Abstract
We employed ABI high-density oligonucleotide microarrays containing 31,700 sixty-mer probes (representing 27,868 annotated human genes) to determine differential gene expression in idiopathic dilated cardiomyopathy (DCM). We identified 626 up-regulated and 636 down-regulated genes in DCM compared to controls. Most significant changes occurred in the tricarboxylic acid cycle, angiogenesis, and apoptotic signaling pathways, among which 32 apoptosis- and 13 MAPK activity-related genes were altered. Inorganic cation transporter, catalytic activities, energy metabolism and electron transport-related processes were among the most critically influenced pathways. Among the up-regulated genes were HTRA1 (6.9-fold), PDCD8(AIFM1) (5.2) and PRDX2 (4.4) and the down-regulated genes were NR4A2 (4.8), MX1 (4.3), LGALS9 (4), IFNA13 (4), UNC5D (3.6) and HDAC2 (3) (pb0.05), all of which have no clearly defined cardiac-related function yet. Gene ontology and enrichment analysis also revealed significant alterations in mitochondrial oxidative phosphorylation, metabolism and Alzheimer’s disease pathways. Concordance was also confirmed for a significant number of genes and pathways in an independent validation microarray dataset. Furthermore, verification by real-time RT-PCR showed a high degree of consistency with the microarray results. Our data demonstrate an association of DCM with alterations in various cellular events and multiple yet undeciphered genes that may contribute to heart muscle disease pathways.
Keywords: Idiopathic dilated cardiomyopathy, Global gene expression, Microarray gene expression, Gene up/down-regulation, Mitochondrial function, Apoptotic signaling
Background
Idiopathic dilated cardiomyopathy (DCM) is the thinning of one or both ventricle(s) from an unknown cause, with the resultant impaired cardiac contractility often leading to overt congestive heart failure or cardiac arrhythmias. While no clear cause is evident in the majority of cases, DCM is probably an end product of myocardial damage triggered by a variety of toxic, metabolic or infectious agents [1]. Besides, some forms of familial DCM, in particular, also appear to be triggered by mutations in genes encoding cytoskeletal, contractile or other myocardial proteins [2–5]. The ensuing progression of heart failure is associated with left ventricular remodeling, which manifests as a gradual increase in left ventricular end-diastolic and end-systolic volumes, wall thinning and alteration in the shape of the chambers to a more spherical and less elongated form [6]. Several molecular and cellular alterations have been identified that contribute to cardiac muscle contractility and relaxation abnormalities in this process.
These include, among others, the cyclic AMP (cAMP)-dependent pathways, calcium (Ca2+) homeostasis, neurohumoral activation and myofibrillar function [7]. Essentially, cAMP-dependent pathways are desensitized due to alterations in β-adrenoceptors (β-AR), β-AR kinases and guanine nucleotide binding proteins (G-proteins) [8]. Calcium ion (Ca2+) homeostasis is impaired, characterized by a reduced sarcoplasmic reticulum Ca2+ reuptake rate, elevated Ca2+ release channel threshold and an increase in sodium ion (Na+)/Ca2+ exchanger expression [9,10]. Myofibrillar function may also be influenced by a decrease in Mg2+-ATPase activity and in troponin I phosphorylation, as well as changes in troponin T isoform expression [9,11–13]. Accumulating data also suggests a link between alterations and/or deficiencies in cytoskeletal proteins and the progression of cardiomyopathy to heart failure. Moreover, the remodeling process appears to be regulated by a number of pathways including cytokines and growth factors [14].
Despite great efforts to understand the mechanism involved in the progress of DCM to overt heart failure, the underlying triggering factors for the disease remain to be elucidated. Accumulating evidence from gene profiling and other studies implicates diverse pathways, including among others, the vascular renin–angiotensin system [15], Gi-coupled receptors [16], TGFβ-activin-A/Smad signaling pathway [17], SH2-containing cytoplasmic tyrosine phosphatase (Shp) [18] and apoptotic signaling [15,17–20], to name a few. While classical opinion might argue that several of these alterations occur independently of the underlying etiology of the disease, it has also become apparent that the greater part of the familiar myocardial changes is probably triggered by chronic neurohumoral activation and abnormal mechanical load [21], which greatly promote the progression of heart failure as part of a vicious circle. However, the molecular basis for this link remains unclear. Several studies have been performed using different microarray-based and other techniques to evaluate alterations in gene expression in DCM [22–26], and recently intraplatform consistency in terms of sample sources as well as a high level of interplatform concordance with respect to genes identified as differentially expressed have been demonstrated [27,28]. Hence, deciphering the pattern of alterations in gene expression in DCM using the microarray system provides a valuable basis for elucidating some of the mechanisms involved in this vicious circle. In particular, the ABI high-density oligonucleotide microarray platform allows analysis of a greater number of genes than most platforms, as it includes annotated genes from both public and Celera databases. ABI platform is also a chemiluminescent based array by which signal is enhanced to fentomol sensitivity which may help to detect rare mRNAs. It has also been shown that ABI 1700 platform has substantially higher sensitivity, detecting four times as many changes in an identical experimental design and results are well correlated (R2>0.7) with qRT-PCR compared to other microarray platforms [29– 31]. In this study, we therefore sought to establish left ventricular differential gene expression in DCM employing the ABI 1700 platform, in order to be able to detect a relatively rare class of mRNAs and obtain further insight into the mechanism of heart muscle disease pathways.
Materials and methods
Study patients
For the gene expression and subsequent experiments, 300 mg of tissue were harvested from left ventricles of five DCM hearts excised from patients (3 male and 2 female; 42.3±6.3 years) with end-stage heart failure undergoing cardiac transplantation at our institution. All samples were procured from identical myocardial loci to ensure optimal uniformity. The patients had New York Heart Association class 3–4 symptoms, and received anti-heart failure treatment and/or inotropic support. None of the patients was on a left ventricle assist device or any other mechanical support. Four healthy hearts procured from organ donors (three male and 1 female; 34.1±4.7 years) who died of traffic accidents with no history of cardiac disease served as controls. The mean age of the controls was not significantly different from that of the patients (p=0.37). These hearts had originally been intended for transplantation, but failed to get suitable matching recipients. At the time of harvesting, whole hearts were explanted after preservation in cold cardioplegia, followed by immediate dissection into small portions, snap-frozen in liquid nitrogen, and maintained at −80 °C until use. Minimum time possible (usually<3 h) was allowed between harvesting donor hearts and freezing the samples in liquid nitrogen. Fully informed consent was obtained from all patients or family members before participating in the study. This study was performed in accordance with the Declaration of Helsinki as adopted and promulgated by the US National Institutes of Health as well as rules and regulations laid down by our Institutional Ethics Committee.
