Abstract
Variability and complexity of phenotypes observed in microdeletion syndromes can be due to deletion of a single gene whose product participates in several aspects of development or can be due to the deletion of a number of tightly linked genes, each adding its own effect to the syndrome. The p6H deletion in mouse chromosome 7 presents a good model with which to address this question of multigene vs. single-gene pleiotropy. Mice homozygous for the p6H deletion are diluted in pigmentation, are smaller than their littermates, and manifest a nervous jerky-gait phenotype. Male homozygotes are sterile and exhibit profound abnormalities in spermiogenesis. By using N-ethyl-N-nitrosourea (EtNU) mutagenesis and a breeding protocol designed to recover recessive mutations expressed hemizygously opposite a large p-locus deletion, we have generated three noncomplementing mutations that map to the p6H deletion. Each of these EtNU-induced mutations has adverse effects on the size, nervous behavior, and progression of spermiogenesis that characterize p6H deletion homozygotes. Because EtNU is thought to induce primarily intragenic (point) mutations in mouse stem-cell spermatogonia, we propose that the trio of phenotypes (runtiness, nervous jerky gait, and male sterility) expressed in p6H deletion homozygotes is the result of deletion of a single highly pleiotropic gene. We also predict that a homologous single locus, quite possibly tightly linked and distal to the D15S12 (P) locus in human chromosome 15q11-q13, may be associated with similar developmental abnormalities in humans.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brannan C. I., Bedell M. A., Resnick J. L., Eppig J. J., Handel M. A., Williams D. E., Lyman S. D., Donovan P. J., Jenkins N. A., Copeland N. G. Developmental abnormalities in Steel17H mice result from a splicing defect in the steel factor cytoplasmic tail. Genes Dev. 1992 Oct;6(10):1832–1842. doi: 10.1101/gad.6.10.1832. [DOI] [PubMed] [Google Scholar]
- Brilliant M. H. The mouse pink-eyed dilution locus: a model for aspects of Prader-Willi syndrome, Angelman syndrome, and a form of hypomelanosis of Ito. Mamm Genome. 1992;3(4):187–191. doi: 10.1007/BF00355717. [DOI] [PubMed] [Google Scholar]
- Buiting K., Greger V., Brownstein B. H., Mohr R. M., Voiculescu I., Winterpacht A., Zabel B., Horsthemke B. A putative gene family in 15q11-13 and 16p11.2: possible implications for Prader-Willi and Angelman syndromes. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5457–5461. doi: 10.1073/pnas.89.12.5457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo M. P., Martinotti A., Howard T. A., Schneider C., D'Eustachio P., Seldin M. F. Localization of growth arrest-specific genes on mouse chromosomes 1, 7, 8, 11, 13, and 16. Mamm Genome. 1992;2(2):130–134. doi: 10.1007/BF00353861. [DOI] [PubMed] [Google Scholar]
