the particles, and surface coverage of parent PAH may all influence the reactivity of PM-bound PAHs.18,21−25 Various artificial substrates, including silica, graphite, diesel soot, fly ash, wood smoke, and kerosene soot, have been used in laboratory experiments to simulate PM-bound PAH reactions and derive heterogeneous rate coefficients.17,19,26−30 We recently reported that PAHs with more than 4 rings sorbed to quartz fiber filters are transformed to NPAHs by reaction with NO3/N2O5.31 However, few laboratory studies have been conducted on the transformation of PAHs and NPAHs on ambient PM,32,33 which can provide important information needed in extrapolating model studies to ambient conditions.
We concluded from our recent study of the formation of NPAH from reactions of ambient particles with NO3/N2O5 that PAH in PM quickly become “deactivated” or unavailable for nitration.33 The evidence included little formation of 1-nitropyrene (1-NP) from NO3/N2O5 exposures of daytime ambient PM samples, compared to nighttime samples with similar pyrene concentration, and relatively little formation of 1-NP in both daytime and nighttime samples from downwind sites (Riverside and Banning, CA) upon exposure to NO3/N2O5, relative to exposure of a downtown Los Angeles, CA, nighttime PM sample.33 We speculated that daytime gas-phase reactions of volatile organic compounds (VOCs) with OH radicals and/or O3 producing products, which then adsorbed onto the PM, were responsible for the apparent “deactivation” of the PM-bound PAHs. In addition, 2-nitrofluoranthene (2-NF), the most abundant NPAH in the unexposed ambient PM samples, was not formed from the heterogeneous exposure of the PM to NO3/N2O5. The lack of heterogeneous formation of 2-NF is consistent with 2-NF being only formed during gas-phase radical-initiated reactions of fluoranthene and confirms its usefulness as a marker of atmospheric “aging” (ref (33) and references therein).
In this earlier work, ambient 24 h samples from Beijing, China, and a Los Angeles nighttime sample were the most reactive samples in terms of 1-NP formation. The objectives of our present research were to (1) confirm the reactivity differences for NO3/N2O5 exposure between samples collected at an urban site, namely, Beijing, China, and the downwind site of Riverside, CA. Beijing PM has very high PAH and NPAH concentrations resulting from strong primary emissions,14 while Riverside can be strongly influenced by chemically aged PM transported from upwind areas in the Los Angeles air basin, in addition to local primary emissions;10,33 (2) extend the study of the reactivity of PM-bound PAHs and NPAHs to include heterogeneous exposures to OH radicals and O3; and (3) examine differences in bacterial mutagenicity of the PM extracts from Beijing prior to and after heterogeneous exposures. The PAH and NPAH measurements were carried out using GC/MS, and the Salmonella mutagenicity assay was conducted with and without microsomal activation. To simulate long-range atmospheric transport conditions in our environmental chamber, the average concentrations of NO3 radicals, OH radicals, and O3 over the ∼8 h chamber exposure periods were ∼420 ppt, ∼0.8 ppt, and ∼800 ppb, respectively. These concentrations were equivalent to ∼7 days exposure to ambient concentrations of NO3 radicals, OH radicals, and O3 (see Supporting Information for further discussion), noting that the N2O5 concentrations in the chamber NO3/N2O5 exposures were at least 2 orders of magnitude higher than average ambient concentration.33
Experimental Section
Chemicals
The 23 parent PAHs and 20 NPAHs measured (and their abbreviations) are listed in Table 1. Deuterium-labeled PAHs and NPAHs were purchased from CDN Isotopes (Point-Claire, Quebec, Canada) and Cambridge Isotope Laboratories (Andover, MA). The isotopically labeled PAH and NPAH surrogates used as recovery controls included fluorene-d10, phenanthrene-d10, pyrene-d10, triphenylene-d12, benzo[a]pyrene-d12, benzo[ghi]perylene-d12, 1-nitronaphthalene-d7, 5-nitroacenaphthene-d9, 9-nitroanthracene-d9, 3-nitrofluoranthene-d9, 1-nitropyrene-d9, and 6-nitrochrysene-d11. The labeled PAH and NPAH internal standards included acenaphthene-d10, fluoranthene-d10, benzo[k]fluoranthene-d12, 2-nitrobiphenyl-d9, and 2-nitrofluorene-d9.
Table 1. List of Parent PAHs and NPAHs (and Their Abbreviations) Measured in This Study.
| PAHsa |
NPAHsb |
||||
|---|---|---|---|---|---|
| # | compound | abbreviation | # | compound | abbreviation |
| 1 | fluorene | FLU | 1 | 2-nitrofluorene | 2-NFL |
| 2 | phenanthrene | PHE | 2 | 9-nitroanthracene | 9-NAN |
| 3 | anthracene | ANT | 3 | 9-nitrophenanthrene | 9-NPH |
| 4 | 2-methylphenanthrene | 2-MPHE | 4 | 2-nitrodibenzothiophene | 2-NDBT |
| 5 | 2-methylanthracene | 2-MANT | 5 | 3-nitrophenanthrene | 3-NPH |
| 6 | 1-methylphenanthrene | 1-MPHE | 6 | 2-nitroanthracene | 2-NAN |
| 7 | 3,6-dimethylphenanthrene | 3,6-DPHE | 7 | 2-nitrofluoranthene | 2-NF |
| 8 | dibenzothiophene | DBT | 8 | 3-nitrofluoranthene | 3-NF |
| 9 | fluoranthene | FLA | 9 | 1-nitropyrene | 1-NP |
| 10 | pyrene | PYR | 10 | 2-nitropyrene | 2-NP |
| 11 | retene | RET | 11 | 7-nitrobenz(a)anthracene | 7-NBaA |
| 12 | benzo[c]fluorene | BcFLU | 12 | 1-nitrotriphenylene | 1-NTR |
| 13 | 1-methylpyrene | 1-MPYR | 13 | 2,8-dinitrodibenzothiophene | 2,8-DNDBT |
| 14 | benz[a]anthracene | BaA | 14 | 6-nitrochrysene | 6-NCH |
| 15 | chrysene + triphenylene | CHR + TRI | 15 | 3-nitrobenzanthrone | 3-NBENZ |
| 16 | 6-methylchrysene | 6-MCHR | 16 | 2-nitrotriphenylene | 2-NTR |
| 17 | benzo(b)fluoranthene | BbF | 17 | 1,3-dinitropyrene | 1,3-DNP |
| 18 | benzo(k)fluoranthene | BkF | 18 | 1,6-dinitropyrene | 1,6-DNP |
| 19 | benzo[e]pyrene | BeP | 19 | 1,8-dinitropyrene | 1,8-DNP |
| 20 | benzo[a]pyrene | BaP | 20 | 6-nitrobenzo[a]pyrene | 6-NBaP |
| 21 | indeno[1,2,3-cd]pyrene | IcdP | |||
| 22 | dibenz[a,h] + (a,c)anthracene | DahA + DacA | |||
| 23 | benzo[ghi]perylene | BghiP | |||
Purchased from AccuStandard (New Haven, CT) and Chem Service (West Chester, PA).