Expression array analysis
Total RNA was isolated from similar left ventricular biopsies using Applied Biosystems (ABI) Totally RNA Isolation Kit (ABI-Ambion, Foster City, CA, USA), quantified with the NanoDrop® ND-1000 Spectrophotometer (Nanodrop Inc., Wilmington, DE, USA) and further analyzed by RNA 6000 Nano Assay using 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA). Digoxigenin-UTP labeled cRNA was generated and amplified from 1 µg of total RNA using Applied Biosystems (ABI) Chemiluminescent RT-IVT Labeling Kit v 1.0. The array hybridization was performed for 16 h at 55 °C and detection, image acquisition and analysis were performed using ABI Chemilu-minescence Detection Kit on ABI 1700 Chemiluminescent Microarray Analyzer (ABI, Foster City, CA, USA).
Real-time RT-PCR
In order to validate our microarray results, confirmatory quantitative real-time RT-PCR (qRT-PCR) was performed using the ABI 7500 Sequence Detection System (ABI, Foster City, CA, USA). For this purpose, 50 ng total RNA procured from the same microarray study samples were transcribed into cDNA using Sensiscript Kit (QIAGEN Inc., Valencia, CA, USA) under the following conditions: 25 °C for 10 min,42 °C for 2 h, and 70 °C for 15 min in a total volume of 20 µl. Six differentially expressed genes (CRYM, NR4A2, PDK4, RASD1, TNNI3K, and AIFM1) were randomly selected and primers designed using Primer3 software. After primer optimization, the PCR assays were performed in 6 µl of the cDNA using the QIAGEN Quantitet SyBR Green Kit, employing GAPDH as the endogenous control gene. All reactions were conducted in triplicates and the data was analyzed using the delta delta CT method [32].
Data analysis
Hybridization images were analyzed using the ABI 1700 Chemiluminescent Microarray Analyzer software v 1.1, with the detection threshold set at signal to noise (S/N) ratio>3 (a value that indicates 99.9% confidence level for the signal being above the background level, “present” probes) and quality flag <5000. The open source Bioconductor packages, ab1700, limma, multtest and affy (Fred Hutchinson Cancer Research Center, Seattle, WA, USA) and Partek Genomics Suite (Partek Inc.) were employed to normalize the data via quantile normalization and to determine significant differences in gene expression levels between DCM patients and normal controls [33]. When comparing DCM patients and normal controls to identify the differentially expressed genes, we used a combination of three criteria. We considered genes that are “present” in at least half of the samples in either group. Given the nature of the data, and statistical tests selected, adjusting for multiple testing errors is critical. We used Benjamini-Hochberg [34] step-up procedure to control the false discovery rate (FDR). As an alternative approach, we employed the two-class SAM procedure to estimate the FDR [35]. Significantly modulated genes were defined as those with absolute fold change (FC)>1.8 and controlling FDR at 5%. A validation data set was generated from an independent study by Barth et al. [22] using Affymetrix HG-U133A array, and the raw data was analyzed by using dChip [36] and open source R/Bioconductor packages. The dChip outlier detection algorithm was used to identify outlier arrays (all arrays passed), and probes “present” in at least 50% of the samples in either group were filtered. The data was normalized by the GC Robust Multi-array Average (GC-RMA) algorithm [37,38]. Unpaired t-tests were performed to determine significant differences in gene expression levels between patients and normal controls, Multi Experiment Viewer (MeV4.0) [39] was used to perform two-dimensional hierarchical clustering employing Euclidean distance as well as Pearson correlation with average linkage clustering. Functional annotation and biological term enrichment analysis were performed using DAVID Bioinformatics Resources [40], Expression Analysis Systematic Explorer (EASE) [41], Protein ANalysis Through Evolutionary Relationships (PANTHER™) classification systems [42], and Ingenuity Pathways Analysis (IPA) 6.3 (Ingenuity Systems, Mountain View, CA). Gene Set Enrichment Analysis/MSigDB was used to determine whether an a priori defined set of genes showed statistically significant, concordant differences between the 2 groups (DCM vs normal). Statistical analyses were performed with the MATLAB software packages (Mathworks, Natick, MA, USA), R and Bioconductor and PARTEK Genomics Suite (Partek Inc., St. Lois, MO, USA).
