Abstract
Background and objectives
No data on the development of conventional indications for RRT (refractory acidosis, hyperkalemia, uremia, oliguria/anuria, and volume overload) related to timing of RRT exist. The prevalence of conventional indications among critically ill patients on RRT for AKI was evaluated, and patients manifesting indications versus patients without indications were compared in terms of crude and adjusted 90-day mortality.
Design, setting, participants, & measurements
In this substudy of the Finnish Acute Kidney Injury study conducted in 2011 and 2012 in 17 intensive care units with 2901 patients, patients were classified as pre-emptive (no conventional indications) and classic (one or more indications) RRT recipients. Patients with classic RRT were divided into classic-urgent (RRT initiated ≤12 hours from manifesting indications) and classic-delayed (RRT >12 hours from first indication). Additionally, 2450 patients treated without RRT were matched to patients with pre-emptive RRT.
Results
Of 239 patients treated with RRT, 134 (56.1%; 95% confidence interval [95% CI], 49.8% to 62.4%) fulfilled at least one conventional indication before commencing RRT. Crude 90-day mortality of 134 patients with classic RRT was 48.5% (95% CI, 40.0% to 57.0%), and it was 29.5% (95% CI, 20.8% to 38.2%) for the 105 patients with pre-emptive RRT. Classic RRT was associated with a higher risk for mortality (adjusted odds ratio, 2.05; 95% CI, 1.03 to 4.09). Forty-four patients with classic–delayed RRT showed higher crude mortality (68.2%; 95% CI, 54.4% to 82.0%) compared with patients with classic–urgent RRT, and this association persisted after adjustment for known confounders (odds ratio, 3.85; 95% CI, 1.48 to 10.22). Crude 90-day mortality of 67 1:1 matched patients with pre-emptive RRT was 26.9% (95% CI, 6.3% to 37.5%), and it was 49.3% (95% CI, 37.3% to 61.2%; P=0.01) for their non-RRT matches.
Conclusions
Patients on RRT after one or more conventional indications had both higher crude and adjusted 90-day mortality compared with patients without conventional indications. These findings require confirmation in an adequately powered, multicenter, randomized controlled trial.
Keywords: AKI, RRT, timing, indications, critically ill
Introduction
The optimal time to initiate RRT is unclear (1). Data from meta-analyses suggest that initiating RRT in a pre-emptive fashion according to arbitrary definitions, such as serum urea concentration or duration of oliguria or anuria, may be beneficial (2,3). Observational studies have reported better outcomes for patients who initiated RRT with milder stages of AKI (4,5) or within 24 hours of stage 3 AKI diagnosis (6). However, in a small randomized controlled trial (RCT), pre-emptive RRT after the discovery of oliguric AKI was not shown to improve survival compared with RRT initiation on the basis of clinical indications (7). Nevertheless, the RCT was underpowered to discern a realistic effect on survival and hence, cannot be viewed as definitive.
Clinical practice has traditionally been predicated on starting RRT only when a life-threatening complication of AKI arises (1,8). These indications include severe metabolic acidosis, refractory hyperkalemia, and fluid overload with pulmonary edema unresponsive to other forms of treatment (1,8,9). Additionally, sustained oliguria/anuria and complications of uremia are considered to be conventional indications (1,8). However, it has become increasingly clear that, in usual clinical practice, many patients with severe AKI commence RRT well before the development of any conventional indications for RRT. This approach may have conceivable benefits (e.g., removal of uremic toxins and improved volume control) but may also expose patients to the harms of RRT while being resource intensive. No data on the manifestation of the conventional indications in relation to intensive care unit (ICU) admission or the time of RRT initiation exist.
Accordingly, we evaluated the circumstances surrounding RRT initiation in a multicenter cohort of patients who commenced RRT for AKI. We aimed to identify characteristics and outcomes of patients who commenced RRT without conventional indications compared with patients who started with one or more indications. Additionally, we compared individuals who started RRT without conventional indications with a matched cohort of general ICU patients who did not commence RRT.
Materials and Methods
This study was a substudy of the prospective observational Finnish Acute Kidney Injury (FINNAKI) study conducted between September 1, 2011, and February 1, 2012, in 17 Finnish ICUs with capacity to provide RRT (10). The Ethics Committee of the Department of Surgery, Helsinki and Uusimaa Hospital District gave approval for the study protocol and a deferred consent policy. Each patient or proxy gave written informed consent as soon as possible. The Finnish National Institutes of Health approved data collection from the medical records of deceased patients if informed consent could not be obtained.
Patients
All adult patients with an emergency admission to study ICUs and elective postoperative patients with an expected stay over 24 hours were eligible for the FINNAKI study. The exclusion criteria were (1) preexisting ESRD requiring maintenance dialysis, (2) previous receipt of RRT while enrolled in the study, (3) organ donor, (4) patient not permanently living in Finland, (5) intermediate care patient, (6) transferred patient who had already participated in the study for 5 days (the data collection period of the study), and (7) absence of consent. For this substudy, we also excluded patients (1) treated for intoxications, (2) transferred from other centers, (3) who commenced RRT for AKI before ICU admission, (4) initially treated with molecular adsorbent recirculation system, and (5) with insufficient data for this analysis (Figure 1).
Figure 1.
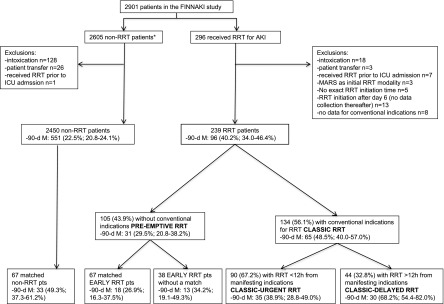
Study flow chart illustrating different patient groups and their crude 90-day mortality with 95% confidence intervals. 90-d M, 90-day mortality; FINNAKI, Finnish Acute Kidney Injury; ICU, intensive care unit; MARS, molecular adsorbent recirculation system; pts, patients. *One hundred ninety-one (7.3%) patients had treatment restrictions or were deceased within the first 5 days in the ICU.
Data Collection
We collected clinical data on patients’ preexisting conditions and medications, pre-ICU treatment from admission to day 5 in the ICU, and aspects of administered RRT with a standardized case report form. We obtained study-specific data on physiologic variables, laboratory parameters, disease severity scores, and diagnoses from the ICU data management system using an existing validated electronic platform of the Finnish Intensive Care Consortium database. The Finnish Population Register Centre provided information on patients’ vital status.