Purchased from Chiron AS (Norway), AccuStandard (New Haven, CT), Chem Service (West Chester, PA), and Sigma-Aldrich Corp. Cambridge Isotope Laboratories (Andover, MA).
Sampling
Beijing, China
The Beijing sampling site was located on the roof of the 7-story (about 25 m above ground) Geology Building on the Peking University Campus (PKU).14,34 This site is located in Northwestern Beijing and is primarily a residential and commercial area. Dominant PAH emission sources near the site include vehicular traffic and fuel combustion for cooking. PM2.5 and PM10 were collected on prebaked (350 °C) quartz fiber filters (No.1851-865, Tisch Environmental, Cleves, OH) using a High Volume Cascade Impactor (Series 230, Tisch Environmental, Cleves, OH). PM was collected continuously over 24 h periods, with the sampler being changed over in the late morning. The average flow rate was ∼1 m3 min–1. PM10 and PM2.5 samples were collected from May 2009 to February 2010 and in April 2011, respectively (Table SI.1, Supporting Information).
Riverside, California
The sampling site and sampling collection have been previously described in detail.33,35,36 Briefly, sampling in Riverside was conducted at a site in the Agricultural Operations area at the University of California, Riverside, campus, approximately 90 km downwind of Los Angeles.33,35 PM2.5 samples were collected during October 1997 using an ultrahigh volume particulate sampler, containing four Teflon-impregnated glass fiber (TIGF) filters (each 40.6 cm × 50.8 cm). After collection, the filters were stored at −20 °C. For this study, three 20.3 cm × 25.4 cm portions were cut out from 40.6 cm × 50.8 cm filters (Table SI.1, Supporting Information). The average flow rate for each cut-out filter portion was ∼1 m3 min–1.
Filter Preparation and Exposures
Beijing PM PAH and NPAH concentrations vary significantly from day-to-day.14 In order to measure changes in the PAH and NPAH concentrations with and without exposure in the chamber for the chemical study, single 24 h 20.3 cm × 25.4 cm filter samples were cut into six equal portions of 8.5 cm × 10.2 cm (Figure SI.1, Supporting Information). Three 8.5 cm × 10.2 cm portions were exposed in the chamber, and the remaining three 8.5 cm × 10.2 cm portions of single 24 h filter samples were used as unexposed controls. In order to measure changes in the mutagenicity of the PM with and without exposure in the chamber for the mutagenicity study, single 24 h 20.3 cm × 25.4 cm filter samples were cut into four equal portions of 10.2 cm × 12.7 cm because the Salmonella assay did not adequately measure the mutagenicity of the 8.5 cm × 10.2 cm portions (Figure SI.1, Supporting Information). Two 10.2 cm × 12.7 cm portions of the single 24 h filter samples were exposed in the chamber, and the remaining two 10.2 cm × 12.7 cm portions were used as unexposed controls. The PAH and NPAH concentrations of the exposed and unexposed 10.2 cm × 12.7 cm portions used for the mutagenicity study were also measured and directly compared to the results of the Salmonella assay. Overall, six PKU and three Riverside 20.3 cm × 25.4 cm filter samples were tested in each exposure.
Chamber Exposures
The PM2.5 and PM10 filters were exposed to NO3/N2O5, OH radicals, and O3 in a ∼7000 L indoor collapsible Teflon film chamber equipped with two parallel banks of blacklamps (used for the OH radical exposures) and a Teflon-coated fan at room temperature (∼296 K) and ∼735 Torr pressure.31,33,36,37 The filters were placed within the Teflon chamber as shown, for example, in Figure SI.2, Supporting Information.31,33 For all exposure experiments, blank, clean filters were also placed in the Teflon chamber to test for background contamination in the chemistry analyses and mutagenicity assays. The details of the NO3/N2O5, OH radical, and O3 exposures are given in the Supporting Information and have been previously reported.31,33
Sample Extraction and Analysis
Details of the sample extraction and analysis have been previously described and are given in the Supporting Information.14
Salmonella Mutagenicity Assay
The basic methodology followed that reported by Maron and Ames38 and used Salmonella typhimurium strain TA98. The experimental details have been described elsewhere.14,31 The positive control doses were 2 μg of 2-aminoanthracene (2-AA) and 20 μg of 4-nitro-1,2-phenylenediamine (NPD) for assays with and without metabolic activation (rat S9 mix), respectively. The negative control (DMSO) dose was 30 μL. All filter extracts were tested in triplicate.
On the basis of preliminary studies and the limit of detection in the Salmonella assay, only the Beijing PM samples were tested for mutagenic activity (Table SI.1, Supporting Information). Positive controls of NPD and 2-AA gave mean revertant counts of ∼3500/plate and ∼1000/plate, respectively. The average background revertant count (DMSO) was ∼25/plate for both assays. The revertant counts for the control blanks were comparable to the background revertant count, indicating no interference from the purified air in the chamber. It should be noted that different sets of filters were used for the mutagenicity and chemical studies (Figure SI.1, Supporting Information) and that the PAH and NPAH concentrations of the PM samples used for the mutagenicity testing were measured and directly compared to the results from the Salmonella assay.
Results and Discussion
Chemical Study
Beijing, China PM
The masses of individual PAHs and NPAHs on each 24 h filter sample cut-out (shown in Figure SI.1, Supporting Information) were measured for the exposed filters (PAHexposed and NPAHexposed) and unexposed filters (PAHunexposed and NPAHunexposed). The amount of individual PAH or NPAH degraded (or formed) after exposure to NO3/N2O5, OH radicals, or O3 was calculated by dividing the exposed mass by the unexposed mass (a ratio close to 1.0 indicates no net degradation or formation of a given individual PAH or NPAH after exposure to the various oxidants). The PAHexposed/PAHunexposed ratios and the NPAHexposed/NPAHunexposed ratios for the Beijing PM after exposure to NO3/N2O5, OH radicals, and O3 are shown in Figure 1A,B, respectively. An asterisk indicates a statistically significant difference in mass after exposure to the various oxidants (p-value <0.05, according to the paired student’s t test). The means and standard errors of PAH and NPAH masses, measured in the Beijing filters before and after exposure to the various oxidants, are given in Tables SI.2–SI.4, Supporting Information. The 3- to 4-ring PAHs exist in both the gas and particulate phases in the atmosphere at ambient temperatures.39 While it is possible that a portion of the PAH initially sorbed to the PM desorbed from the PM into the gas phase of the chamber during the experiments, it is expected that PAH available for volatilization would have done so in the atmosphere and/or during collection of the PM. Therefore, we believe that volatilization of these compounds from the PM, during the course of the experiments, was minimal. The temperature in the chamber was maintained at room temperature (∼296 K) and held constant to ±2 K; no formation of NPAHs due to gas-phase reactions was observed (see below), and the blank filters installed in the reactor at the same time as the PM filter samples had PAH concentrations below the detection limit.