Results
Global gene expression analysis
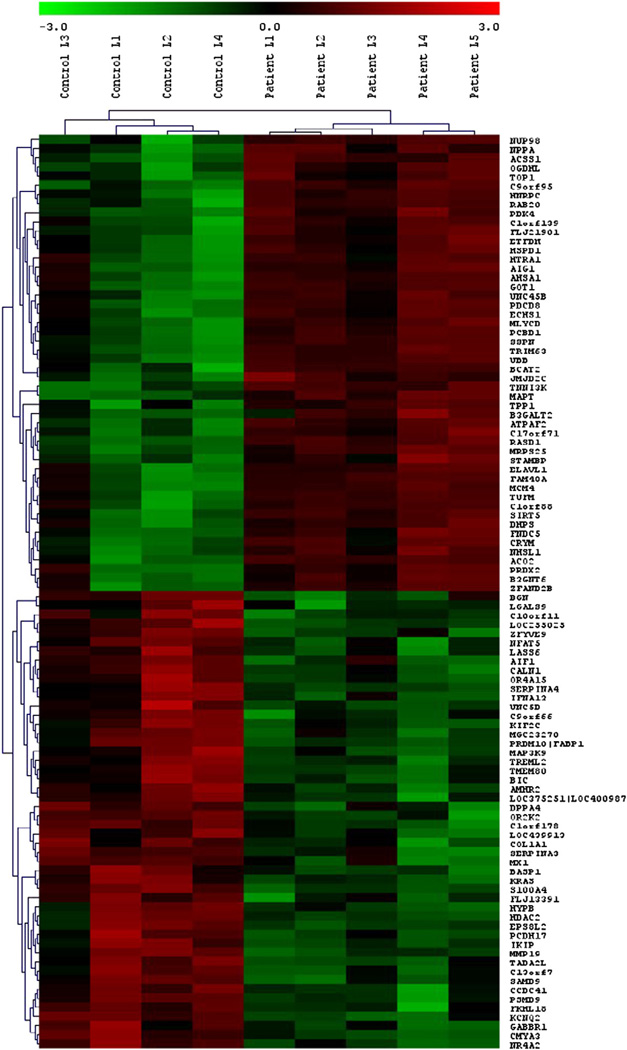
The mRNA expression was analyzed using the ABI human whole genome array version 2. The ABI Human Genome Survey Microarray contains 31,700 sixty-mer oligonucleotide probes representing 27,868 individual human genes. Approximately 19,000 of these probes were detectable based on the above criteria. We have found 1309 probes, of which 655 probes (626 genes) were up-regulated and 654 probes (636 genes) were down-regulated, whose expression varied at least 1.8-fold and were statistically significant at a false discovery rate of <5% between DCM patients and normal controls (Supplementary Table 1). The hierarchical clustering in both dimensions (samples and genes) clearly distinguished individuals as either DCM or controls (Fig. 1). The 50 most significantly altered genes (>3-fold change) are listed in Tables 1A and 1B.
Fig. 1.
Heatmap of genes that were significantly modulated due to DCM. Hierarchical clustering clearly separated individuals as either DCM patients or normal controls. Highly expressed genes are indicated in red, intermediate in black, and weakly expressed in green. Only 100 of the most significantly altered genes are shown for readability.
Table 1.
|
A Top 50 genes that were most significantly increased in expression in DCM. | ||||
|---|---|---|---|---|
| Probe ID | UniGene ID | Symbol | Gene_name | FCa |
| 184159 | Hs.75640 | NPPA | natriuretic peptide precursor A | 24.27 |
| 101060 | Hs.8364 | PDK4 | pyruvate dehydrogenase kinase, isozyme 4 | 16.44 |
| 127385 | Hs.461571 | MLYCD | malonyl-CoA decarboxylase | 9.23 |
| 212992 | Hs.356190 | UBB | ubiquitin B | 7. 21 |
| 115431 | Hs.25829 | RASD1 | RAS, dexamethasone-induced 1 | 7.18 |
| 199204 | Hs.567501 | AIG1 | androgen-induced 1 | 7.1 2 |
| 119696 | Hs.3192 | PCBD1 | pterin-4 alpha-carbinolamine dehydratase/dimerization cofactor of hepatocyte nuclear factor 1 alpha (TCF1) | 7.03 |
| 168813 | Hs.17860 | OGDHL | oxoglutarate dehydrogenase-like | 6.94 |
| 11174 8 | Hs.501280 | HTRA1 | HtrA serine peptidase 1 | 6.87 |
| 117167 | Hs.204041 | AHSA1 | AHA1, activator of heat shock 90 kDa protein ATPase homolog 1 (yeast) | 6.73 |
| 118421 | Hs.480085 | TNNI3K | TNNI3 interacting kinase | 6.68 |
| 146066 | Hs.494186 | C9orf95 | chromosome 9 open reading frame 95 | 5.85 |
| 211439 | Hs.500756 | GOT1 | glutamic-oxaloacetic transaminase 1, soluble (aspartate aminotransferase 1) | 5.83 |
| 162178 | Hs.512670 | BCAT2 | branched chain aminotransferase 2, mitochondrial | 5.71 |
| 209321 | Hs.518834 | B3GALT2 | UDP-Gal:betaGlcNAc beta 1,3-galactosyltransferase, polypeptide 2 | 5.54 |
| 122333 | Hs.529276 | FLJ21901(FASTKD1) | 5.46 | |
| 112942 | Hs.479491 | C1orf139(GPR177) | chromosome 1 open reading frame 139 | 5.46 |
| 161710 | Hs.924 | CRYM | crystallin, mu | 5.31 |
| 205457 | Hs.424932 | AIFM1 | programmed cell death 8 (apoptosis-inducing factor) | 5.25 |
| 146299 | Hs.567431 | SIRT5 | sirtuin (silent mating type information regulation 2 homolog) 5 (S. cerevisiae) | 5.24 |
| 214987 | Hs.12084 | TUFM | Tu translation elongation factor, mitochondrial | 5.06 |
| 214864 | Hs.184492 | ELAVL1 | ELAV (embryonic lethal, abnormal vision, Drosophila)-like 1 (Hu antigen R) | 4.98 |
| 200831 | Hs.499916 | FAM48A | family with sequence similarity 48, member A | 4.93 |
| 158867 | Hs.523454 | TPP1 | tripeptidyl peptidase I | 4.92 |
| 155357 | Hs.183428 | SSPN | sarcospan (Kras oncogene-associated gene) | 4.91 |
| 202365 | Hs.524234 | FNDC5 | fibronectin type III domain containing 5 | 4.87 |
| 115589 | Hs.460184 | MCM4 | MCM4 minichromosome maintenance deficient 4 (S. cerevisiae) | 4.79 |
| 210061 | Hs.486596 | NHSL1 | NHS-like 1 | 4.79 |
| 195766 | Hs.155729 | ETFDH | electron-transferring-flavoprotein dehydrogenase | 4.77 |
| 182995 | Hs.379636 | UNC45B | unc-45 homolog B (C. elegans) | 4.76 |
| 124513 | Hs.172510 | C1orf88 | chromosome 1 open reading frame 88 | 4.74 |
| 217302 | Hs.524750 | NUP98 | nucleoporin 98 kDa | 4.56 |
| 202618 | Hs.8526 | B3GNT6 | UDP-GlcNAc:betaGal beta-1,3-N-acetylglucosaminyltransferase 6 | 4.51 |
| 158513 | Hs.432121 | PRDX2 | peroxiredoxin 2 | 4.43 |
| 102257 | Hs.508848 | HNRPC(HNRNPC) | heterogeneous nuclear ribonucleoprotein C (C1/C2) | 4.21 |
| 190596 | Hs.529353 | ACSS1 | acyl-CoA synthetase short-chain family member 1 | 4.17 |
| 213069 | Hs.279709 | TRIM63 | tripartite motif-containing 63 | 4.16 |
| 107417 | Hs.13434 | ATPAF2 | ATP synthase mitochondrial F1 complex assembly factor 2 | 4.05 |
| 115807 | Hs.76394 | ECHS1 | enoyl Coenzyme A hydratase, short chain, 1, mitochondrial | 4.05 |
| 100079 | Hs.113684|Hs.567290 | HSPD1 | heat shock 60 kDa protein 1 (chaperonin) | 4.04 |
| 218816 | Hs.101174|Hs.569810 | MAPT | microtubule-associated protein tau | 4.00 |