- Culiat C. T., Stubbs L. J., Montgomery C. S., Russell L. B., Rinchik E. M. Phenotypic consequences of deletion of the gamma 3, alpha 5, or beta 3 subunit of the type A gamma-aminobutyric acid receptor in mice. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2815–2818. doi: 10.1073/pnas.91.7.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Culiat C. T., Stubbs L., Nicholls R. D., Montgomery C. S., Russell L. B., Johnson D. K., Rinchik E. M. Concordance between isolated cleft palate in mice and alterations within a region including the gene encoding the beta 3 subunit of the type A gamma-aminobutyric acid receptor. Proc Natl Acad Sci U S A. 1993 Jun 1;90(11):5105–5109. doi: 10.1073/pnas.90.11.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eicher E. M. The Position of ru-2 and qv with Respect to the FLECKED Translocation in the Mouse. Genetics. 1970 Mar;64(3-4):495–510. doi: 10.1093/genetics/64.3-4.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman R. A. From white spots to stem cells: the role of the Kit receptor in mammalian development. Trends Genet. 1993 Aug;9(8):285–290. doi: 10.1016/0168-9525(93)90015-a. [DOI] [PubMed] [Google Scholar]
- Gluecksohn-Waelsch S. Genetic control of morphogenetic and biochemical differentiation: lethal albino deletions in the mouse. Cell. 1979 Feb;16(2):225–237. doi: 10.1016/0092-8674(79)90001-1. [DOI] [PubMed] [Google Scholar]
- Handel M. A. Genetic control of spermatogenesis in mice. Results Probl Cell Differ. 1987;15:1–62. doi: 10.1007/978-3-540-47184-4_1. [DOI] [PubMed] [Google Scholar]
- Handel M. A., Washburn L. L., Rosenberg M. P., Eicher E. M. Male sterility caused by p6H and qk mutations is not corrected in chimeric mice. J Exp Zool. 1987 Jul;243(1):81–92. doi: 10.1002/jez.1402430111. [DOI] [PubMed] [Google Scholar]
- Holdener B. C., Brown S. D., Angel J. M., Nicholls R. D., Kelsey G., Magnuson T. Encyclopedia of the mouse genome III. October 1993. Mouse chromosome 7. Mamm Genome. 1993;4(Spec No):S110–S120. doi: 10.1007/BF00360833. [DOI] [PubMed] [Google Scholar]
- Hunt D. M., Johnson D. R. Abnormal spermiogenesis in two pink-eyed sterile mutants in the mouse. J Embryol Exp Morphol. 1971 Aug;26(1):111–121. [PubMed] [Google Scholar]
- Kelsey G., Ruppert S., Beermann F., Grund C., Tanguay R. M., Schütz G. Rescue of mice homozygous for lethal albino deletions: implications for an animal model for the human liver disease tyrosinemia type 1. Genes Dev. 1993 Dec;7(12A):2285–2297. doi: 10.1101/gad.7.12a.2285. [DOI] [PubMed] [Google Scholar]
- Klebig M. L., Russell L. B., Rinchik E. M. Murine fumarylacetoacetate hydrolase (Fah) gene is disrupted by a neonatally lethal albino deletion that defines the hepatocyte-specific developmental regulation 1 (hsdr-1) locus. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1363–1367. doi: 10.1073/pnas.89.4.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kot M. C., Handel M. A. Spermatogenesis in XO,Sxr mice: role of the Y chromosome. J Exp Zool. 1990 Oct;256(1):92–105. doi: 10.1002/jez.1402560112. [DOI] [PubMed] [Google Scholar]