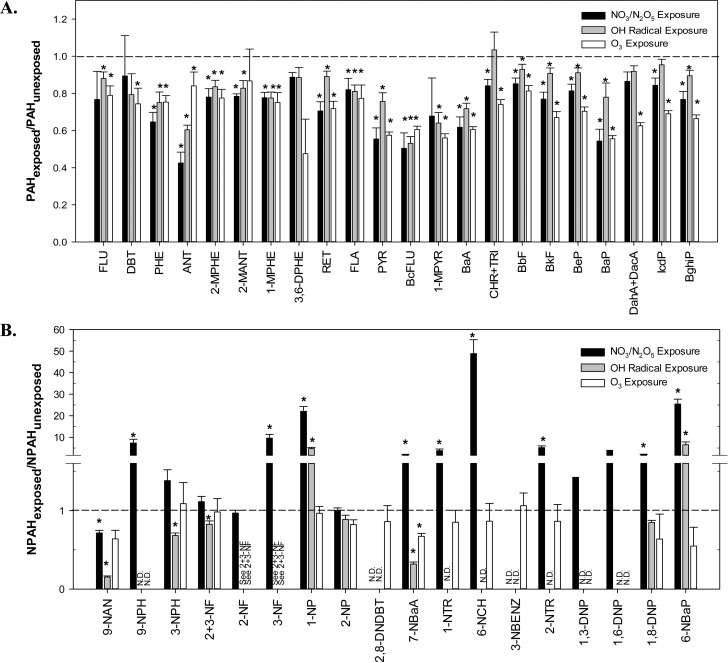
Figure 1.
(A) PAHexposed/PAHunexposed and (B) NPAHexposed/NPAHunexposed of Beijing PM filters (n = 9 for NO3/N2O5 and OH radical exposures, and n = 8 for O3 exposure) used for the chemical study. An asterisk denotes the statistically significant difference between the unexposed and exposed masses (N.D. = not detected).
As shown in Figure 1A and in Tables SI.2–SI.4, Supporting Information, all of the PAHs underwent some degradation upon exposure to NO3/N2O5, OH radicals, and O3, apart from CHR + TRI exposed to OH radicals. The data denoted by asterisks in Figure 1A identify those PAHs which had statistically significant losses. The highest percent removal was 57% for ANT exposed to NO3/N2O5, and a number of other PAHs (including PYR, BcFLU, BaA, and BaP) had up to ∼50% removal from one or more of the exposures (Figure 1A).
Noteworthy is the observation that none of the PAHs were completely removed from exposures to NO3/N2O5, OH radicals, or O3. Our data from the O3 exposure are consistent with a previous study where ambient PM was exposed to ∼4 ppm of O3 for 60 min,32 with the PYR and BaA concentrations being observed to plateau at ∼70% of their initial concentrations following exposure to O3.32 In contrast, a study examining what can be considered “fully available” PYR (coated on silica particles) found that the PYR reacted completely, within 10 min, after exposure to ∼1.3 ppm of O3 in a flow reactor.26 There have now been a number of studies26,32,33 that indicate that the reactivity of particle-bound PAHs is substrate-specific and can be inhibited by the decreased availability of these PAHs in the ambient PM.
Of the PAHs containing more than 4 rings measured on the Beijing PM, BaP exhibited the greatest relative degradation upon exposure to NO3/N2O5, OH radicals, and O3 (Figure 1A). This is consistent with the high previously reported photochemical reactivity of BaP28,40−43 and a study of ambient PM collected in Paris, France, which identified BaP as the most reactive PAH toward O3, OH radicals, and NO2/O3.32
Figure 1B shows the NPAHexposed /NPAHunexposed ratios for the Beijing PM samples. The means and standard errors of NPAH masses measured on the Beijing PM samples, before and after exposure to the various oxidants, are given in Tables SI.2–SI.4, Supporting Information. 2-NF and 3-NF were chromatographically separated and quantified using a 30 m DB-17 GC column in all Beijing PM samples that were exposed to NO3/N2O5, as well as in one-third of the Beijing PM samples that were exposed to OH radicals and O3. The 3-NF concentrations in the Beijing PM samples, both before and after exposure to OH radicals and O3, were below the detection limit, suggesting no marked increase in concentration upon exposure. In all of the Beijing PM exposures, there was no significant increase in the masses of 2-NF and 2-NP, which are products of gas-phase radical-initiated reactions.44 This provides further evidence that there was no significant volatilization of 4-ring PAHs from the ambient PM into the gas phase in the chamber and implies that the increase in the mass of other nitro-PAHs during the exposure experiments was likely due to heterogeneous formation.
A number of the NPAHs were formed to a statistically significant degree during the NO3/N2O5 exposure, with the NPAHexposed/NPAHunexposed ratios reaching 49 for 6-NCH (Figure 1B and Table SI.2, Supporting Information). While 1-NP and 6-NBaP were formed from both the NO3/N2O5 and OH radical exposures, 9-NPH, 3-NF, 7-NBaA, 1-NTR, 6-NCH, 2-NTR, and 1,8-DNP were only formed during the NO3/N2O5 exposure (Figure 1B). Our measured formation of 3-NF and 1-NP is consistent with results from previous studies of the reaction of FLA and PYR adsorbed on TIGF filters with gaseous N2O5.33,41 These previous studies also showed that the major NPAH isomers formed from the heterogeneous reactions of FLA and PYR with N2O5 (3-, 8-, 7-, and 1-NF and 1-NP) are distinct from the 2-NF and 2-NP formed from gas-phase reactions of NO3 radicals with FLA and PYR. While 2-NP is formed in ambient air from the OH radical-initiated reaction of PYR, it is only formed from the NO3 radical-initiated reaction at NO2 concentrations much higher than those observed in ambient air.36,41 The major NPAH isomers formed by heterogeneous nitration have the NO2 group added to the highest electron density position.31 The radical-initiated reactions of FLA and PYR are expected to follow a different mechanism where the OH or NO3 radical attacks FLA or PYR at the highest electron density position, followed by NO2 addition at the ortho position and subsequent loss of H2O or HNO3, yielding 2-NF and 2-NP as the major NPAH products.33,36,44,45 In addition, the N2O5 concentration and the N2O5/NO2 ratio in the photochemical chamber during our NO3/N2O5 exposure experiments were at least 2 orders of magnitude higher than the average ambient concentration,33 suggesting a higher potential for heterogeneous reactions with N2O5.