| 203523 | Hs.79064 | DHPS | deoxyhypusine synthase | 3.98 |
| 134459 | Hs.508720 | RAB20 | RAB20, member RAS oncogene family | 3.95 |
| 179728 | Hs.472737 | TOP1 | topoisomerase (DNA) I | 3.88 |
| 101676 | Hs.7296 | C17orf71 | chromosome 17 open reading frame 71 | 3.88 |
| 169529 | Hs.555973 | MRPS25 | mitochondrial ribosomal protein S25 | 3.79 |
| 169750 | Hs.183070 | STAMBP | STAM binding protein | 3.73 |
| 144908 | Hs.474982 | ACO2 | aconitase 2, mitochondrial | 3.71 |
| 195306 | Hs.157106 | JMJD2C | jumonji domain containing 2C | 3.65 |
| 162286 | Hs.534540 | ZFAND2B | zinc finger, AN1-type domain 2B | 3.65 |
|
B Top 50 gene that were most significantly decreased in expression in DCM. | ||||
|---|---|---|---|---|
| Probe ID | UniGene ID | Gene symbol | Gene_name | FCa |
| 123450 | Hs.165258 | NR4A2 | nuclear receptor subfamily 4, group A, member 2 | −4.83 |
| 221732 | Hs.161851 | KCNQ2 | potassium voltage-gated channel, KQT-like subfamily, member 2 | −4.78 |
| 179577 | Hs.535591| | LOC375251 | −4.72 | |
| 652781 | Hs.388313 | MIRHG2 | −4.67 | |
| 155850 | Hs.81256 | S100A4 | S100 calcium binding protein A4 (calcium protein, calvasculin, metastasin, murine placental homolog) | −4.39 |
| 215752 | Hs.65641 | SAMD9 | sterile alpha motif domain containing 9 | −4.29 |
| 209986 | Hs.517307 | MX1 | myxovirus (influenza virus) resistance 1, interferon-inducible protein p78 (mouse) | −4.25 |
| 162446 | Hs.275086|Hs.380135 | PRDM10|FABP1 | PR domain containing 10|fatty acid binding protein 1, liver | −4.23 |
| 217544 | Hs.352220 | MGC23270 | −4.23 | |
| 207085 | Hs.445496 | MAP3K9 | mitogen-activated protein kinase kinase kinase 9 | −4.11 |
| 171927 | Hs.154057 | MMP19 | matrix metallopeptidase 19 | −4.11 |
| 159046 | Hs.81337 | LGALS9 | lectin, galactoside-binding, soluble, 9 (galectin 9) | −4.10 |
| 200670 | Hs.500066 | TADA2L | transcriptional adaptor 2 (ADA2 homolog, yeast)-like | −4.03 |
| 132165 | Hs.533471 | IFNA13 | interferon, alpha 13 | −4.02 |
| 224571 | Hs.516971 | FKHL18(FOXS1) | forkhead-like 18 (Drosophila) | −4.02 |
| 145309 | Hs.532345 | ZFYVE9 | zinc finger, FYVE domain containing 9 | −4.02 |
| 206143 | Hs.437877 | AMHR2 | anti-Mullerian hormone receptor, type II | −3.96 |
| 223807 | Hs.252543 | IKIP | −3.90 | |
| 691815 | Hs.196484 | C1orf178 | chromosome 1 open reading frame 178 | −3.86 |
| 223796 | Hs.190877 | C9orf66 | chromosome 9 open reading frame 66 | −3.80 |
| 102968 | KRAS | v-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog | −3.69 | |
| 158586 | Hs.317659 | DPPA4 | developmental pluripotency associated 4 | −3.66 |
| 141970 | Hs.238889 | UNC5D | unc-5 homolog D (C. elegans) | −3.64 |
| 126259 | Hs.567576 | RNF219 | chromosome 13 open reading frame 7 | −3.60 |
| 114684 | Hs.506829 | LASS6 | LAG1 longevity assurance homolog 6 (S. cerevisiae) | −3.58 |
| 107450 | Hs.448664 | TMEM80 | transmembrane protein 80 | −3.57 |
| 129347 | Hs.381312 | OR2K2 | olfactory receptor, family 2, subfamily K, member 2 | −3.51 |
| 177348 | Hs.159628 | SERPINA4 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 4 | −3.45 |
| 141595 | Hs.164797 | TREML2 | triggering receptor expressed on myeloid cells-like 2 | −3.39 |
| 127023 | Hs.554529 | OR4A15 | olfactory receptor, family 4, subfamily A, member 15 | −3.39 |
| 145949 | Hs.371987 | NFAT5 | nuclear factor of activated T-cells 5, tonicity-responsive | −3.37 |
| 171525 | Hs.821 | BGN | biglycan | −3.32 |
| 169674 | Hs.131151 | PSMD9 | proteasome (prosome, macropain) 26S subunit, non-ATPase, 9 | −3.32 |
| 102572 | Hs.279209 | CCDC41 | coiled-coil domain containing 41 | −3.28 |
| 224005 | Hs.73680|Hs.470488 | CMYA3(XIRP2) | cardiomyopathy associated 3 | −3.27 |
| 111499 | Hs.434720|Hs.55016 | EPS8L2 | EPS8-like 2 | −3.24 |
| 198318 | Hs.201641 | BASP1 | brain abundant, membrane attached signal protein 1 | −3.21 |
| 110723 | Hs.549368 | LOC439913 | −3.19 | |
| 134951 | Hs.106511 | PCDH17 | protocadherin 17 | −3.18 |
| 212531 | Hs.69360 | KIF2C | kinesin family member 2C | −3.10 |
| 194003 | Hs.172928 | COL1A1 | collagen, type I, alpha 1 | −3.08 |
| 171591 | Hs.552755 | LOC255025 | −3.08 | |
| 165806 | Hs.534293 | SERPINA3 | serpin peptidase inhibitor, clade A (alpha-1 antiproteinase, antitrypsin), member 3 | −3.05 |
| 195643 | Hs.167017 | GABBR1 | gamma-aminobutyric acid (GABA) B receptor, 1 | −3.02 |
| 167343 | Hs.576884|Hs.118161 | C10orf11 | chromosome 10 open reading frame 11 | −3.01 |
| 180037 | Hs.76364 | AIF1 | allograft inflammatory factor 1 | −3.00 |
| 143136 | Hs.333274 | CALN1 | calneuron 1 | −2.99 |
| 133640 | Hs.302346 | FLJ13391(FAM176A) | −2.98 | |
| 230512 | HDAC2 | histone deacetylase 2 | −2.97 | |
| 135102 | Hs.517941 | HYPB(SETD2) | −2.97 | |
FC was calculated between the mean values of control and DCM.