Definitions
We screened the patients for presence of AKI from ICU admission to day 5 using the Kidney Disease Improving Global Outcomes (KDIGO) criteria (1) and considering daily measured creatinine values and hourly urine output (all patients had a urinary catheter) (10). For patients without creatinine measurements in the ICU before RRT initiation (65 of 239; 27.2%), we used the highest creatinine value obtained within 48 hours before ICU admission. Treating clinicians screened patients daily for the presence of severe sepsis according to the American College of Chest Physicians/Society of Critical Care Medicine definition (11).
RRT
The decision to initiate RRT and all aspects of the RRT prescription were at the discretion of the attending intensivist, who recorded the indications for RRT initiation. The provided RRT modality and dose (12) were in agreement with current guidelines (1).
Conventional Indications
We considered the following factors as conventional indications for RRT (8,9) (Table 1): (1) hyperkalemia (serum potassium≥6 mEq/L), (2) severe acidosis (pH≤7.15), (3) plasma urea>36 mmol/L (equals BUN=100.8 mg/dl), (4) oliguria or anuria (urine output<0.3 ml/kg per hour for ≥24 hours or anuria for ≥12 hours) (1), and (5) fluid overload with pulmonary edema as defined by the presence of all the following factors: (1) >10% fluid accumulation (cumulative fluid balance/baseline weight>10%), (2) oliguria (urine output<0.5 ml/kg per hour for ≥12 hours), and (3) severely impaired oxygenation (PaO2/FiO2<200 indicated by respiratory Sequential Organ Failure Assessment [SOFA] score≥3) (13).
Table 1.
Conventional indications for RRT
| Indication | Reported Any Time before RRT (134) | First Indication | ||
|---|---|---|---|---|
| All (134) | Classic-Urgent (90) | Classic-Delayed (44) | ||
| Time from admission to indication (h) | — | 0.5 [0.0–3.3] | 0.4 [0.0–1.6] | 1.8 [0.3–16.7] |
| Time from indication to RRT (h) | — | 4.8 [1.9–17.7] | 2.5 [0.9–4.9] | 23.8 [17.7–43.2] |
| Hyperkalemia K≥6 mEq/L | 41 (30.6) | 25 (18.7) | 20 (22.2) | 5 (11.4) |
| Severe acidosis (pH≤7.15) | 70 (52.2) | 48 (35.8) | 26 (28.9) | 22 (50.0) |
| Plasma urea>36 mmol/La | 26 (19.4)b | 15 (11.2) | 14 (15.6) | 1 (2.3) |
| Oliguria or anuria (urine output<0.3 ml/kg per hour for ≥24 h or anuria for ≥12 h) | 40 (29.9) | 39 (29.1) | 28 (31.1) | 11 (25.0) |
| Fluid overload with pulmonary edemac | 12 (9.0) | 7 (5.2) | 2 (2.2) | 5 (11.4) |
Data are expressed as number (percentage) or median [interquartile range].
Equals BUN of 100.8 mg/dl.
Thirteen of 26 (50.0%) patients also had at least one other conventional indication.
All components listed had to be met: fluid accumulation>10% of weight, respiratory Sequential Organ Failure Assessment score≥3, and oliguria (urine output criteria for AKI stage 2 or 3 present). Stage 2, urine output<0.5 ml/kg per hour for ≥12 hours; stage 3, urine output<0.3 ml/kg per hour for ≥24 hours or anuria for ≥12 hours.
For each patient, we recorded the first value that fulfilled the criteria and its measurement time. If no measurements before RRT initiation were available, we considered the indication not to be met if the clinician had not reported that the particular indication was present. Patients with urine output measurements before ICU admission were screened for fulfillment of the oliguria criterion at admission. Thereafter, all patients were screened continuously on the basis of hourly urine output measurements.
RRT Patient Groups According to Timing
We defined RRT initiated without any conventional indications as pre-emptive RRT and RRT initiated in the presence of any of the five indications as classic RRT. For additional analysis, we defined patients with classic RRT as either classic–urgent RRT (RRT initiated within 12 hours from manifestation of indications) or classic-delayed RRT (RRT >12 hours from indications).
Identification of Controls Who Did Not Commence RRT
We matched patients with pre-emptive RRT to patients enrolled in the FINNAKI study who did not receive RRT (Figure 1). We conducted 1:1 matching on the basis of (1) age (±5 years), (2) Simplified Acute Physiology Score II (SAPS II) score without age and renal components (±5 points), and (3) propensity score (14) to receive pre-emptive RRT (stratum width of 0.2 SD of the logit of the propensity score). Matching was done at random and without replacement. The propensity score model (Supplemental Table 1) included the following variables associated both with the commencing of pre-emptive RRT and the outcome (15): sex, Acute Physiology and Chronic Health Evaluation II main diagnosis group, source of admission, underlying CKD, presence of severe sepsis at admission, first lactate measured in the ICU, cumulative urine output and highest dose of norepinephrine within the first 24 hours, highest KDIGO AKI stage before RRT initiation or within 24 hours of ICU admission (for patients not on RRT), and pre-ICU administration of diuretics.
Statistical Analyses
We report continuous data as medians with interquartile ranges and categorical data as counts and percentages. We used the Mann–Whitney U test to compare continuous data and the Fisher's exact test to compare categorical data. The matched patients were compared with the Wilcoxon test (continuous data) and McNemar test (categorical data). We calculated the confidence interval (CI) for the mortality difference in the matched cohort with the method by Newcombe (16). We used logistic regression models for 90-day mortality to adjust for clinically relevant confounders and independent factors with observed differences between patients with pre-emptive and classic RRT and patients with classic–urgent and classic–delayed RRT. We set the significance level at 0.05 and did not correct for multiple comparisons. We performed the analyses with Excel for Mac 2011 (Microsoft, Redmond, WA) and SPSS 20.0 (IBM, Armonk, NY) with a supplemental algorithm Fuzzy for case-control matching.