The heterogeneous and gas-phase reactions of TRI were previously reported to both form 1- and 2-NTR,36 and in this study, 1-NTR and 2-NTR were formed in equal amounts upon exposure to NO3/N2O5 (Figure 1B). Our measured formation of 7-NBaA, 6-NCH, and 6-NBaP (Figure 1B) was in agreement with previous results showing their formation when PAHs associated with diesel soot were reacted with N2O5.19
Ratios of 1-NPexposed /1-NPunexposed (mean of 4.8) and 6-NBaPexposed /6-NBaPunexposed (mean of 6.5) were measured when the Beijing PM was exposed to OH radicals (Figure 1B). Nitration of PYR by the radical-initiated reaction discussed above would be expected to lead to 2-NP. Therefore, the formation of 1-NP and 6-NBaP is attributed to heterogeneous nitration of parent PAHs by HNO3/NO2.36 During the OH radical exposure experiments, NO2 was formed from the reaction of HO2 and organic peroxy radicals with NO after the photolysis of methyl nitrite (the OH radical precursor) and reaction of NO2 with OH radicals leads to formation of HNO3. In contrast to 1-NP and 6-NBaP, 3-NPH and 2 + 3-NF, and especially 9-NAN and 7-NBaA, were degraded on the Beijing PM during the OH radical exposure (Figure 1B). Because these same NPAHs were not as significantly degraded during the dark exposure with O3 (Figure 1B), it is possible that the degradation observed during the OH radical exposure could be due, in part, to direct photolysis. Previously, Pitts et al.46 observed the direct photolysis of 9-NAN adsorbed on silica gel and identified quinones as degradation products. In another study, the direct photolysis of 7-NBaA resulted in increased direct-acting mutagenicity to TA98 in the Salmonella assay with increasing irradiation time.47 The orientation of the nitro group has been related to the photochemical stability of NPAH,48 and both 9-NAN and 7-NBaA have nitro group orientations that are out of the aromatic plane, reducing the steric effects exerted by two peri-hydrogens. This structure makes these PAHs less photochemically stable and may explain their significant degradation during the OH radical exposure (Figure 1B).48 Although 6-NBaP also has a structure where the nitro group is out of the aromatic plane and is therefore expected to be photolabile, there was still a net formation after exposure of Beijing PM to OH radicals (mean NBaPexposed/NBaPunexposed = 6.5) (Figure 1B). As expected, exposure of the Beijing PM to O3 did not lead to significant NPAH formation (Figure 1B and Table SI.4, Supporting Information) and, except for the degradation of 7-NBaA, the NPAHs sorbed to Beijing PM were not significantly degraded during O3 exposure (Figure 1B).
Riverside, California PM
The PAHexposed/PAHunexposed ratios and the NPAHexposed/NPAHunexposed ratios for the Riverside PM samples after exposure to NO3/N2O5, OH radicals, and O3 are shown in Figures SI.3A and SI.3B, respectively, Supporting Information. The means and standard errors of PAH and NPAH masses measured in the Riverside filters, before and after exposure to the various oxidants, are given in Tables SI.5–SI.7, Supporting Information. Compared to the Beijing PM samples, a smaller number of individual PAH and NPAH in the Riverside PM samples was present at concentrations above their detection limits. PAHexposed/PAHunexposed ratios slightly above 1.0 and statistically significant (p-value <0.05) were measured for 2-MPHE, 1-MPHE, FLA, PYR, and CHR + TRI upon exposure of Riverside PM to OH radicals and similarly for PHE upon exposure to NO3/N2O5 (ratio = 2.9) and OH radicals (ratio = 3.3) (Figure SI.3A, Supporting Information). However, these small changes in mass (Tables SI.5 and SI.6, Supporting Information) are attributed to experimental uncertainty (for example, possible inhomogeneity in the particles across the filter), since PAH formation in these experiments was not possible. Compared to the Beijing PM samples, the higher-ring PAHs in the Riverside PM samples were more resistant to degradation during the NO3/N2O5 and OH radical exposures (Figures 1A and SI.3A, Supporting Information). Exposure of the Riverside PM samples to O3 did not result in significant degradation of PAHs, including the higher-ring PAHs (Figure SI.3A, Supporting Information). Consistent with the absence of significant PAH degradation on the Riverside PM, there was no significant formation of NPAHs in any of the Riverside PM exposures, including the NO3/N2O5 exposure (Figure SI.3B, Supporting Information). A small, but statistically significant (p-value <0.05), reduction in mass was measured for 2 + 3-NF after exposure to OH radicals and for 3-NPH, 2 + 3-NF, and 1-NP after exposure to O3 (Figure SI.3B, Supporting Information). Although artifacts during filter collection may occur,49,50 it has previously been shown that 2-NF is not formed during collection and the artifactual formation of 1-NP is minimal;51 therefore, heterogeneous formation of these NPAH can be examined using PM-bound PAHs on filters. Furthermore, our recent study utilizing 16 samples collected during a 1997 photochemical pollution episode showed an excellent correlation between the naphthalene and other volatile PAHs analyzed immediately after collection and PAH measured on the archived filters from this study.33 For example (see Figure S10 in ref (33)), the highest naphthalene, BghiP, and BeP concentrations occurred in the morning (6–12 h) and evening (18–06 h) Los Angeles samples and the concentrations decreased monotonically at the increasingly downwind sites of Azusa, Riverside, and Banning.
Reactivity for NPAH Formation
Because 1-NP has been consistently reported to form from heterogeneous reactions of PYR adsorbed to a surface or particles,33,52 we used 1-NP formation as a measure of the reactivity of PM to NPAH formation. The percent reactivity of each of the Beijing PM samples was calculated as
| 1 |
where Δ[1-NP] = ([1-NP]exposed – [1-NP]unexposed) and [PYR]0 = [PYR]unexposed.33 We also calculated the [2-NF]/[BeP] ratio of the unexposed PM samples to indicate the degree to which the PM had undergone atmospheric processing (or aging) in the atmosphere prior to collection. 2-NF is formed from the gas-phase reaction of FLA with OH and NO3 radicals in the presence of NO2,33,44 while BeP is a relatively stable PAH.53 A larger [2-NF]/[BeP] ratio therefore indicates a greater contribution of photochemical atmospheric aging in comparison to direct emissions. The relationship between the reactivity toward nitration upon NO3/N2O5 exposure and the degree of aging has been previously discussed in Zimmermann et al.33
A plot of the reactivity versus [2-NF]/[BeP] is shown in Figure SI.4, Supporting Information, for the present study, and the reactivity decreases monotonically as the atmospheric aging of the PM increases, as measured by the [2-NF]/[BeP] ratio. Beijing PM samples with lower reactivity had higher [2-NF]/[BeP] ratios, suggesting that the PAHs sorbed to Beijing PM that had undergone more aging in the atmosphere were less available for reaction with NO3/N2O5. The lack of reactivity of the Riverside PM samples has been explained by a higher extent of aging in the atmosphere prior to sample collection.33
Although the Riverside samples were archived filters, we found a similar lack of reactivity to NO3/N2O5 for Riverside samples collected in 2012 with minimal storage times (<4 days), and we also encountered a reactive Riverside nighttime sample (presumably with local PAH emissions and little aging, based on its 2-NF/BeP ratio).33 Additionally, a set of 16 archived daytime/nighttime PM samples, collected from four sites along the Los Angeles air basin during a photochemical pollution episode, showed a range of reactivities, with the nighttime urban LA sample having the highest reactivity.33 Therefore, we have no evidence that cold-storage of the PM on filters affects the PAH reactivity toward heterogeneous nitration.