FC was calculated between the mean values of control and DCM.
Gene ontology analysis
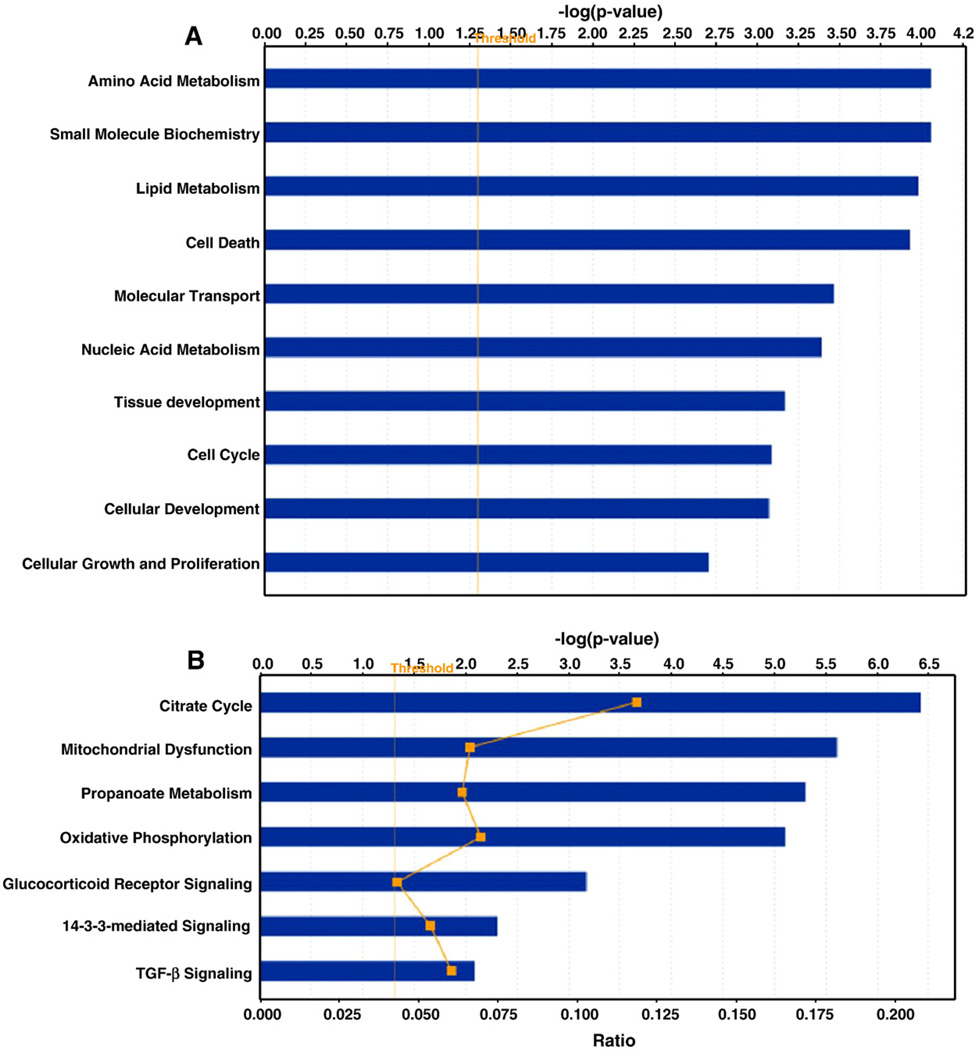
The gene ontology and functional analysis of DCM specific up/ down-regulated genes were performed using the Ingenuity knowledge base (Fig. 2A). The biological functions assigned to the data set are ranked by significance (-log P value). As demonstrated in Fig. 2A, highly significant functions include lipid metabolism, cell death, amino acid metabolism, small molecule biochemistry, molecular transport, cellular growth and proliferation, nucleic acid metabolism, tissue development, and cellular development. We further identified altered biological processes, molecular functions and pathways among the differentially expressed genes using PANTHER™ classification systems [42]. The numbers of genes identified in each of the three categories were calculated and compared using the binomial test to determine if there were more genes than expected in the differentially regulated list [43]. Based on this analysis, genes related to electron transport (p=1.9×10−16), oxidative phosphorylation (p=4.7×10−16), protein metabolism and modification (p=3.4×10−9), carbohydrate metabolism (p=1.3×10−8), fatty acid metabolism (p=1.2×10−7), cell proliferation and differentiation (p=1.7×10−3) belonged to the most significantly enriched among the up-regulated genes. Genes related to signal transduction (p=6.1×10−7), cell communication (p=5.6×10−5), mesoderm development (p=4.2×10−3), mRNA transcription (p=1. 4×10−4), cell surface receptor-mediated signaling (p=1.5×10−3), developmental processes (p=1.3×10−2) and cell adhesion (p=8.7×10−3) were the most significantly enriched among the down-regulated genes (data not shown). Biological themes associated with the differentially expressed genes were also identified by using three gene ontology categories of biological processes, molecular functions and cellular components. The most significantly overrepresented GO categories (EASE score<0.01) among the up-regulated genes were related to catalytic activity, electron transport, mitochondrial metabolism and energy pathways, whereas among the down-regulated genes were those associated with cell adhesion, signal transduction, binding and transcription, which was consistent with the categories identified by PANTHER.