Results
We identified 239 eligible patients treated with RRT and 2450 patients without RRT (Figure 1). Of 239 RRT recipients, 134 (56.1%; 95% CI, 49.8% to 62.4%) patients fulfilled at least one conventional indication and were classified as classic RRT recipients, whereas 105 (43.9%) patients without any conventional indications were classified as patients with pre-emptive RRT. Table 1 presents the frequencies of each indication. Of 134 patients, 23 (17.2%) fulfilled the first conventional indication after 12 hours from ICU admission. In both groups, oliguria was the most prevalent indication for RRT defined by the attending intensivists (Supplemental Table 2).
Pre-emptive Versus Classic RRT
Patient characteristics are compared in Table 2. The 90-day mortality among 105 patients with pre-emptive RRT was 31 (29.5%; 95% CI, 20.8% to 38.2%), and the 90-day mortality among 134 patients with classic RRT was 65 (48.5%; 95% CI, 40.0% to 57.0%). Classic RRT remained associated with a higher risk (odds ratio, 2.05; 95% CI, 1.03 to 4.09) for 90-day mortality after adjusting for multiple confounders, including disease severity and presence of severe sepsis (Table 3). The frequency of RRT-related complications did not differ (data not shown).
Table 2.
Patient characteristics according to the presence of conventional indications (pre-emptive without and classic with) and commencement of RRT in relation to them (classic-urgent versus classic-delayed)
| Characteristic | Pre-emptive (n=105) | Classic (n=134) | P Value | Classic-Urgent (n=90) | Classic-Delayed (n=44) | P Value |
|---|---|---|---|---|---|---|
| Age (yr) | 64.0 [50.0–75.5] | 65.5 [56.8–73.0] | 0.32 | 65.0 [56.8–72.0] | 69.5 [55.5–77.8] | 0.29 |
| Men | 69/105 (65.7) | 90/134 (67.2) | 0.89 | 59/90 (65.6) | 21/44 (47.7) | 0.70 |
| CKD | 137104 (12.5) | 26/132 (19.7) | 0.16 | 16/88 (18.2) | 10/44 (22.7) | 0.64 |
| Number of preexisting chronic illnesses | 1 [0–2] | 2 [1–2] | 0.03 | 1 [0–1] | 2 [1–3] | 0.21 |
| Operative admission | 52/105 (49.5) | 28/134 (20.9) | <0.001 | 13/90 (14.4) | 15/44 (34.1) | 0.01 |
| Emergency admission | 90/105 (85.7) | 131/134 (97.8) | 0.001 | 89/90 (98.9) | 42/44 (95.5) | 0.25 |
| Admitted froma | <0.001 | 0.20 | ||||
| Operating room or recovery room | 52/105 (49.5) | 24/134 (17.9) | 11/90 (12.2) | 13/44 (29.5) | ||
| Emergency room | 26/105 (24.8) | 60/134 (44.8) | 45/90 (50.0) | 15/44 (34.1) | ||
| Ward | 21/107 (20.0) | 36/134 (26.9) | 24/90 (26.7) | 12/44 (27.3) | ||
| Other | 6/105 (5.7) | 14/134 (10.4) | 10/90 (11.1) | 4/44 (9.1) | ||
| SAPS II score | 47.0 [36.0–60.0] | 56.0 [47.0–69.5] | <0.001 | 56.0 [47.0–68.3] | 58.5 [46.5–72.0] | 0.58 |
| SAPS II score without age points | 38.0 [25.5–46.5] | 45.0 [35.8–59.3] | <0.001 | 44.5 [35.8–59.3] | 46.0 [35.5–62.8] | 0.71 |
| SOFA score, first 24 h | 10.0 [8.0–13.0] | 11.0 [9.0–14.0] | 0.24 | 11.0 [8.8–14.0] | 11.0 [9.0–14.0] | 0.35 |
| SOFA score, maximum | 12.0 [10.0–15.0] | 12.5 [10.0–15.0] | 0.58 | 12.0 [9.0–15.0] | 14.0 [11.0–17.0] | 0.01 |
| Mechanical ventilation (ICU stay) | 91/105 (86.7) | 87/134 (64.9) | <0.001 | 51/90 (56.7) | 36/44 (81.8) | 0.004 |
| Vasoactive drugs (ICU stay) | 92/105 (87.6) | 112/134 (83.6) | 0.46 | 71/90 (78.9) | 41/44 (93.2) | 0.05 |
| Severe sepsis until day 5 | 48/105 (45.7) | 72/134 (53.7) | 0.24 | 44/90 (48.9) | 28/44 (63.6) | 0.14 |
| AKI before RRT | 92/104 (88.5) | 128/134 (95.5) | 0.05 | 84/90 (93.3) | 44/44 (100.0) | 0.18 |
| Highest AKI stage before RRTa | <0.001 | 0.86 | ||||
| Stage 1 | 19/92 (20.7) | 5/128 (3.9) | 2/84 (2.4) | 3/44 (6.8) | ||
| Stage 2 | 29/92 (31.5) | 26/128 (20.3) | 15/84 (17.9) | 11/44 (25.0) | ||
| Stage 3 | 44/92 (47.8) | 97/128 (75.8) | 67/84 (79.8) | 30/44 (68.2) | ||
| Time from hospital admission to RRT (d) | 2 [1–4] | 1 [0–3] | 0.002 | 1 [0–1] | 3 [1–6] | <0.001 |
| Time from ICU admission to RRT (h) | 22.5 [10.5–44.6] | 7.3 [2.6–28.7] | <0.001 | 3.3 [2.0–7.4] | 35.5 [22.7–59.8] | <0.001 |
| CRRT as initial modality | 80/105 (76.2) | 102/134 (76.1) | >0.99 | 66/90 (73.3) | 36/44 (81.8) | 0.39 |
| At RRT initiation | ||||||
| K (mEq/L) | 4.2 [3.9–4.7] | 4.8 [4.1–5.5] | <0.001 | 5.1 [4.3–6.1] | 4.5 [4.0–5.1] | 0.004 |
| pH | 7.35 [7.27–7.40] | 7.23 [7.12–7.34] | <0.001 | 7.20 [7.10–7.33] | 7.27 [7.18–7.36] | 0.02 |
| BE (mmol/L) | −5.5 [−9.5 to −2.6] | −11.2 [−16.9 to −7.0] | <0.001 | −13.0 [−19.8 to −4.9] | −9.8 [−12.9 to −4.9] | 0.003 |
| Lactate (mmol/L) | 1.8 [1.2–3.9] | 3.4 [1.5–9.2] | <0.001 | 3.4 [1.7–10.3] | 3.4 [1.4–6.5] | 0.38 |
| Creatinine before RRT (mg/dl) | 2.6 [1.9–3.6] | 3.7 [2.3–6.3] | <0.001 | 4.5 [2.4–7.9] | 2.9 [1.9–4.4] | 0.003 |
| Urea (mmol/L) | 19.1 [12.1–25.8] | 23.2 [13.9–33.4] | 0.01 | 25.9 [17.6–38.9] | 16.7 [10.6–27.2] | 0.01 |
| Urine output (ml/24 h)b | 380 [110–1075] | 234 [56–780] | 0.17 | 315 [105–1190] | 109 [28–482] | 0.003 |
| Fluid accumulation (%) | 5.1 [1.6–10.8] | 5.7 [1.7–10.4] | 0.72 | 3.3 [0.5–8.4] | 9.3 [6.2–14.0] | <0.001 |
| Diuretics before RRT | 79/105 (75.2) | 90/134 (67.2) | 0.20 | 49/90 (54.4) | 41/44 (93.2) | <0.001 |
| Duration of RRT among 90-d survivors (d) at 90 d | 7.0 [3.0–28.0] | 4.0 [2.0–23.0] | 0.13 | 4.0 [1.8–21.0] | 5.0 [3.3–25.0] | 0.27 |
Data are expressed as median [interquartile range] or number/total number (percentage). SAPS, Simplified Acute Physiology Score; SOFA, Sequential Organ Failure Assessment; ICU, intensive care unit; CRRT, continuous RRT; BE, base excess.