Mutagenicity Study
Direct-Acting Mutagenicity
Most NPAHs are known to be direct-acting mutagens, requiring no exogenous bioactivation to convert them into the active form.54 The direct-acting mutagenic activity (without addition of S9) of the paired Beijing PM samples (with and without exposure to NO3/N2O5, OH radical, and O3) is shown in Figure 2. Figure SI.5, Supporting Information, shows the PAHexposed/PAHunexposed ratios and the NPAHexposed/NPAHunexposed ratios for the Beijing PM used in the mutagenicity assay after exposure to NO3/N2O5, OH radicals, and O3. In general, the results were comparable to the Beijing PM used in the chemical study in that there was significant formation of NPAHs upon exposure of the Beijing PM-bound PAHs to NO3/N2O5 (Figures 1B and SI.5B, Supporting Information).
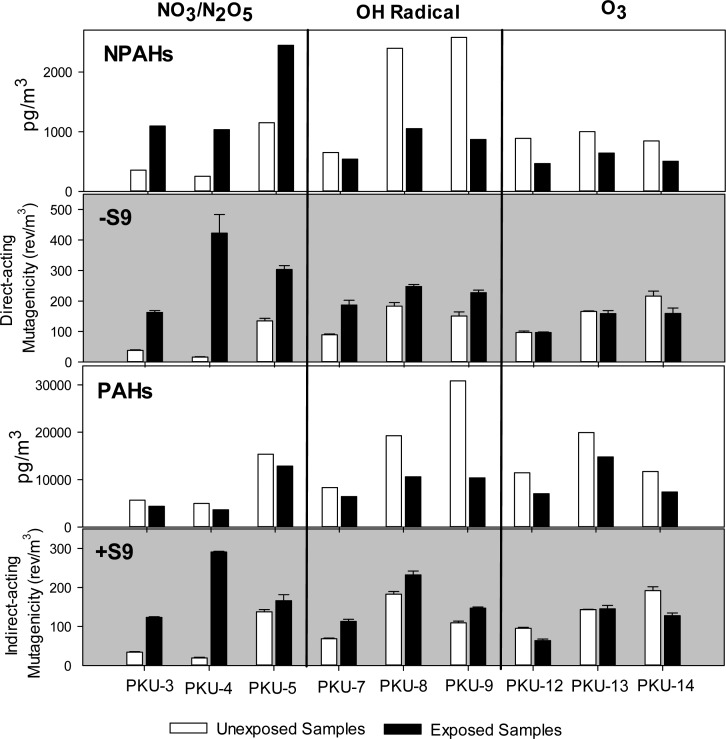
Figure 2.
Direct-acting and indirect-acting mutagen densities (rev/m3) of exposed and unexposed Beijing PM extracts and the associated total PAH and NPAH concentrations (pg/m3).
Lower direct-acting mutagenic activity was measured in the two unexposed Beijing extracts with lower NPAH concentrations (PKU-3 and PKU-4), compared to the unexposed Beijing PM extract with higher NPAH concentrations (PKU-5) (Figure 2 and Table SI.8, Supporting Information). This is consistent with a previous study from our laboratory that showed significant daily variation in the direct-acting mutagenicity, and in the corresponding NPAH concentrations, of Beijing PM.14 After NO3/N2O5 exposure, the direct-acting mutagenicity of the Beijing PM increased 2- to 26-fold (Figure 2). Of the Beijing PM samples exposed to NO3/N2O5, the largest increase in direct-acting mutagenicity (26-fold) was measured in sample PKU-4, which also had the largest increases in 1,3-, 1,6-, and 1,8-DNP masses after exposure to NO3/N2O5 (Table SI.8, Supporting Information). Among these DNPs, the greatest increase in mass was for 1,8-DNP (10.2 ng) (Table SI.8, Supporting Information). The 1,8-DNP concentrations measured in the other two mutagenicity study samples exposed to NO3/N2O5 (PKU-3 and PKU-5) were either below the detection limit (PKU-3) or three times less than that measured in the PKU-4 sample (PKU-5). In previous mutagenicity assay studies, DNPs, especially 1,8-DNP, were found to be powerful direct-acting mutagens.55 Small amounts of DNPs have been shown to contribute significantly to the total direct-acting mutagenicity of diesel particles.16
For the OH radical exposure in the direct-acting mutagenicity study, two of the Beijing PM samples had no 1-NP formation (and hence zero reactivity according to eq 1) (PKU-8 and PKU-9) and one sample had only 4.1% reactivity (PKU-7) (Figure 2 and Table SI.9, Supporting Information). Overall, the increase in direct-acting mutagenicity after OH radical exposure (mean of 1.7-fold) was lower than the increase in direct-acting mutagenicity after NO3/N2O5 exposure (mean of 11-fold). The NPAHexposed /NPAHunexposed profiles, from both the mutagenicity and chemical studies, showed the formation of 1-NP and 6-NBaP from OH radical exposure (Figures 1B and SI.5B, Supporting Information). Given the significant 6-NBaP formation in all of the Beijing PM samples from exposure to OH radicals, resulting from the formation of HNO3 in the chamber (Figures 1B and SI.5B, Supporting Information), and the minimal corresponding increase in direct-acting mutagenicity of these same samples, 6-NBaP does not appear to contribute significantly to the overall direct-acting mutagenicity of the Beijing PM. This is consistent with the structure of 6-NBaP which has a NO2 group nearly perpendicular to the aromatic ring that does not allow for favorable nitro-reduction.55 However, an increase in direct-acting mutagenicity and a decrease in total NPAH mass, as well as a decrease in mutagenic 1-NP mass, was observed after PKU-8 and PKU-9 were exposed to OH radicals (Figure 2). This suggests that other mutagenic degradation products, not measured in this study or below the detection limit of our analytical method, may have contributed to the enhanced direct-acting mutagenicity of the Beijing PM after exposure to OH radicals.
For the Beijing PM samples, Figure SI.5B, Supporting Information, shows that 9-NAN, 3-NPH, 2 + 3-NF, 1-NP, and 2-NP were degraded significantly during the O3 exposure in the mutagenicity study, while only 7-NBaA was significantly degraded during the O3 exposure in the chemical study (Figures 1B and SI.5B, Supporting Information). Only PKU-14 showed a significant decrease in direct-acting mutagenicity of the Beijing PM after exposure to O3, which is consistent with the corresponding decrease in the total NPAH mass (Figure 2 and Table SI.10, Supporting Information).