Fig. 2.
Functional (A) and canonical pathway (B) analysis of DCM specific genes. X-axis indicates the significance (-log P value) of the functional/pathway association that is dependent on the number of genes in a class as well as biologic relevance. The threshold line represents a P value of 0.05.
We also investigated biological pathways significantly represented among the differentially expressed genes. The most significantly overrepresented pathways included the TCA cycle, asparagine and aspartate biosyntheses, apoptosis signaling, Parkinson’s disease, cell cycle, and salvage pyrimidine ribonucleotide pathways enriched among the up-regulated genes, and TGF-β signaling, p53, apoptosis signaling, Ras, integrin signaling and Alzheimer's disease-presenilin pathways among the down-regulated genes (Table 2). The IPA analysis of DCM specific genes (up/down-regulated) also revealed that citrate cycle, mitochondrial dysfunction, oxidative phosphorylation and TGF-β signaling are among the most significantly altered canonical pathways (Fig. 2B). The Gene Set Enrichment Analysis/MSigDB further complements the ontology analysis with significant enrichment of gene sets or pathways related to cytoplasm, mitochondrial genes, Alzheimer disease, metabolism and oxidative phosphorylation.
Table 2.
Overrepresented pathways among up/down-regulated genes.
| Pathways | List hitsa | Expected valueb | P valuec |
|---|---|---|---|
| Overrepresented pathways among up-regulated genes | |||
| TCA cycle | 7 | 0.44 | 4.40E–07 |
| Asparagine and aspartate biosynthesis | 4 | 0.2 | 5.32E–05 |
| Fructose galactose metabolism | 4 | 0.32 | 3.37E–04 |
| ATP synthesis | 4 | 0.37 | 5.74E–04 |
| Methylmalonyl pathway | 2 | 0.1 | 4.54E–03 |
| Glycolysis | 4 | 0.74 | 6.88E–03 |
| Vitamin B6 metabolism | 2 | 0.12 | 6.98E–03 |
| Methylcitrate cycle | 2 | 0.12 | 6.98E–03 |
| Acetate utilization | 2 | 0.22 | 2.12E–02 |
| Threonine biosynthesis | 1 | 0.05 | 4.81E–02 |
| Cell cycle | 3 | 0.81 | 4.91E–02 |
| Salvage pyrimidine ribonucleotides | 2 | 0.42 | 6.66E–02 |
| Parkinson disease | 6 | 2.86 | 6.97E–02 |
| Overrepresented pathways among down-regulated genes | |||
| TGF-beta signaling pathway | 11 | 3.67 | 1.42E–03 |
| p53 pathway feedback loops 2 | 5 | 1.53 | 1.97E–02 |
| Ornithine degradation | 1 | 0.02 | 2.43E–02 |
| EGF receptor signaling pathway | 8 | 3.57 | 2.92E–02 |
| Interleukin signaling pathway | 13 | 7.49 | 4.12E–02 |
| Angiogenesis | 10 | 5.49 | 5.25E–02 |
| Integrin signaling pathway | 10 | 5.54 | 5.51E–02 |
| Alzheimer disease-presenilin pathway | 7 | 3.4 | 5.72E–02 |
The number of genes in respective PANTHER classification categories.
The expected value is the number of genes expected in the differentially expressed genes for this PANTHER category, based on the reference list.
P values for each category were calculated from the binomial test statistic.
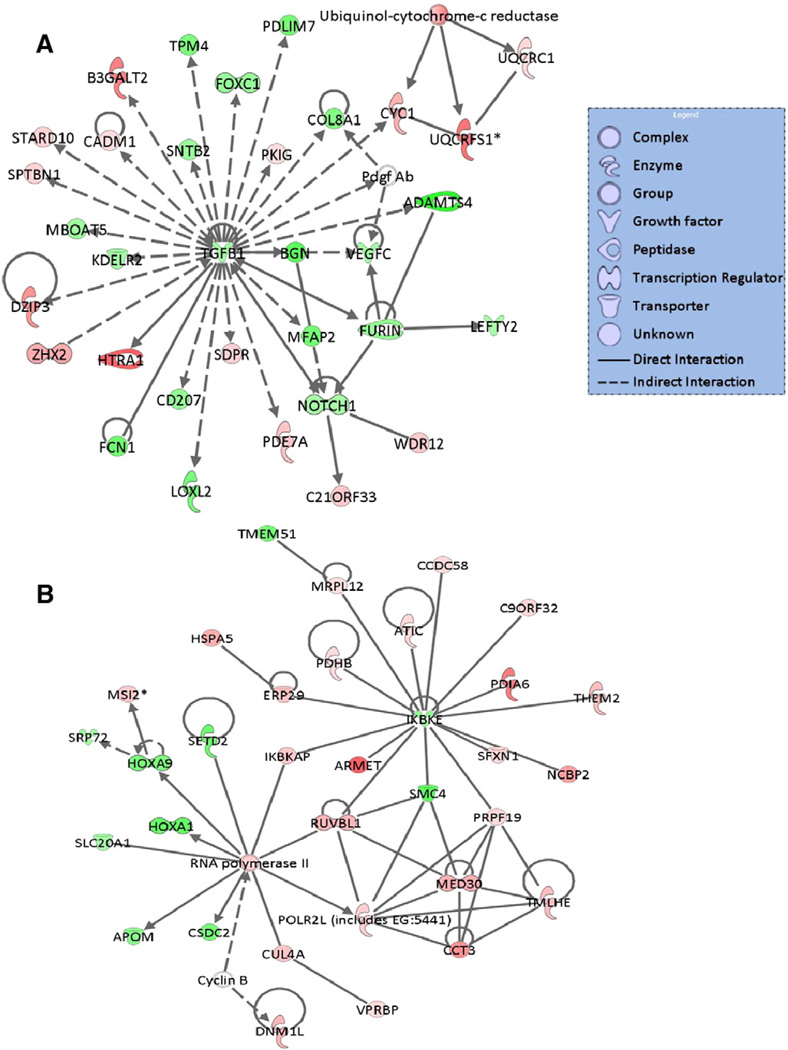
Gene interaction network analysis
To obtain a deeper insight into the interactions of the dysregulated genes among the various pathways, the DCM specific genes were mapped to the gene networks using the Ingenuity knowledge base. These genes were mapped primarily to top networks (Fig. 3A and B) related to, among others, cell death, cellular growth and proliferation, cardiovascular and nervous system development and function, post-translational modification, protein folding, cell cycle, tissue development, lipid metabolism and small molecule biochemistry.