Comparison across groups.
On the day of RRT initiation.
Table 3.
Logistic regression models for 90-day mortality among all patients with RRT and among patients with classic RRT
| Covariate | Univariate Odds Ratio (95% CI) | P Value | Multivariate Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| All patients on RRTa | ||||
| Age (5 yr) | 1.18 (1.07 to 1.30) | 0.001 | 1.20 (1.06 to 1.36) | 0.01 |
| Number of preexisting chronic illnessesb | 1.03 (0.84 to 1.27) | 0.77 | 0.94 (0.72 to 1.23) | 0.66 |
| Study site | — | 0.15 | — | 0.90 |
| Source of admission | — | 0.18 | — | 0.54 |
| SAPS II score without age points | 1.07 (1.05 to 1.09) | <0.001 | 1.06 (1.04 to 1.09) | <0.001 |
| Mechanical ventilation (ICU stay) | 3.65 (1.82 to 7.32) | <0.001 | 2.93 (1.23 to 6.96) | 0.02 |
| Vasoactive drugs (ICU stay) | 3.10 (1.29 to 7.41) | 0.01 | 1.30 (0.41 to 4.12) | 0.66 |
| Severe sepsis until day 5 | 1.99 (1.18 to 3.37) | 0.01 | 1.16 (0.61 to 2.24) | 0.58 |
| Time from ICU admission to RRT (h) | 1.00 (0.99 to 1.01) | 0.93 | 1.01 (0.98 to 1.02) | 0.17 |
| CRRT as initial modality | 0.50 (0.26 to 0.95) | 0.04 | 0.97 (0.42 to 2.21) | 0.94 |
| Classic RRT (versus pre-emptive RRT) | 2.25 (1.31 to 3.86) | 0.003 | 2.05 (1.03 to 4.09) | 0.04 |
| Patients on classic RRTc | ||||
| Age (5 yr) | 1.21 (1.03 to 1.41) | 0.02 | 1.32 (1.09 to 1.62) | 0.01 |
| Study site | — | 0.61 | — | 0.75 |
| SAPS II score without age points | 1.07 (1.04 to 1.10) | <0.001 | 1.08 (1.04 to 1.12) | <0.001 |
| Mechanical ventilation (ICU stay) | 6.36 (2.79 to 14.49) | <0.001 | 4.14 (1.57 to 12.39) | 0.01 |
| Vasoactive drugs (ICU stay) | 2.30 (0.87 to 6.08) | 0.09 | 0.42 (0.09 to 1.76) | 0.40 |
| Severe sepsis until day 5 | 1.85 (0.93 to 3.68) | 0.08 | 1.19 (0.48 to 2.94) | 0.71 |
| CRRT as initial modality | 0.47 (0.20 to 1.06) | 0.07 | 0.96 (0.31 to 3.04) | 0.95 |
| Classic-delayed RRT (versus classic–urgent RRT) | 3.37 (1.57 to 7.22) | 0.002 | 3.85 (1.48 to 10.22) | 0.01 |
CRRT, continuous RRT; 95 % CI, 95% confidence interval.
Model included all 239 patients. Hosmer–Lemeshow test=9.82; P=0.28.
In total, 10 of 239 (4.2%) patients with missing data were assumed to have no comorbidities.
Model included all 134 patients. Hosmer–Lemeshow test=3.13; P=0.93.
Classic-Urgent Versus Classic-Delayed RRT
Of 134 patients with conventional indications, 90 (67.2%) patients commenced RRT within 12 hours of manifesting indications (classic–urgent RRT), and 44 (32.8%) patients initiated RRT >12 hours after manifesting indications (classic–delayed RRT). Table 2 presents the comparison of patient characteristics. Of patients with classic–urgent RRT, 35 (38.9%; 95% CI, 28.8% to 49.0%) patients were deceased by day 90 compared with 30 (68.2%; 95% CI, 54.4% to 82.0%) patients in the classic-delayed group. After adjustments, classic–delayed RRT was associated with a higher risk (odds ratio, 3.85; 95% CI, 1.48 to 10.22) for 90-day mortality (Table 3).
Pre-emptive RRT Versus Non-RRT
The propensity score model for pre-emptive RRT had an area under the curve of 0.92 (0.89–0.95). Of 105 recipients of pre-emptive RRT, 67 (63.8%) patients were matched to 67 patients not on RRT. The postmatching balance was mainly satisfactory, but patients on pre-emptive RRT reached higher maximum SOFA scores, and patients not on RRT more commonly had treatment restrictions (Table 4). Matched patients on pre-emptive RRT had lower crude 90-day mortality compared with matched patients not on RRT (26.9% versus 49.3%; P=0.01; absolute difference=22.4%; 95% CI, 7.5% to 35.9%) (Figure 1). Patients with pre-emptive RRT left without a match (n=38) did not significantly differ regarding disease severity (Supplemental Table 3) and crude 90-day mortality (Figure 1) compared with matched patients on pre-emptive RRT.
