Abstract
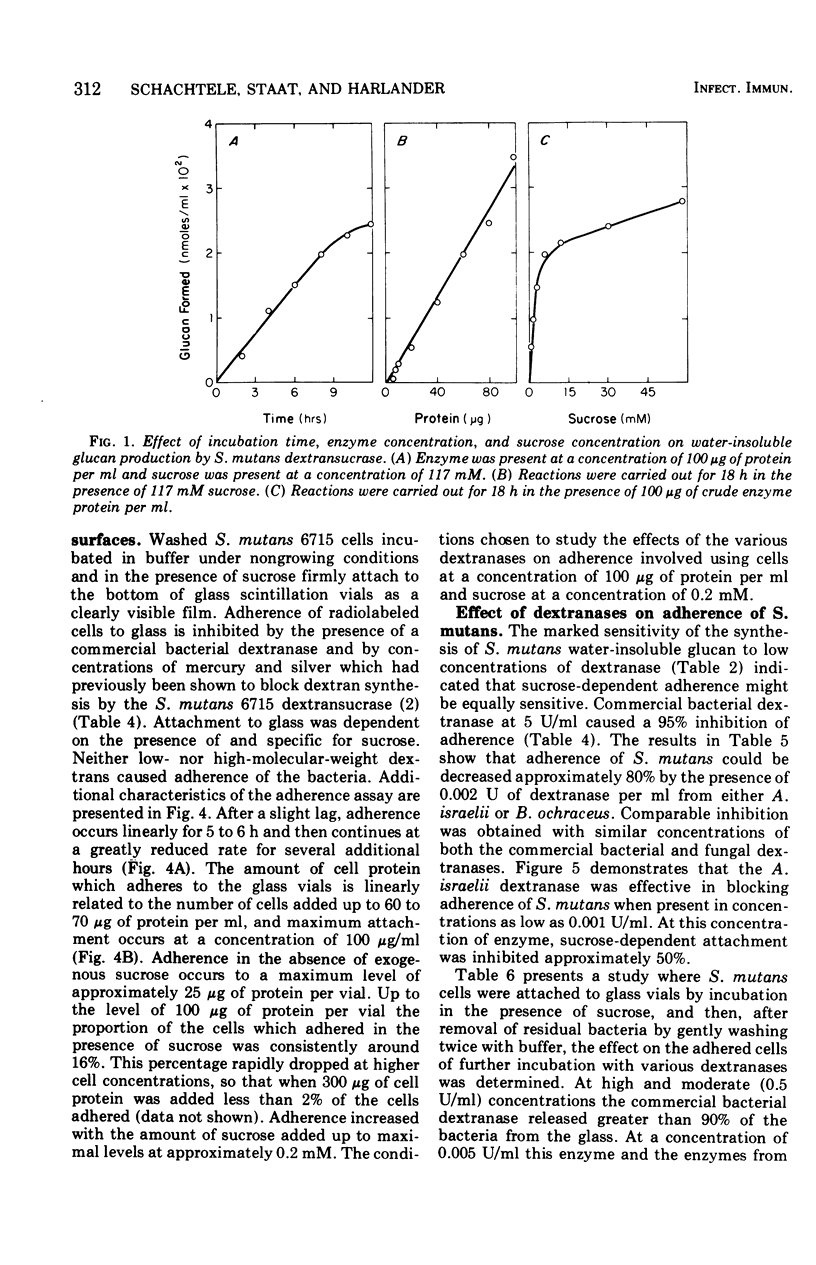
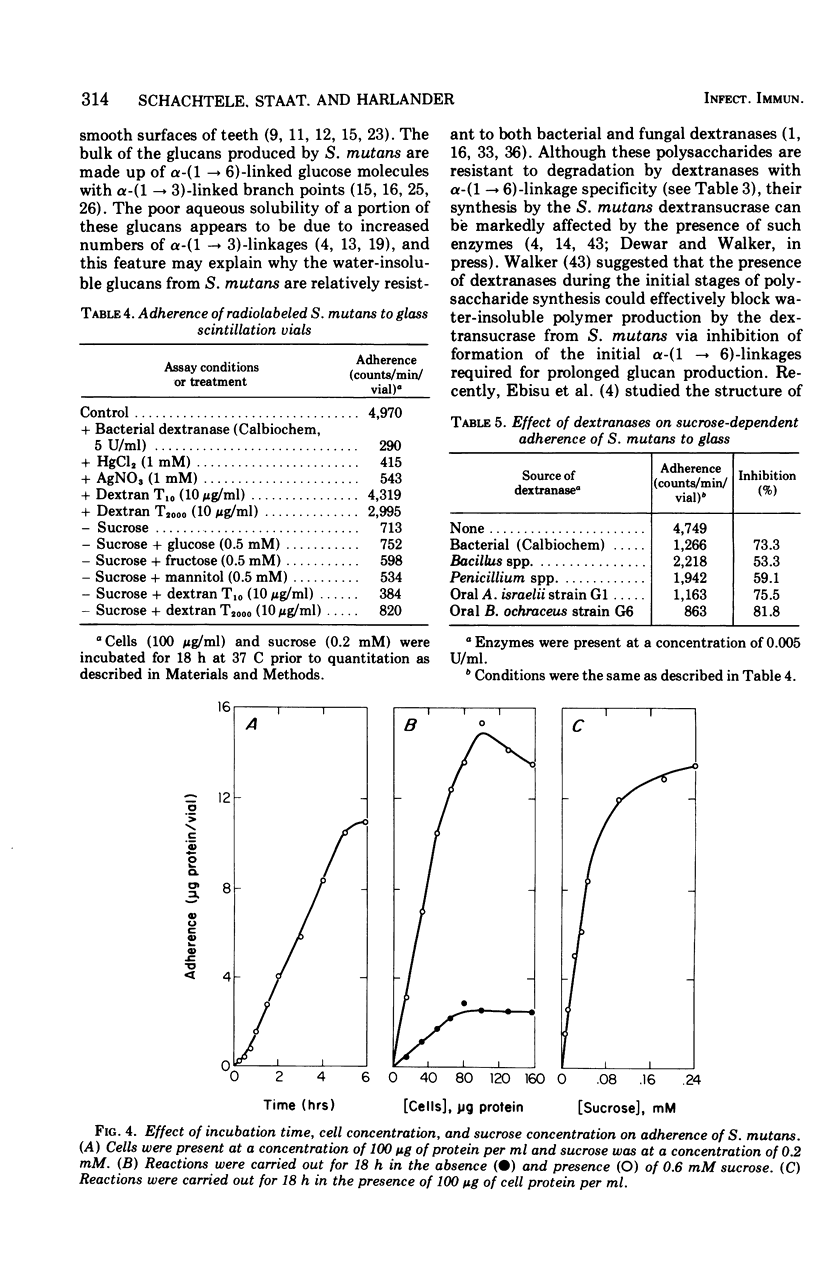
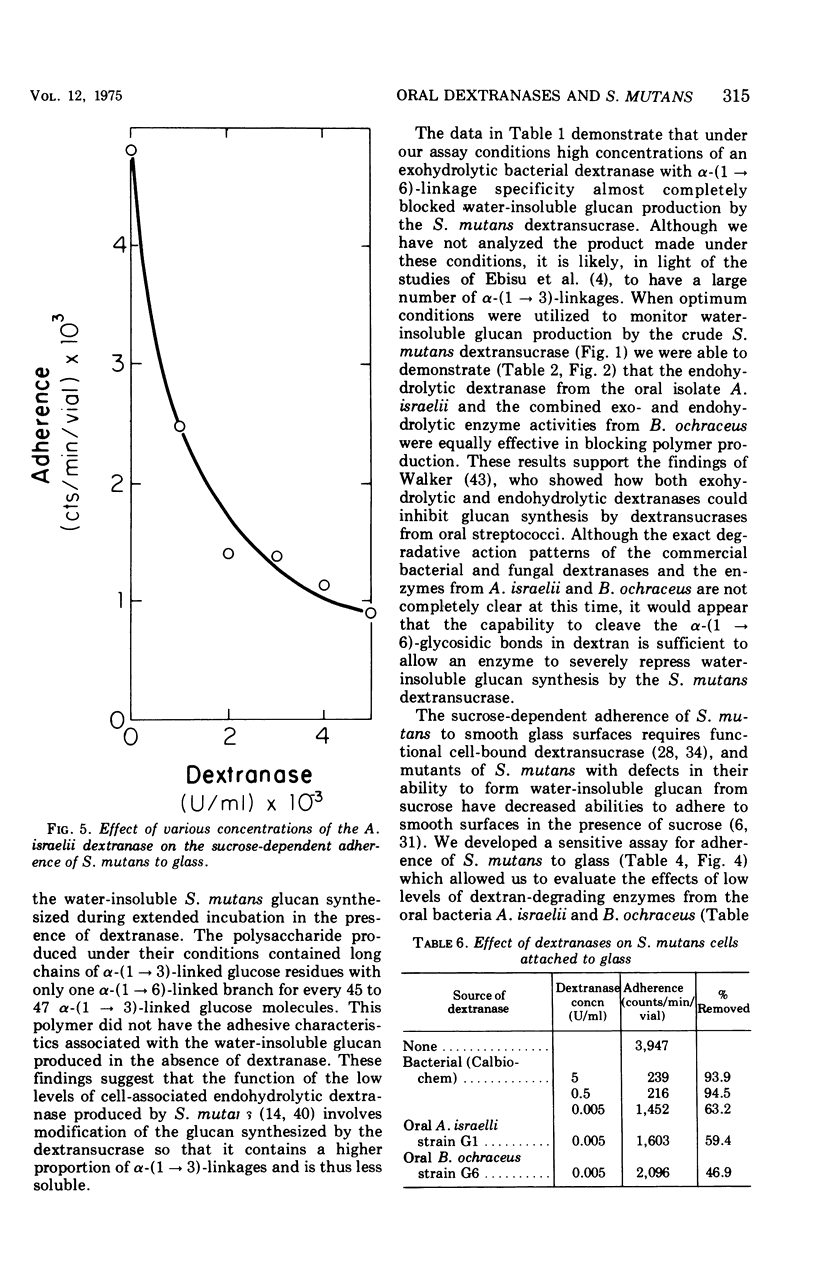
The effect of dextranases (EC 3.2.1.11) from the oral isolates Actinomyces israelii and Bacteroides ochraceus on water-insoluble glucan production by the Streptococcus mutans dextransucrase (EC 2.4.1.5) and sucrose-dependent adherence to smooth glass surfaces by S. mutans was studied. Collection on membrane filters of water-insoluble polysaccharides synthesized from radioactive sucrose was used to demonstrate the marked sensitivity of insoluble glucan formation to the presence of dextranase. Concentrations of A. israelii dextranase as low as 0.002 U/ml inhibited insoluble glucan formation by 60%. Similar results were obtained the the B. ochraceus enzyme. An assay for sucrose-stimulated adherence of S. mutans to smooth surfaces involved attachment of radioactively labeled nongrowing cells to the bottom of glass scintillation vials. This facile and sensitive assay was utilized to demonstrate that sucrose-dependent adherence was affected by low levels of dextranase from either A. israelii or B. ochraceus. Enzyme at 0.005 U/ml reduced adherence of S. mutans by 80%. Treatment of S. mutans cells previously attached to glass with low concentrations of the dextranases resulted in removal of 50% to 60% of the bacteria. The results indicate that dextranase-producing oral bacteria may affect sucrose-dependent colonization of S. mutans on the tooth surface and offer a possible explanation for both the difficulties involved in implanting this bacterium into the human mouth and the limited intraoral transmission of S. mutans from one tooth surface to another.