Fig. 3.
Functional network analysis of DCM specific genes. Top two scoring gene interaction networks with high relevancy scores (significance: score=48) for the DCM specific genes. A score of three indicates that there is 1/1000 (score=−log (P value)) chance that the focus genes are assigned to a network randomly. Green indicates down-regulated, red, up-regulated. The color intensity is correlated with fold change. Straight lines are for direct gene to gene interactions, dashed lines are for indirect ones.
Among the differentially expressed genes, 32 were apoptosis-related and 13 were associated with mitogen-activated protein kinase (MAPK) activities. Also noteworthy was the large number of up-regulated genes pertaining to oxidoreductase activity, synthases, ribosomal proteins, nucleic acid binding, mitochondrial function and metabolism on the one hand, and the down-regulated genes involved in homeobox transcription, signal transduction, receptor signaling, growth, extracellular matrix or DNA-binding on the other. Furthermore, the most highly (>4-fold change) elevated genes included pyruvate dehydrogenase kinase, isozyme 4 (PDK4), malonyl-CoA decarboxylase (MLYCD), the programmed cell death (AIFM1 also known as apoptosis-inducing factor or mitochondrial programmed cell-death protein 8), ubiquitin B (UBB), human troponin I subtype 3 (TNNI3) interacting kinase (TNNI3K), mitochondrial branched chain aminotransferase 2 (BCAT2), crystalline-mu (CRYM), and peroxiredoxin 2 (PRDX2), while the most significantly down-regulated genes included the pyruvate dehydrogenase kinase, isoenzyme 4 (NR4A2), dexamethasome-induced RAS encoding subtype 1 gene (RASD1), B-cell receptor-inducible gene BIC(MIRHG2), myxovirus 1 (MX1), interferon A13 (IFNA13), unc-5 homolog D (UNC5D), histone deacetylase 2 (HDAC2) and potassium voltage-gated channel, member 2 (KCNQ2) genes, just to name a few.
Independent validation set analysis
As a validation of our results, we analyzed an independently performed microarray dataset for DCM from Barth et al. [22] using Affymetrix short oligo array using the analysis procedure defined in the “Materials and methods” section on the new dataset. The data is composed of 12 samples for non-failing heart (n=5) and DCM heart patients (n = 7). We found 1223 genes differentially expressed between DCM and normal controls. The validation dataset showed a significant number of genes (p<10−5) in common with our analysis results. Significance of overlaps was calculated using hypergeometric distributional assumption [44] and P values were adjusted using Bonferroni correction for multiple comparisons [45]. In addition, unsupervised clustering was performed using our signature gene list to cluster Barth et al. [22] data. We found that using our signature gene list was sufficient to separate individuals in Barth et al.'s study as either DCM patients or normal controls (Supplemental Fig. 1).
Furthermore, almost 70% of all GO categories from our analysis were retained in the validation dataset (p~0), and a significant number of overrepresented GO categories was in common. Of note, metabolism, catalytic activity, ribosome, energy derivation by oxidation, protein metabolism, muscle development, and biosynthesis come up as significantly enriched GO categories in both the validation and our analyses. The gene set enrichment analysis (GSEA) showed highly significant enrichment of sets related to cytoplasm, neuronal stem cell, Alzheimer’s disease and protein metabolism. The similarities between our results and the independent validation results argues against random chance accounting for the observed enrichment of these gene sets.