Indirect-Acting Mutagenicity
Some parent PAHs and NPAHs, including 6-NBaP and 1-nitrocoronene, contribute to the indirect-acting mutagenicity of PM.56 On average, there was a ∼7-fold and ∼1.4-fold increase in the indirect-acting mutagenicity of the Beijing PM after exposure to NO3/N2O5 and OH radicals, respectively (Figure 2). Because most parent PAHs were degraded after exposure to NO3/N2O5 and OH radicals (Figure 2 and Tables SI.8 and SI.9, Supporting Information), it is possible that the NPAHs formed contributed to the increase in indirect-acting mutagenicity. Increased indirect-acting mutagenicity after parent PAH exposure to NO3/N2O5 and OH radicals was observed in our previous studies.31 Kamens et al.8 found that the exposure of wood soot to NO2 and O3 resulted in an increase in both direct- and indirect-acting mutagenicity of the NPAH fraction. In the same study, the most polar fraction made the largest contribution to the total indirect-acting mutagenicity. This suggests that other, more polar, transformation products may contribute significantly to the indirect-acting mutagenicity of the extracts. Moreover, the high molecular weight PAHs (MW 302), including dibenzo[a,l]pyrene which is 30 times more toxic than BaP,57 may also play a significant role in the indirect-acting mutagenicity and were shown to be a significant contributor to the inhalation cancer risk in Beijing air.58 The results of our previous study on the heterogeneous nitration of dibenzo[a,l]pyrene adsorbed on filters showed that the indirect-acting mutagenicity increased 2.5-fold after the NO3/N2O5 exposure and that 6-nitrodibenzo[a,l]pyrene was the only nitro product identified.31
For the O3 exposure, the PAHexposed/PAHunexposed profiles of Beijing PM samples were comparable in the mutagenicity and chemical studies (Figures 1A and SI.5A, Supporting Information). The reduction in the mutagenicity (33%) of the two Beijing samples exposed to O3 (PKU-12 and PKU-14) may be associated with the degradation of the total parent PAH and NPAH masses (Figure 2). However, there was no significant change in the indirect-acting mutagenicity in PKU-13 when total PAH and NPAH masses decreased (Figure 2).
Atmospheric Implications
Our present and previous data33 suggest that the Riverside PM had undergone more aging prior to collection and was less reactive than the Beijing PM. The decreased reactivity of the Riverside PM, compared to the Beijing PM, may be because the Riverside sampling site is located downwind of Los Angeles and receives photochemically “aged” air masses from this major urban source region.33 Moreover, we previously observed that a night-time PM sample collected from downtown Los Angeles had a similar reactivity and [2-NF]/[BeP] ratio as some of the Beijing PM samples.33 In contrast to Riverside (but like downtown Los Angeles at night), the Beijing sampling site is located within a major urban source region and appears to receive air masses that are not as photochemically aged and more influenced by direct emissions. One can therefore conclude that aged particles exhibit decreased reactivity, most probably due to a lower availability of the PAHs for reaction and due at least in part to the accumulation of atmospheric reaction products on the surface of ambient PM. On the basis of the observed decrease in reactivity of PM collected along an upwind–downwind trajectory in the Los Angeles air basin,33 it appears that, at least in that air basin at that time, photochemical aging occurred rapidly, on a time-scale of a few hours during daytime, and that little or no additional heterogeneous reactions would occur for additional atmospheric transport times.
The extent of PAH transformation observed in this study may be limited by the multilayer coverage of the ambient PM on the filters. In reality, the transformation of PAHs on PM may be more significant in the atmosphere, where the PAHs are present on individual particles in the atmosphere rather than on PM layered onto filters. However, the resistance of the Riverside PM samples to chemical reaction suggests that secondary pollutant formation plays an important role in making PM-bound PAHs less available for chemical reactions. Overall, our present and previous33 results suggest that heterogeneous PAH degradation and concurrent NPAH formation on ambient PM occurs mainly, or only, for particles with minimal photochemical aging and that photochemically aged particles are nonreactive toward heterogeneous reactions of PAHs.
Acknowledgments
This publication was made possible in part by grant number RD83375201 from the U.S. Environmental Protection Agency (USEPA), grant number P30ES00210 from the National Institute of Environmental Health Sciences (NIEHS), NIH and NIEHS Grant P42 ES016465, and the U.S. National Science Foundation (ATM-0841165). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the USEPA, NIEHS, or NIH. Salmonella assays were conducted in the Cancer Chemoprotection Program (CCP) Core Laboratory of the Linus Pauling Institute, Oregon State University.
Supporting Information Available
Appendix I: Chamber Exposures; Appendix II: Sample Extraction and Analysis; additional tables and figures. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Albinet A.; Leoz-Garziandia E.; Budzinski H.; Villenave E.; Jaffrezo J. L. Nitrated and oxygenated derivatives of polycyclic aromatic hydrocarbons in the ambient air of two French alpine valleys: Part 1: Concentrations, sources and gas/particle partitioning. Atmos. Environ. 2008, 42, 43–54. [Google Scholar]
- Björseth A.; Lunde G.; Lindskog A. Long-range transport of polycyclic aromatic hydrocarbons. Atmos. Environ. 1979, 13, 45–53. [Google Scholar]
- Lammel G.; Novák J.; Landlová L.; Dvorská A.; Klánová J.; Čupr P.; Kohoutek J.; Reimer E.; Škrdlíková L.. Sources and distributions of polycyclic aromatic hydrocarbons and toxicity of polluted atmosphere aerosols. In Urban Airborne Particulate Matter:Origins, Chemistry;Zereini F., Wiseman C. L. S., Eds.; Springer: Berlin, Heidelberg, 2011; pp 39–62. [Google Scholar]
- Welch H. E.; Muir D. C. G.; Billeck B. N.; Lockhart W. L.; Brunskill G. J.; Kling H. J.; Olson M. P.; Lemoine R. M. Brown snow: A long-range transport event in the Canadian Arctic. Environ. Sci. Technol. 1991, 25, 280–286. [Google Scholar]
- Killin R. K.; Simonich S. L.; Jaffe D. A.; DeForest C. L.; Wilson G. R. Transpacific and regional atmospheric transport of anthropogenic semivolatile organic compounds to Cheeka Peak Observatory during the spring of 2002. J. Geophys. Res. 2004, 109, D23S15. [Google Scholar]
- Genualdi S. A.; Killin R. K.; Woods J.; Wilson G.; Schmedding D.; Simonich S. L. M. Trans-pacific and regional atmospheric transport of polycyclic aromatic hydrocarbons and pesticides in biomass burning emissions to western North America. Environ. Sci. Technol. 2009, 43, 1061–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primbs T.; Piekarz A.; Wilson G.; Schmedding D.; Higginbotham C.; Field J.; Simonich S. M. Influence of Asian and western United States urban areas and fires on the atmospheric transport of polycyclic aromatic hydrocarbons, polychlorinated biphenyls, and fluorotelomer alcohols in the western United States. Environ. Sci. Technol. 2008, 42, 6385–6391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens R.; Bell D.; Dietrich A.; Perry J.; Goodman R.; Claxton L.; Tejada S. Mutagenic transformations of dilute wood smoke systems in the presence of ozone and nitrogen dioxide. Analysis of selected high-pressure liquid chromatography fractions from wood smoke particle extracts. Environ. Sci. Technol. 1985, 19, 63–69. [Google Scholar]