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowen W. H. Effects of dextranase on cariogenic and non-cariogenic dextrans. Br Dent J. 1968 Apr 16;124(8):347–349. [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Stoppelaar J. D., Van Houte J., Backer DIRKS O. The effect of carbohydrate restriction on the presence of Streptococcus mutans, Streptococcus sanguis and iodophilic polysaccharide-producing bacteria in human dental plaque. Caries Res. 1970;4(2):114–123. doi: 10.1159/000259633. [DOI] [PubMed] [Google Scholar]
- Ebisu S., Misaki A., Kato K., Kotani S. The structure of water-insoluble glucans of cariogenic Streptococcus mutans, formed in the absence and presence of dextranase. Carbohydr Res. 1974 Dec;38:374–381. doi: 10.1016/s0008-6215(00)82375-7. [DOI] [PubMed] [Google Scholar]
- Fitzgerald R. J., Spinell D. M., Stoudt T. H. Enzymatic removal of artificial plaques. Arch Oral Biol. 1968 Jan;13(1):125–128. doi: 10.1016/0003-9969(68)90042-3. [DOI] [PubMed] [Google Scholar]
- Freedman M. L., Tanzer J. M. Dissociation of plaque formation from glucan-induced agglutination in mutants of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):189–196. doi: 10.1128/iai.10.1.189-196.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Schachtele C. F., Chludzinski A. M. Rapid filter paper assay for the dextransucrase activity from Streptococcus mutans. J Dent Res. 1974 Nov-Dec;53(6):1355–1360. doi: 10.1177/00220345740530061101. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Depaola P. F., Spinell D. M., Skobe Z. Interdental localization of Streptococcus mutans as related to dental caries experience. Infect Immun. 1974 Mar;9(3):481–488. doi: 10.1128/iai.9.3.481-488.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Fitzgerald R. J. Dextran-induced agglutination of Streptococcus mutans, and its potential role in the formation of microbial dental plaques. J Bacteriol. 