Table 4.
Characteristics of matched patients on pre-emptive RRT compared with matched patients who did not receive RRT
| Characteristic | Pre-emptive RRT | Non-RRT | P Value | SMD |
|---|---|---|---|---|
| Age (yr) | 64.0 [56.0–76.0] | 67.0 [55.0–75.0] | 0.92 | −0.5 |
| Men | 44/67 (65.7) | 46//67 (68.7) | 0.03 | −6.4 |
| CKD | 10/66 (14.9) | 7/67 (10.4) | 0.61 | 13.6 |
| Operative admission | 33/67 (49.3) | 28/67 (41.8) | 0.52 | 15.1 |
| Emergency admission | 56/67 (83.6) | 58/67 (86.6) | 0.79 | −8.4 |
| Admitted froma | 0.92 | |||
| Operating room or recovery room | 32/67 (47.8) | 28/67 (41.8) | ||
| Emergency room | 14/67 (20.9) | 21/67 (31.3) | ||
| Ward | 15/67 (22.4) | 14/67 (20.9) | ||
| Other | 6/67 (9.0) | 4/67 (6.0) | ||
| SAPS II score | 45.0 [35.0–59.0] | 41.0 [32.0–58.0] | 0.11 | 13.5 |
| SAPS II score without age and renal components | 24.0 [16.0–30.0] | 24.0 [17.0–30.0] | 0.83 | −0.4 |
| SOFA score, first 24 h | 10.0 [8.0–13.0] | 9.0 [7.0–12.0] | 0.24 | 16.9 |
| SOFA score, maximum | 12.0 [9.0–14.0] | 9.0 [8.0–13.0] | 0.001 | 52.6 |
| Mechanical ventilation (ICU stay) | 58/67 (86.8) | 47/67 (70.1) | 0.04 | 20.6 |
| Severe sepsis until day 5 | 31/67 (46.3) | 29/67 (43.3) | 0.03 | 6.0 |
| AKI before RRT or within days 1–5 | 55/67 (82.1) | 59/67 (88.1) | 0.13 | −16.9 |
| Highest AKI stage before RRT or within 24 h of ICU admission (patients not on RRT)a | 0.35 | |||
| Stage 1 | 15/67 (22.4) | 15/67 (22.4) | ||
| Stage 2 | 21/67 (31.3) | 23/67 (34.3) | ||
| Stage 3 | 19/67 (28.4) | 20/67 (29.9) | ||
| Treatment restrictionsb | 16/66 (24.2) | 29/67 (43.3) | 0.02 | −41.2 |
| At ICU admission | ||||
| Vasoactives | 57/67 (85.1) | 60/67 (89.6) | 0.61 | −13.6 |
| Severe sepsis | 28/67 (41.8) | 23/67 (34.3) | 0.47 | 15.5 |
| Highest norepinehprine dose within first 24 h (μg/kg per minute) | 0.22 [0.06–0.44] | 0.18 [0.07–0.35] | 0.28 | 5.0 |
| Cumulative urine output within the first 24 h (ml) | 865 [460–1760] | 1135 [546–1720] | 0.78 | 0.9 |
| First creatinine (mg/dl) | 1.6 [1.2–2.2] | 1.4 [1.0–2.7] | 0.95 | −2.5 |
| First lactate (mmol/L) | 2.8 [1.5–5.8] | 2.5 [1.3–5.2] | 0.71 | 7.7 |
| Received diuretics | 35/64 (54.7) | 30/65 (46.2) | 0.31 | 17.1 |
| 90-d mortality | 18/67 (26.9) | 33/67 (49.3) | 0.01 | −47.4 |
Data are expressed as median [interquartile range] or number/total number (percentage). SMD, standardized mean difference.
Comparison across groups with marginal homogeneity test.
Treatment restrictions include one or several of the following restrictions: (1) do not resuscitate, (2) do not increase the intensity of treatment, and (3) withdrawal of intensive care.
Discussion
In this substudy of general ICU patients treated with RRT, 56% of patients presented with at least one conventional indication for RRT before commencing RRT. These more severely ill patients showed higher crude 90-day mortality compared with patients having RRT started pre-emptively without any conventional indication. This association persisted after adjustment for disease severity and other known confounders. Matched patients with pre-emptive RRT showed a signal to lower 90-day mortality compared with patients who did not receive RRT, suggesting that pre-emptive RRT is unlikely to add excessive harm.
The question of RRT timing is fundamental in the management of critically ill patients with AKI. To the best of our knowledge, this study is the first to assess the timing of RRT in regards to the manifestation of conventional indications for RRT. Unlike many previous studies that have assessed RRT timing in regard to a single factor, we combined a comprehensive set of clinically relevant indications for RRT. Moreover, relating timing of RRT to the time of manifestation of these indications provides a practical viewpoint to RRT timing. It is likely that, in many centers, RRT is initiated only after one or more of these indications are fulfilled (17,18). Thus, what we defined as classic RRT is plausibly close to the standard practice in many centers. Moreover, in this analysis, patients manifesting conventional indications commenced RRT earlier in terms of time from ICU admission than patients without conventional indications. Thus, because patients are admitted to the ICU in varying phases of critical illness, defining RRT initiation temporally according to ICU admission seems biased.
Our set of criteria classified the patients into groups with crude 90-day mortality varying from 30% to 68%. Patients with classic RRT showed significantly higher crude 90-day mortality compared with patients with pre-emptive RRT. After adjusting for disease severity and other confounders, classic RRT remained associated with a higher risk of 90-day mortality. These findings support a hypothesis that commencing RRT before the appearance of AKI complications might be beneficial. However, despite meticulous data recording, the presence of unmeasured confounders and potentially differing courses of disease between patients with pre-emptive and classic RRT cannot be excluded. Earlier initiation of RRT in the course of AKI may have prevented the worsening of electrolyte, acid-base, and fluid-balance disturbances, which may explain the lower SAPS II scores of patients on pre-emptive RRT. Moreover, on a practical level, commencing RRT earlier among the patients on classic–urgent RRT would not have been possible, because these patients presented with complications of AKI and advanced critical illness already at ICU admission and started RRT within approximately 3 hours. One half of them were admitted directly from the emergency room, but the rest came from a ward or operating room, and therefore, recognizing their worsening clinical status earlier might have improved their outcome.