- Lyon M. F., King T. R., Gondo Y., Gardner J. M., Nakatsu Y., Eicher E. M., Brilliant M. H. Genetic and molecular analysis of recessive alleles at the pink-eyed dilution (p) locus of the mouse. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6968–6972. doi: 10.1073/pnas.89.15.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls R. D. Genomic imprinting and candidate genes in the Prader-Willi and Angelman syndromes. Curr Opin Genet Dev. 1993 Jun;3(3):445–456. doi: 10.1016/0959-437x(93)90119-a. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D., Gottlieb W., Russell L. B., Davda M., Horsthemke B., Rinchik E. M. Evaluation of potential models for imprinted and nonimprinted components of human chromosome 15q11-q13 syndromes by fine-structure homology mapping in the mouse. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2050–2054. doi: 10.1073/pnas.90.5.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popp R. A., Bailiff E. G., Skow L. C., Johnson F. M., Lewis S. E. Analysis of a mouse alpha-globin gene mutation induced by ethylnitrosourea. Genetics. 1983 Sep;105(1):157–167. doi: 10.1093/genetics/105.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinchik E. M., Bultman S. J., Horsthemke B., Lee S. T., Strunk K. M., Spritz R. A., Avidano K. M., Jong M. T., Nicholls R. D. A gene for the mouse pink-eyed dilution locus and for human type II oculocutaneous albinism. Nature. 1993 Jan 7;361(6407):72–76. doi: 10.1038/361072a0. [DOI] [PubMed] [Google Scholar]
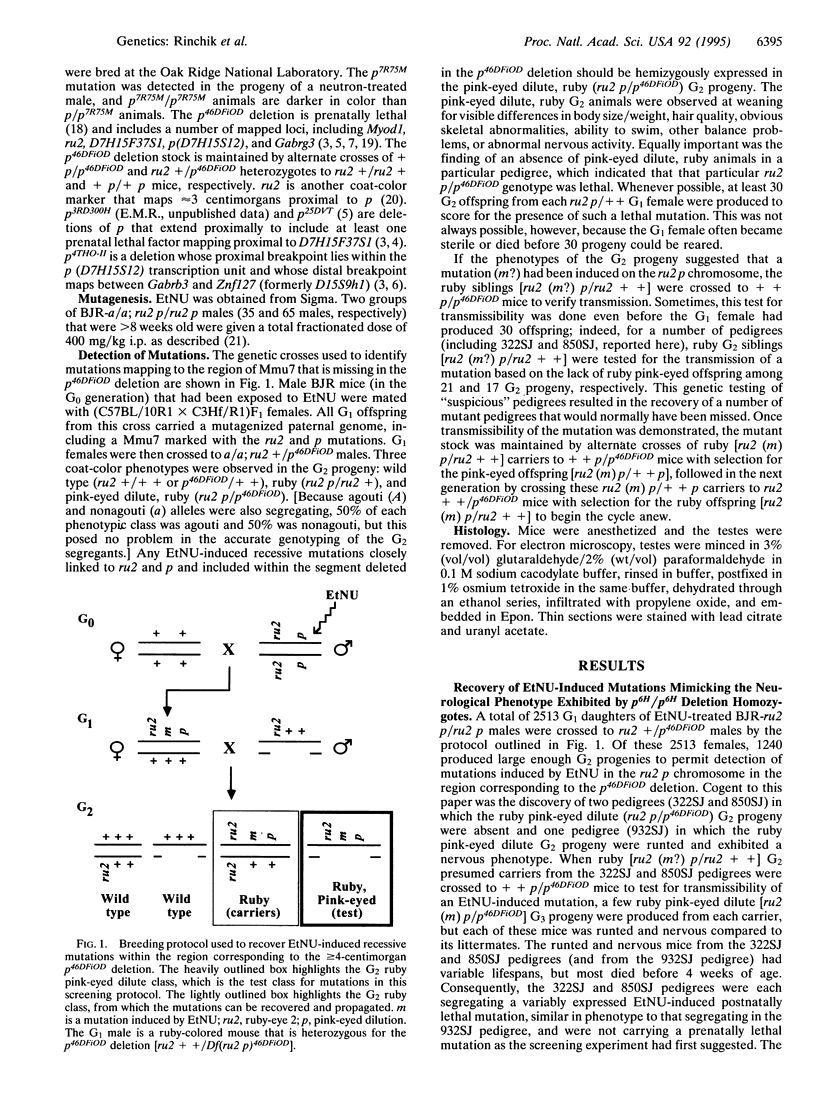
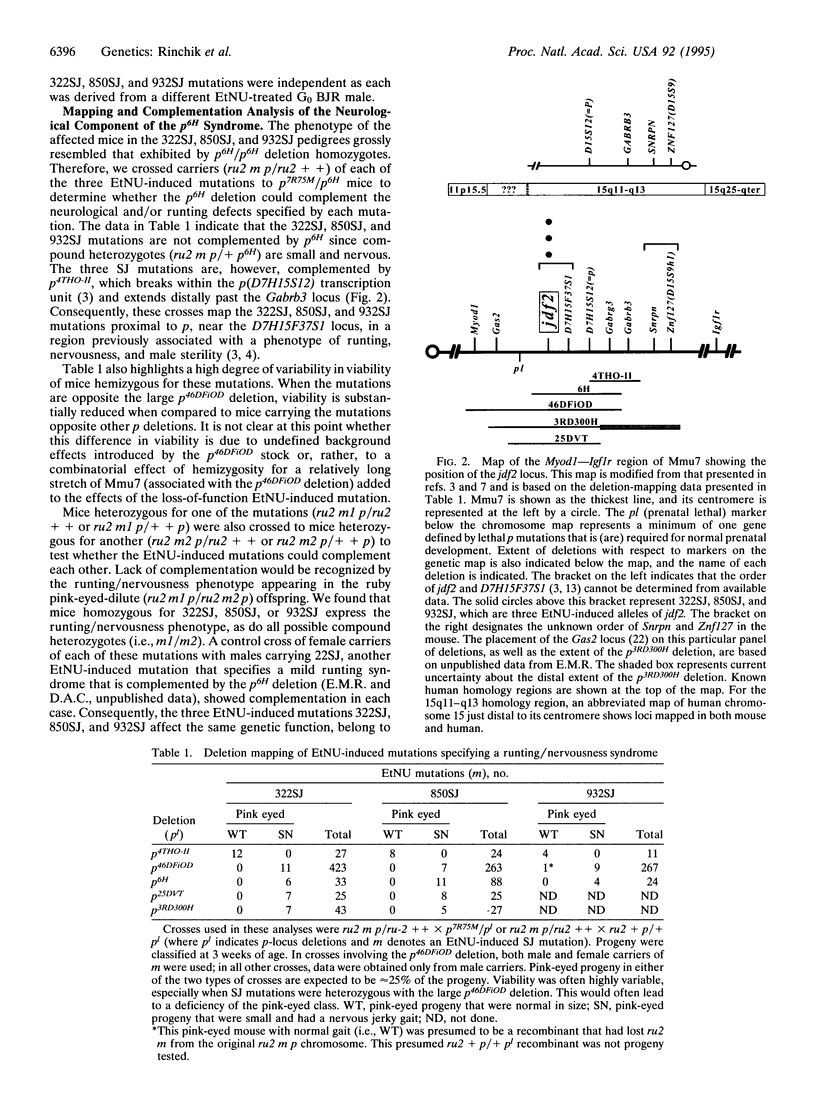
- Rinchik E. M., Carpenter D. A., Selby P. B. A strategy for fine-structure functional analysis of a 6- to 11-centimorgan region of mouse chromosome 7 by high-efficiency mutagenesis. Proc Natl Acad Sci U S A. 1990 Feb;87(3):896–900. doi: 10.1073/pnas.87.3.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruppert S., Kelsey G., Schedl A., Schmid E., Thies E., Schütz G. Deficiency of an enzyme of tyrosine metabolism underlies altered gene expression in newborn liver of lethal albino mice. Genes Dev. 1992 Aug;6(8):1430–1443. doi: 10.1101/gad.6.8.1430. [DOI] [PubMed] [Google Scholar]
- Schmickel R. D. Contiguous gene syndromes: a component of recognizable syndromes. J Pediatr. 1986 Aug;109(2):231–241. doi: 10.1016/s0022-3476(86)80377-8. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Johnson D. K., Rinchik E. M., Cavenee W. K. Rhabdomyosarcoma-associated locus and MYOD1 are syntenic but separate loci on the short arm of human chromosome 11. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2182–2186. doi: 10.1073/pnas.87.6.2182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zdarsky E., Favor J., Jackson I. J. The molecular basis of brown, an old mouse mutation, and of an induced revertant to wild type. Genetics. 1990 Oct;126(2):443–449. doi: 10.1093/genetics/126.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]