- Yang X.-Y.; Igarashi K.; Tang N.; Lin J.-M.; Wang W.; Kameda T.; Toriba A.; Hayakawa K. Indirect- and direct-acting mutagenicity of diesel, coal and wood burning-derived particulates and contribution of polycyclic aromatic hydrocarbons and nitropolycyclic aromatic hydrocarbons. Mutat. Res., Genet. Toxicol. Environ. Mutagen. 2010, 695, 29–34. [DOI] [PubMed] [Google Scholar]
- Reisen F.; Arey J. Atmospheric reactions influence seasonal PAH and nitro-PAH concentrations in the Los Angeles basin. Environ. Sci. Technol. 2004, 39, 64–73. [PubMed] [Google Scholar]
- Dimashki M.; Harrad S.; Harrison R. M. Measurements of nitro-PAH in the atmospheres of two cities. Atmos. Environ. 2000, 34, 2459–2469. [Google Scholar]
- Bamford H. A.; Baker J. E. Nitro-polycyclic aromatic hydrocarbon concentrations and sources in urban and suburban atmospheres of the Mid-Atlantic region. Atmos. Environ. 2003, 37, 2077–2091. [Google Scholar]
- Albinet A.; Leoz-Garziandia E.; Budzinski H.; ViIlenave E. Polycyclic aromatic hydrocarbons (PAHs), nitrated PAHs and oxygenated PAHs in ambient air of the Marseilles area (South of France): Concentrations and sources. Sci. Total Environ. 2007, 384, 280–292. [DOI] [PubMed] [Google Scholar]
- Wang W.; Jariyasopit N.; Schrlau J.; Jia Y.; Tao S.; Yu T.-W.; Dashwood R. H.; Zhang W.; Wang X.; Simonich S. L. M. Concentration and photochemistry of PAHs, NPAHs, and OPAHs and toxicity of PM2.5 during the Beijing Olympic Games. Environ. Sci. Technol. 2011, 45166887–6895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer (IARC) Monograph in the series Evaluation of Carcinogenic Risks to Humans: Vol 105, Diesel and gasoline engine exhausts and some nitroarenes; World Health Organization: Lyon, 2013. [Google Scholar]
- Hayakawa K.; Nakamura A.; Terai N.; Kizu R.; Ando K. Nitroarene concentration and direct-acting mutagenicity of diesel exhaust particulates fractionated by silica-gel column chromatography. Chem. Pharm. Bull. 1997, 45, 1820–1822. [DOI] [PubMed] [Google Scholar]
- Grosjean D.; Fung K.; Harrison J. Interactions of polycyclic aromatic hydrocarbons with atmospheric pollutants. Environ. Sci. Technol. 1983, 17, 673–679. [DOI] [PubMed] [Google Scholar]
- McDow S. R.; Sun Q.; Vartiainen M.; Hong Y.; Yao Y.; Fister T.; Yao R.; Kamens R. M. Effect of composition and state of organic components on polycyclic aromatic hydrocarbon decay in atmospheric aerosols. Environ. Sci. Technol. 1994, 28, 2147–2153. [DOI] [PubMed] [Google Scholar]
- Kamens R. M.; Guo J.; Guo Z.; McDow S. R. Polynuclear aromatic hydrocarbon degradation by heterogeneous reactions with N2O5 on atmospheric particles. Atmos. Environ. 1990, 24, 1161–1173. [Google Scholar]
- Esteve W.; Budzinski H.; Villenave E. Relative rate constants for the heterogeneous reactions of NO2 and OH radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles. Part 2: PAHs adsorbed on diesel particulate exhaust SRM 1650a. Atmos. Environ. 2006, 40, 201–211. [Google Scholar]
- Zelenyuk A.; Imre D.; Beránek J.; Abramson E.; Wilson J.; Shrivastava M. Synergy between secondary organic aerosols and long-range transport of polycyclic aromatic hydrocarbons. Environ. Sci. Technol. 2012, 46, 12459–12466. [DOI] [PubMed] [Google Scholar]
- Behymer T. D.; Hites R. A. Photolysis of polycyclic aromatic hydrocarbons adsorbed on simulated atmospheric particulates. Environ. Sci. Technol. 1985, 19, 1004–1006. [Google Scholar]
- Zhou S.; Lee A.; McWhinney R.; Abbatt J. Burial effects of organic coatings on the heterogeneous reactivity of particle-borne benzo [a] pyrene (BaP) toward ozone. J. Phys. Chem. A 2012, 116, 7050–7056. [DOI] [PubMed] [Google Scholar]
- Pöschl U.; Letzel T.; Schauer C.; Niessner R. Interaction of ozone and water vapor with spark discharge soot aerosol particles coated with benzo [a] pyrene: O3 and H2O adsorption, benzo [a] pyrene degradation, and atmospheric implications. J. Phys. Chem. A 2001, 105, 4029–4041. [Google Scholar]
- Kwamena N. O. A.; Thornton J. A.; Abbatt J. P. D. Kinetics of surface-bound benzo [a] pyrene and ozone on solid organic and salt aerosols. J. Phys. Chem. A 2004, 108, 11626–11634. [Google Scholar]
- Miet K.; Le Menach K.; Flaud P. M.; Budzinski H.; Villenave E. Heterogeneous reactions of ozone with pyrene, 1-hydroxypyrene and 1-nitropyrene adsorbed on particles. Atmos. Environ. 2009, 43, 3699–3707. [Google Scholar]
- Esteve W.; Budzinski H.; Villenave E. Relative rate constants for the heterogeneous reactions of OH, NO2 and NO radicals with polycyclic aromatic hydrocarbons adsorbed on carbonaceous particles. Part 1: PAHs adsorbed on 1–2 μm calibrated graphite particles. Atmos. Environ. 2004, 38, 6063–6072. [Google Scholar]
- Perraudin E.; Budzinski H.; Villenave E. Kinetic study of the reactions of ozone with polycyclic aromatic hydrocarbons adsorbed on atmospheric model particles. J. Atmos. Chem. 2007, 56, 57–82. [Google Scholar]
- Nguyen M.; Bedjanian Y.; Guilloteau A. Kinetics of the reactions of soot surface-bound polycyclic aromatic hydrocarbons with NO2. J. Atmos. Chem. 2009, 62, 139–150. [DOI] [PubMed] [Google Scholar]
- Bedjanian Y.; Nguyen M. L.; Le Bras G. Kinetics of the reactions of soot surface-bound polycyclic aromatic hydrocarbons with the OH radicals. Atmos. Environ. 2010, 44, 1754–1760. [DOI] [PubMed] [Google Scholar]
- Jariyasopit N.; McIntosh M.; Zimmermann K.; Arey J.; Atkinson R.; Cheong P. H.-Y.; Carter R. G.; Yu T.-W.; Dashwood R. H.; Simonich S. L. Nitro-PAH product formation from heterogeneous reactions of PAHs with NO2, NO3/N2O5, O3 and OH radicals: Prediction, laboratory studies and mutagenicity. Environ. Sci. Technol. 2013, 48, 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringuet J.; Albinet A.; Leoz-Garziandia E.; Budzinski H.; Villenave E. Reactivity of polycyclic aromatic compounds (PAHs, NPAHs and OPAHs) adsorbed on natural aerosol particles exposed to atmospheric oxidants. Atmos. Environ. 2012, 61, 15–22. [Google Scholar]
- Zimmermann K.; Jariyasopit N.; Massey Simonich S. L.; Tao S.; Atkinson R.; Arey J. Formation of nitro-PAHs from the heterogeneous reaction of ambient particle-bound PAHs with N2O5/NO3/NO2. Environ. Sci. Technol. 2013, 47, 8434–8442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Primbs T.; Tao S.; Simonich S. L. M. Atmospheric particulate matter pollution during the 2008 Beijing Olympics. Environ. Sci. Technol. 2009, 43, 5314–5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson R.; Arey J.; Dodge M. C.; Harger W. P.; McElroy P.; Phousongphouang P. T.. Yields and Reactions of Intermediate Compounds Formed from the Initial Atmospheric Reactions of Selected VOCs; Contract No. 96-306; Final Report to the California Air Resouces Board; 2001; http://www.arb.ca.gov (accessed February 2014).