Approximately one third of patients with conventional indications were initiated on RRT >12 hours after manifesting the indication (classic–delayed RRT) and had the highest crude mortality rates. Compared with patients with classic–urgent RRT, these patients showed a higher degree of fluid accumulation. Severe acidosis was the most prevalent indication present among them. Possibly, proving severe acidosis refractory may take more time and require large amounts of fluid therapy. Consequently, in the presence of oliguric AKI, these patients accumulate a marked amount of fluid, which has been shown to associate with a worse outcome (12,19,20). Classic-delayed RRT was significantly associated with a higher risk for 90-day mortality, suggesting that delaying RRT initiation among patients who present conventional indications might be harmful.
Patients with pre-emptive RRT were less severely ill, had milder disturbances in the acid–base balance at the time of RRT initiation, were more often admitted after surgery, and had stayed longer in the hospital and the ICU before commencing RRT compared with patients with classic RRT. Thus, patients on pre-emptive RRT had been in treatment longer, which may have provided the attending intensivists with better insight on the progression of disease. According to recommendations (1,21) and survey data (22), the trend in severity of illness is an important factor affecting the decision to start RRT. Moreover, pre-emptive initiation of RRT may be viewed as organ support that provides respite for injured kidneys (23). One may speculate that this group may include patients who might have recovered without RRT. Because the population-based incidence of RRT treatment in Finland (24) is well within range of reports from other regions (25,26), we believe that providing unnecessary RRT in Finland is unlikely. Moreover, the proportion of patients (17%) with classic RRT who developed conventional indications >12 hours after ICU admission was surprisingly small, likely because of the large proportion of patients with pre-emptive RRT.
Matched patients with pre-emptive RRT showed a signal toward a lower crude 90-day mortality (point estimate for absolute difference=−22%) compared with control patients treated without RRT. This finding suggests that pre-emptive RRT, even if not beneficial, is unlikely to add excessive harm. Patients left without a match had a worse outcome, which may dilute our finding. This phenomenon has also been encountered in other propensity-matched studies (27,28). Furthermore, patients with severe AKI who did not commence RRT have been observed to be fundamentally different from patients with RRT (29). A recent propensity-matched analysis comparing patients with RRT with patients without RRT did not find RRT to be beneficial (30), and a previous observational study found RRT to associate with worse survival compared with conservative treatment of AKI (31). However, unmeasurable factors leading a clinician to prescribe or not prescribe RRT may have driven these findings.
We reported a lower prevalence of conventional indications (56%) than a previous report (74%) that included RIFLE class (32). We evaluated each of the five conventional indications as equally important, although in the clinical setting, the presence of refractory hyperkalemia is a far more urgent indication than an elevated urea concentration (22). In this study, one half of patients whose indication for RRT was elevated urea concentration also had other conventional indications for RRT. Moreover, we had to use a complex definition for organ edema and fluid overload because of the retrospective screening for the presence of indications, and, potentially, more patients may have fulfilled this indication had the screening been prospective.
The optimal time to start RRT is a top priority in AKI research (1,9), and thus, RCTs focused on the timing of RRT are clearly warranted (1,2,33). Our study provides valuable data for planning an RCT. Most importantly, one half of the patients treated with RRT presented with conventional indications at ICU admission, and many of them would, therefore, not be eligible for randomization in an RCT that is in development (34). Defining appropriate laboratory inclusion criteria for an RCT might be challenging, because the ability of novel biomarkers to predict the receipt of RRT in patients with AKI is poor. Unfortunately, we were unable to estimate the number of patients who developed conventional indications but did not receive RRT, because no data regarding the refractoriness of acidosis, hyperkalemia, and fluid overload were recorded. Previously reported reasons for not starting RRT, despite the presence of indications, include treatment restrictions (7,29), death before commencing RRT, spontaneous recovery of renal function (7), or use of a watch-and-wait approach (29). Of note, in the study cohort, very few patients (7%) died or did not start RRT because of treatment limitations within the first 5 days.
The detailed collection of both clinical and laboratory data that enabled precise screening for the presence of indications and their timing in addition to a well captured clinical end point of unequivocal clinical relevance are obvious strengths of our study. However, some limitations should be addressed. First, because the measured laboratory values were captured for only the first 5 days in the ICU, we had to exclude a few patients (n=13) who initiated RRT after day 6. Second, the proportion of patients with fluid accumulation may be underestimated, because no reliable data on fluid balance before ICU admission were available. Third, the precise cutoffs for conventional indications have not been validated, although the thresholds that we used represent established clinical practice. Fourth, among patients with classic–delayed RRT with a maximum delay of 5 days commencing RRT after meeting conventional indications, evolving clinical situations and potentially different indications for RRT cannot be excluded. Fifth, because this study was observational, unmeasured confounding factors and bias from competing risks (related to the exclusion of the few patients who died before potential classic start of RRT) cannot be excluded.
In conclusion, in a large multicenter cohort, approximately one half of the critically ill patients who received RRT for AKI commenced RRT in the absence of any conventional indications for RRT. This pre-emptive approach to the initiation of RRT was associated with a lower risk for 90-day mortality compared with the initiation of RRT after the development of one or more conventional indications. The actual benefits of this strategy will require confirmation in a definitive RCT.
Disclosures
R.W. has received grant support, has served as a paid speaker for Alere, and serves as a consultant to Thrasos. S.M.B. has consulted for Gambro and Alere. The other authors declare no conflict of interest.
Supplementary Material
Acknowledgments
We thank Tieto Healthcare and Welfare for database management.
The Academy of Finland, the Sigrid Juselius Foundation, the Päivikki and Sakari Sohlberg Foundation, and Helsinki University Central Hospital EVO grants have supported this study. S.T.V. has received a grant from the Finnish Society of Anaesthesiologists. S.M.B. is supported by a Canada Research Chair in Critical Care Nephrology and a Clinical Investigator Award from Alberta Innovates–Health Solutions.