1969 May;98(2):341–346. doi: 10.1128/jb.98.2.341-346.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Guggenheim B., Burckhardt J. J. Isolation and properties of a dextranase from streptococcus mutans OMZ 176. Helv Odontol Acta. 1974 Oct;18(2):101–113. [PubMed] [Google Scholar]
- Guggenheim B. Enzymatic hydrolysis and structure of water-insoluble glucan produced by glucosyltransferases from a strain of streptococcus mutans. Helv Odontol Acta. 1970 Nov;14(Suppl):89+–89+. [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Guggenheim B., Schroeder H. E. Biochemical and morphological aspects of extracellular polysaccharides produced by cariogenic streptococci. Helv Odontol Acta. 1967 Oct;11(2):131–152. [PubMed] [Google Scholar]
- Hoffman S., Tow H. D., Cole J. S., 3rd Scanning electron microscope studies of dextranase-treated plaque streptococci. J Dent Res. 1973 May-Jun;52(3):551–557. doi: 10.1177/00220345730520032901. [DOI] [PubMed] [Google Scholar]
- Hotz P., Guggenheim B., Schmid R. Carbohydrates in pooled dental plaque. Caries Res. 1972;6(2):103–121. doi: 10.1159/000259783. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J., Bradley E. L., Jr Changes in Streptococcus mutans and lactobacilli in plaque in relation to the initiation of dental caries in Negro children. Arch Oral Biol. 1973 Apr;18(4):555–566. doi: 10.1016/0003-9969(73)90076-9. [DOI] [PubMed] [Google Scholar]
- Ikeda T., Sandham H. J. Prevalence of Streptococcus mutans on various tooth surfaces in Negro children. Arch Oral Biol. 1971 Oct;16(10):1237–1240. doi: 10.1016/0003-9969(71)90053-7. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Englander H. R., Engler W. O., Kulczyk S. Observations on the implantation and transmission of Streptococcus mutans in humans. J Dent Res. 1972 Mar-Apr;51(2):515–518. doi: 10.1177/00220345720510024501. [DOI] [PubMed] [Google Scholar]