- Zimmermann K.; Atkinson R.; Arey J.; Kojima Y.; Inazu K. Isomer distributions of molecular weight 247 and 273 nitro-PAHs in ambient samples, NIST diesel SRM, and from radical-initiated chamber reactions. Atmos. Environ. 2012, 55, 431–439. [Google Scholar]
- Nishino N.; Atkinson R.; Arey J. Formation of nitro products from the gas-phase OH radical-initiated reactions of toluene, naphthalene, and biphenyl: Effect of NO2 concentration. Environ. Sci. Technol. 2008, 42, 9203–9209. [DOI] [PubMed] [Google Scholar]
- Maron D. M.; Ames B. N. Revised methods for the Salmonella mutagenicity test. Mutat. Res. 1983, 113, 173–215. [DOI] [PubMed] [Google Scholar]
- Finlayson-Pitts B. J.; Pitts J. N. Jr.. Chemistry of the upper and lower atmosphere, 1st ed.; Academic Press: San Diego, 2000. [Google Scholar]
- Behymer T. D.; Hites R. A. Photolysis of polycyclic aromatic hydrocarbons adsorbed on simulated atmospheric particulates. Environ. Sci. Technol. 1985, 19, 1004–1006. [Google Scholar]
- Pitts J. N. Jr.; Sweetman J. A.; Zielinska B.; Atkinson R.; Winer A. M.; Harger W. P. Formation of nitroarenes from the reaction of polycyclic aromatic hydrocarbons with dinitrogen pentoxide. Environ. Sci. Technol. 1985, 19, 1115–1121. [DOI] [PubMed] [Google Scholar]
- Carrara M.; Wolf J.-C.; Niessner R. Nitro-PAH formation studied by interacting artificially PAH-coated soot aerosol with NO2 in the temperature range of 295–523 K. Atmos. Environ. 2010, 44, 3878–3885. [Google Scholar]
- Alebic-Juretic A.; Cvitas T.; Klasinc L. Heterogeneous polycyclic aromatic hydrocarbon degradation with ozone on silica gel carrier. Environ. Sci. Technol. 1990, 24, 62–66. [Google Scholar]
- Atkinson R.; Arey J.; Zielinska B.; Aschmann S. M. Kinetics and nitro-products of the gas-phase OH and NO3 radical-initiated reactions of naphthalene-d8, fluoranthene-d10, and pyrene. Int. J. Chem. Kinet. 1990, 22, 999–1014. [Google Scholar]
- Pitts J. N. Jr.; Sweetman J. A.; Zielinska B.; Winer A. M.; Atkinson R. Determination of 2-nitrofluoranthene and 2-nitropyrene in ambient particulate organic matter: Evidence for atmospheric reactions. Atmos. Environ. 1985, 19, 1601–1608. [Google Scholar]
- Pitts J. N. Jr.; Van Cauwenberghe K. A.; Grosjean D.; Schmid J. P.; Fitz D. R.; Belser W.; Knudson G.; Hynds P. M. Atmospheric reactions of polycyclic aromatic hydrocarbons: Facile formation of mutagenic nitro derivatives. Science 1978, 202, 515–519. [DOI] [PubMed] [Google Scholar]
- White G.; Fu P.; Heflich R. Effect of nitro substitution on the light-mediated mutagenicity of polycyclic aromatic hydrocarbons in Salmonella typhimurium TA 98. Mutat. Res. 1985, 144, 1–7. [DOI] [PubMed] [Google Scholar]
- Fan Z.; Kamens R. M.; Hu J.; Zhang J.; McDow S. Photostability of nitro-polycyclic aromatic hydrocarbons on combustion soot particles in sunlight. Environ. Sci. Technol. 1996, 30, 1358–1364. [Google Scholar]
- Schauer C.; Niessner R.; Pöschl U. Polycyclic aromatic hydrocarbons in urban air particulate matter: Decadal and seasonal trends, chemical degradation, and sampling artifacts. Environ. Sci. Technol. 2003, 37, 2861–2868. [DOI] [PubMed] [Google Scholar]
- Tsapakis M.; Stephanou E. G. Collection of gas and particle semi-volatile organic compounds: Use of an oxidant denuder to minimize polycyclic aromatic hydrocarbons degradation during high-volume air sampling. Atmos. Environ. 2003, 37, 4935–4944. [Google Scholar]
- Arey J.; Zielinska B.; Atkinson R.; Winer A. M. Formation of nitroarenes during ambient high-volume sampling. Environ. Sci. Technol. 1988, 22, 457–462. [Google Scholar]
- Miet K.; Le Menach K.; Flaud P. M.; Budzinski H.; Villenave E. Heterogeneous reactivity of pyrene and 1-nitropyrene with NO2: Kinetics, product yields and mechanism. Atmos. Environ. 2009, 43, 837–843. [Google Scholar]
- van Pinxteren D.; Bruggemann E.; Gnauk T.; Iinuma Y.; Muller K.; Nowak A.; Achtert P.; Wiedensohler A.; Herrmann H. Size- and time-resolved chemical particle characterization during CAREBeijing-2006: Different pollution regimes and diurnal profiles. J. Geophys. Res. 2009, 114, D00G09. [Google Scholar]
- Fu P. P. Metabolism of nitro-polycyclic aromatic hydrocarbons. Drug Metab. Rev. 1990, 22, 209–268. [DOI] [PubMed] [Google Scholar]
- Jung H.; Heflich R. H.; Fu P. P.; Shaikh A. U.; Hartman P. Nitro group orientation, reduction potential, and direct-acting mutagenicity of nitro-polycyclic aromatic hydrocarbons. Environ. Mol. Mutagen. 1991, 17, 169–180. [DOI] [PubMed] [Google Scholar]
- Rosenkranz H. S.; Mermelstein R.. The mutagenic and carcinogenic properties of nitrated polyclic aromatic hydrocarbons. In Nitrated Polycyclic Aromatic Hydrocarbons; White C. M., Ed.; Huethig: Heidelberg, 1985; pp 267–297. [Google Scholar]
- USEPA Development of a Relative Potency Factor (RPF) Approach for Polycyclic Aromatic Hydrocarbon (PAH) Mixtures, an external review draft; U.S. Environmental Protection Agency, Integrated Risk Information System (IRIS): Washington, DC, 2010. [Google Scholar]
- Jia Y.; Stone D.; Wang W.; Schrlau J.; Tao S.; Massey Simonich S. L. Estimated reduction in cancer risk due to PAH exposures if source control measures during the 2008 Beijing Olympics were sustained. Environ. Health Perspect. 2011, 119, 815–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.