The Finnish Acute Kidney Injury Study Group includes Central Finland Central Hospital: Raili Laru-Sompa, Anni Pulkkinen, Minna Saarelainen, Mikko Reilama, Sinikka Tolmunen, Ulla Rantalainen, and Marja Miettinen; East Savo Central Hospital: Markku Suvela, Katrine Pesola, Pekka Saastamoinen, and Sirpa Kauppinen; Helsinki University Central Hospital: Ville Pettilä, Kirsi-Maija Kaukonen, Anna-Maija Korhonen, Sara Nisula, Suvi Vaara, Raili Suojaranta-Ylinen, Leena Mildh, Mikko Haapio, Laura Nurminen, Sari Sutinen, Leena Pettilä, Helinä Laitinen, Heidi Syrjä, Kirsi Henttonen, Elina Lappi, and Hillevi Boman; Jorvi Central Hospital: Tero Varpula, Päivi Porkka, Mirka Sivula, Mira Rahkonen, Anne Tsurkka, Taina Nieminen, and Niina Pirttinen; Kanta-Häme Central Hospital: Ari Alaspää, Ville Salanto, Hanna Juntunen, and Teija Sanisalo; Kuopio University Hospital: Ilkka Parviainen, Ari Uusaro, Esko Ruokonen, Stepani Bendel, Niina Rissanen, Maarit Lång, Sari Rahikainen, Saija Rissanen, Merja Ahonen, Elina Halonen, and Eija Vaskelainen; Lapland Central Hospital: Meri Poukkanen, Esa Lintula, and Sirpa Suominen; Länsi-Pohja Central Hospital: Jorma Heikkinen, Timo Lavander, Kirsi Heinonen, and Anne-Mari Juopperi; Middle Ostrobothnia Central Hospital: Tadeusz Kaminski, Fiia Gäddnäs, Tuija Kuusela, and Jane Roiko; North Karelia Central Hospital: Sari Karlsson, Matti Reinikainen, Tero Surakka, Helena Jyrkönen, Tanja Eiserbeck, and Jaana Kallinen; Oulu University Hospital: Tero Ala-Kokko, Jouko Laurila, and Sinikka Sälkiö; Satakunta Hospital District: Vesa Lund, Päivi Tuominen, Pauliina Perkola, Riikka Tuominen, Marika Hietaranta, and Satu Johansson; South Karelia Central Hospital: Seppo Hovilehto, Anne Kirsi, Pekka Tiainen, Tuija Myllärinen, Pirjo Leino, and Anne Toropainen; Tampere University Hospital: Anne Kuitunen, Jyrki Tenhunen, Ilona Leppänen, Markus Levoranta, Sanna Hoppu, Jukka Sauranen, Atte Kukkurainen, Samuli Kortelainen, and Simo Varila; Turku University Hospital: Outi Inkinen, Niina Koivuviita, Jutta Kotamäki, and Anu Laine; and Vaasa Central Hospital: Simo-Pekka Koivisto, Raku Hautamäki, and Maria Skinnar.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.12691213/-/DCSupplemental.
See related editorial, “A Policy of Preemption: The Timing of Renal Replacement Therapy in AKI,” on pages 1510–1512.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group : KDIGO clinical practice guideline for acute kidney injury. Kidney Int [Suppl]2: 1–138, 2012 [Google Scholar]
- 2.Karvellas CJ, Farhat MR, Sajjad I, Mogensen SS, Leung AA, Wald R, Bagshaw SM: A comparison of early versus late initiation of renal replacement therapy in critically ill patients with acute kidney injury: A systematic review and meta-analysis. Crit Care 15: R72, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seabra VF, Balk EM, Liangos O, Sosa MA, Cendoroglo M, Jaber BL: Timing of renal replacement therapy initiation in acute renal failure: A meta-analysis. Am J Kidney Dis 52: 272–284, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Bell M, Liljestam E, Granath F, Fryckstedt J, Ekbom A, Martling CR: Optimal follow-up time after continuous renal replacement therapy in actual renal failure patients stratified with the RIFLE criteria. Nephrol Dial Transplant 20: 354–360, 2005 [DOI] [PubMed] [Google Scholar]
- 5.Shiao CC, Wu VC, Li WY, Lin YF, Hu FC, Young GH, Kuo CC, Kao TW, Huang DM, Chen YM, Tsai PR, Lin SL, Chou NK, Lin TH, Yeh YC, Wang CH, Chou A, Ko WJ, Wu KD, National Taiwan University Surgical Intensive Care Unit-Associated Renal Failure Study Group : Late initiation of renal replacement therapy is associated with worse outcomes in acute kidney injury after major abdominal surgery. Crit Care 13: R171, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leite TT, Macedo E, Pereira SM, Bandeira SR, Pontes PH, Garcia AS, Militão FR, Sobrinho IM, Assunção LM, Libório AB: Timing of renal replacement therapy initiation by AKIN classification system. Crit Care 17: R62, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J: Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: A prospective, randomized trial. Crit Care Med 30: 2205–2211, 2002 [DOI] [PubMed] [Google Scholar]
- 8.Bagshaw SM, Cruz DN, Gibney RT, Ronco C: A proposed algorithm for initiation of renal replacement therapy in adult critically ill patients. Crit Care 13: 317, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gibney N, Hoste E, Burdmann EA, Bunchman T, Kher V, Viswanathan R, Mehta RL, Ronco C: Timing of initiation and discontinuation of renal replacement therapy in AKI: Unanswered key questions. Clin J Am Soc Nephrol 3: 876–880, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Nisula S, Kaukonen KM, Vaara ST, Korhonen AM, Poukkanen M, Karlsson S, Haapio M, Inkinen O, Parviainen I, Suojaranta-Ylinen R, Laurila JJ, Tenhunen J, Reinikainen M, Ala-Kokko T, Ruokonen E, Kuitunen A, Pettilä V, FINNAKI Study Group : Incidence, risk factors and 90-day mortality of patients with acute kidney injury in Finnish intensive care units: The FINNAKI study. Intensive Care Med 39: 420–428, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ, The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine : Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Chest 101: 1644–1655, 1992 [DOI] [PubMed] [Google Scholar]