- Jordan H. V., Keyes P. H. In vitro methods for the study of plaque formation and carious lesions. Arch Oral Biol. 1966 Aug;11(8):793–802. doi: 10.1016/0003-9969(66)90005-7. [DOI] [PubMed] [Google Scholar]
- Krasse B., Edwardsson S., Svensson I., Trell L. Implantation of caries-inducing streptococci in the human oral cavity. Arch Oral Biol. 1967 Feb;12(2):231–236. doi: 10.1016/0003-9969(67)90042-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Long L. W., Edwards J. R. Detailed structure of a dextran from a cariogenic bacterium. Carbohydr Res. 1972 Sep;24(1):216–217. doi: 10.1016/s0008-6215(00)82285-5. [DOI] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nalbandian J., Freedman M. L., Tanzer J. M., Lovelace S. M. Ultrastructure of Mutants of Streptococcus mutans with Reference to Agglutination, Adhesion, and Extracellular Polysaccharide. Infect Immun. 1974 Nov;10(5):1170–1179. doi: 10.1128/iai.10.5.1170-1179.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbrun E. Extracellular polysaccharides synthesized by glucosyltransferases of oral streptococci. Composition and susceptibility to hydrolysis. Caries Res. 1972;6(2):132–147. doi: 10.1159/000259785. [DOI] [PubMed] [Google Scholar]
- Olson G. A., Bleiweis A. S., Small P. A., Jr Adherence inhibition of Streptococcus mutans: an assay reflecting a possible role of antibody in dental caries prophylaxis. Infect Immun. 1972 Apr;5(4):419–427. doi: 10.1128/iai.5.4.419-427.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Knudson D. J. Preferential utilization of the glucosyl moiety of sucrose by a cariogenic strain of Streptococcus mutans. Infect Immun. 1972 Apr;5(4):531–536. doi: 10.1128/iai.5.4.531-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F., Loken A. E., Schmitt M. K. Use of specifically labeled sucrose for comparison of extracellular glucan and fructan metabolism by oral streptococci. Infect Immun. 1972 Feb;5(2):263–266. doi: 10.1128/iai.5.2.263-266.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Gawronski T. H., Schachtele C. F. Detection and preliminary studies on dextranase-producing microorganisms from human dental plaque. Infect Immun. 1973 Dec;8(6):1009–1016. doi: 10.1128/iai.8.6.1009-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staat R. H., Schachtele C. F. Evaluation of dextranase production by the cariogenic bacterium Streptococcus mutans. Infect Immun. 1974 Feb;9(2):467–469. doi: 10.1128/iai.9.2.467-469.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TSUCHIYA H. M., JEANES A., BRICKER H. M., WILHAM C. A. Dextran-degrading enzymes from molds. J Bacteriol. 1952 Oct;64(4):513–519. doi: 10.1128/jb.64.4.513-519.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Houte J., Green D. B. Relationship between the concentration of bacteria in saliva and the colonization of teeth in humans. Infect Immun. 1974 Apr;9(4):624–630. doi: 10.1128/iai.9.4.624-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker G. J., Pulkownik A. Degradation of dextrans by an alpha-1,6-glucan glucohydrolase from Streptococcus mitis. Carbohydr Res. 1973 Jul;29(1):1–14. doi: 10.1016/s0008-6215(00)82066-2. [DOI] [PubMed] [Google Scholar]
- Walker G. J. Some properties of a dextranglucosidase isolated from oral streptococci and its use in studies on dextran synthesis. J Dent Res. 1972 Mar-Apr;51(2):409–414. doi: 10.1177/00220345720510022901. [DOI] [PubMed] [Google Scholar]