- 12.Vaara ST, Korhonen AM, Kaukonen KM, Nisula S, Inkinen O, Hoppu S, Laurila JJ, Mildh L, Reinikainen M, Lund V, Parviainen I, Pettilä V, The FINNAKI study group : Fluid overload is associated with an increased risk for 90-day mortality in critically ill patients with renal replacement therapy: Data from the prospective FINNAKI study. Crit Care 16: R197, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vincent JL, de Mendonça A, Cantraine F, Moreno R, Takala J, Suter PM, Sprung CL, Colardyn F, Blecher S: Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Working group on “sepsis-related problems” of the European Society of Intensive Care Medicine. Crit Care Med 26: 1793–1800, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Gayat E, Pirracchio R, Resche-Rigon M, Mebazaa A, Mary JY, Porcher R: Propensity scores in intensive care and anaesthesiology literature: A systematic review. Intensive Care Med 36: 1993–2003, 2010 [DOI] [PubMed] [Google Scholar]
- 15.Heinze G, Jüni P: An overview of the objectives of and the approaches to propensity score analyses. Eur Heart J 32: 1704–1708, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Newcombe RG: Improved confidence intervals for the difference between binomial proportions based on paired data. Stat Med 17: 2635–2650, 1998 [PubMed] [Google Scholar]
- 17.Bellomo R, Cass A, Cole L, Finfer S, Gallagher M, Lo S, McArthur C, McGuinness S, Myburgh J, Norton R, Scheinkestel C, Su S, RENAL Replacement Therapy Study Investigators : Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 361: 1627–1638, 2009 [DOI] [PubMed] [Google Scholar]
- 18.Palevsky PM, Zhang JH, O’Connor TZ, Chertow GM, Crowley ST, Choudhury D, Finkel K, Kellum JA, Paganini E, Schein RM, Smith MW, Swanson KM, Thompson BT, Vijayan A, Watnick S, Star RA, Peduzzi P, VA/NIH Acute Renal Failure Trial Network : Intensity of renal support in critically ill patients with acute kidney injury. N Engl J Med 359: 7–20, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bouchard J, Soroko SB, Chertow GM, Himmelfarb J, Ikizler TA, Paganini EP, Mehta RL, Program to Improve Care in Acute Renal Disease (PICARD) Study Group : Fluid accumulation, survival and recovery of kidney function in critically ill patients with acute kidney injury. Kidney Int 76: 422–427, 2009 [DOI] [PubMed] [Google Scholar]
- 20.Modem V, Thompson M, Gollhofer D, Dhar AV, Quigley R: Timing of continuous renal replacement therapy and mortality in critically ill children. Crit Care Med 42: 943–953, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Ostermann M, Dickie H, Barrett NA: Renal replacement therapy in critically ill patients with acute kidney injury—when to start. Nephrol Dial Transplant 27: 2242–2248, 2012 [DOI] [PubMed] [Google Scholar]
- 22.Thakar CV, Rousseau J, Leonard AC: Timing of dialysis initiation in AKI in ICU: International survey. Crit Care 16: R237, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chawla LS, Kellum JA, Ronco C: Permissive hypofiltration. Crit Care 16: 317, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vaara ST, Pettilä V, Reinikainen M, Kaukonen KM, Finnish Intensive Care Consortium : Population-based incidence, mortality and quality of life in critically ill patients treated with renal replacement therapy: A nationwide retrospective cohort study in Finnish intensive care units. Crit Care 16: R13, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Prescott GJ, Metcalfe W, Baharani J, Khan IH, Simpson K, Smith WC, MacLeod AM: A prospective national study of acute renal failure treated with RRT: Incidence, aetiology and outcomes. Nephrol Dial Transplant 22: 2513–2519, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Waikar SS, Curhan GC, Wald R, McCarthy EP, Chertow GM: Declining mortality in patients with acute renal failure, 1988 to 2002. J Am Soc Nephrol 17: 1143–1150, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Bucaloiu ID, Kirchner HL, Norfolk ER, Hartle JE, 2nd, Perkins RM: Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int 81: 477–485, 2012 [DOI] [PubMed] [Google Scholar]
- 28.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, Ray JG, University of Toronto Acute Kidney Injury Research Group : Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Bagshaw SM, Uchino S, Kellum JA, Morimatsu H, Morgera S, Schetz M, Tan I, Bouman C, Macedo E, Gibney N, Tolwani A, Oudemans-van Straaten HM, Ronco C, Bellomo R, Beginning and Ending Supportive Therapy for the Kidney (B.E.S.T. Kidney) Investigators : Association between renal replacement therapy in critically ill patients with severe acute kidney injury and mortality. J Crit Care 28: 1011–1018, 2013 [DOI] [PubMed] [Google Scholar]
- 30.Clec’h C, Darmon M, Lautrette A, Chemouni F, Azoulay E, Schwebel C, Dumenil AS, Garrouste-Orgeas M, Goldgran-Toledano D, Cohen Y, Timsit JF: Efficacy of renal replacement therapy in critically ill patients: A propensity analysis. Crit Care 16: R236, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elseviers MM, Lins RL, Van der Niepen P, Hoste E, Malbrain ML, Damas P, Devriendt J, SHARF investigators : Renal replacement therapy is an independent risk factor for mortality in critically ill patients with acute kidney injury. Crit Care 14: R221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Piccinni P, Cruz DN, Gramaticopolo S, Garzotto F, Dal Santo M, Aneloni G, Rocco M, Alessandri E, Giunta F, Michetti V, Iannuzzi M, Belluomo Anello C, Brienza N, Carlini M, Pelaia P, Gabbanelli V, Ronco C, NEFROINT Investigators : Prospective multicenter study on epidemiology of acute kidney injury in the ICU: A critical care nephrology Italian collaborative effort (NEFROINT). Minerva Anestesiol 77: 1072–1083, 2011 [PubMed] [Google Scholar]
- 33.Joannidis M, Forni LG: Renal replacement therapy: To treat, or not to treat, that is the question... Crit Care 17: 125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith OM, Wald R, Adhikari NK, Pope K, Weir MA, Bagshaw SM, Canadian Critical Care Trials Group : Standard versus accelerated initiation of renal replacement therapy in acute kidney injury (STARRT-AKI): Study protocol for a randomized controlled trial. Trials 14: 320, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