Validation of selected differentially expressed genes
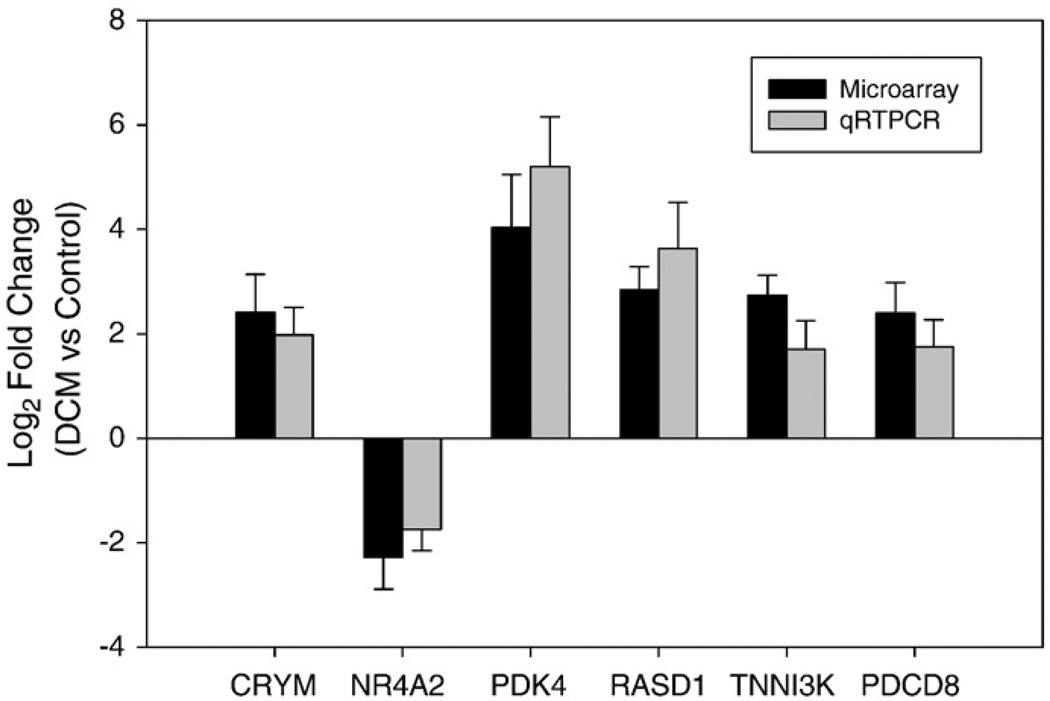
We next used quantitative real-time PCR (qRT-PCR) to validate selected differentially expressed genes, CRYM, NR4A2, PDK4, RASD1, TNNI3K and AIFM1. QRT-PCR analysis revealed a highly significant correlation (r=0.97, P value<0.01) between the microarray and the qRT-PCR data (Fig. 4), thus demonstrating the reliability of our gene expression measurements. These sets of genes and their interaction networks are shown (Supplementary Fig. 1A – E).
Fig. 4.
Confirmation of the microarray gene expression for six randomly selected differentially expressed genes by qRT-PCR. Ratio of expression for each gene in DCM group to normal control (fold change) was log2 transformed for microarray data and real-time RT-PCR. Dark bars represent microarray hybridizations, and, and grey bars represent values from qRT-PCR. The error bar represents standard deviation (SD) over three experiments.
Discussion
The present study investigates alterations in gene expression associated with heart muscle disease using DCM as a study model. While the sample sizes employed in this study are comparatively small, it should be noted that all tissues were procured from identical myocardial loci, to ensure optimal uniformity in cell content. This procedure should produce comparatively reliable and informative results with the microarray platform employed, in particular, if considered together with similar gene expression studies employing different platforms [46–48]. Most importantly, a validation analysis demonstrated great concordance of our results with other data sets using a different microarray platform. Besides ABI 1700 system has a unique approach in identifying the dysregulated genes since it targets genes from both Celera and Public databases and utilizes chemiluminescently enhanced detection that is likely to determine relatively rare mRNAs. Also, our confirmatory qRT-PCR experiments displayed very good correlation with the microarray results, adding to the validity of the present observations. This is in concordance with some recent studies showing linear relationship for real-time and conventional reverse transcription and therefore validity of robustness of mRNA quantification using either microarrays or quantitative RT-PCR [49]. Hence the aim of the present work is to identify potentially novel and signature genes for DCM, in order to gain further insight into the mechanism of heart muscle disease pathways.
We identified differentially expressed probes satisfying the set criteria of S/N ratio >3 in >50% of the samples, a 5% FDR and absolute fold change >1.8 in DCM patients compared to controls. These observations are consistent with the study of Guo et al. [28], in which gene lists ranked by fold change and filtered with non-stringent statistically significant tests were more reproducible across platforms than those generated through other analytical procedures. Our study reveals several differentially and highly expressed genes and gene families, the majority of which encode apoptotic, cell proliferation and differentiation, homeostatic and mitochondrial energy metabolizing proteins. In general, genes and pathways involved in apoptosis, growth, communication and cardiomyoctye structure were down-regulated, whereas those involved in energy metabolic processes, cell survival, cell cycle, ion transport were up-regulated. Thus, our study furnishes supporting evidence for the prevalence of apoptosis in DCM, as indicated by the presence of DNA fragmentation in conjunction with changes in apoptotic signaling components [50,51]. These results are also consistent with other microarray and small-scale studies demonstrating changes in related genes, including cytokines, tumor necrosis factor (TNF)-induced genes, MAPKs, cell survival and stress response genes as well as other components of apoptotic signaling pathways [52–54]. Taken together, these data strongly implicate perturbations in death-related signaling in the pathogenesis of DCM.
Other similarly described changes in DCM thus far involve genes contributing to diverse cellular processes, including transcription [55], sarcomeric and cytoskeletal function [24,56], extracellular matrix remodeling [57], membrane transport [23], ion channels [55] and immune responses [22]. Also consistently implicated in this disease is the alteration in myofilament function in conjunction with depressed myofibrillar ATPase activity [58] and increased myofilament Ca2+ sensitivity, which presumably contribute to slowed or incomplete muscle relaxation, and therefore depression of the force-frequency relation [12]. In this regard, it is noteworthy that altered post-translational modification of particularly the phosphorylation state of troponins I and T, and possibly myosin light chain, has been postulated as the most important mechanism of myofilament dysfunction in DCM, with possible contributions of other modifications, such as oxidation and glycation [13]. Therefore, the down-regulation of extracellular matrix genes and the increase in cell structural and myofilament gene expression established in this and various other studies substantiate the potential involvement of cardiomyocyte modification(s) as an integral component of events occurring in DCM.
Apart from modifications of the contractile muscle structure, changes in mitochondrial gene expression were also evident, ranging from the up-regulation of genes involved in energy metabolism, such as the citric acid cycle and ATP synthesis, and those important in maintaining energy metabolic pathways, such as MLYCD or PDK4, to alterations at the level of oxidative phosphorylation (e.g. TNNI3K) and transamination (e.g. BCAT1). Similar changes have also been described by other investigators in genes related to oxidative phosphorylation [59], mitochondrial ADP/ATP transport and adenine nucleotide translocase (SLC25A4) [60,61], for example. In this regard, it should be noted that changes in DCM have also been associated with more economical and efficient energy utilization by the contractile machinery, which may offer myocardial protection [58]. Interestingly, in our study, metabolic pathways were by far the most significantly altered and well-represented among the over-expressed biological processes, implicitly placing them at the centre of events occurring in this disease. Therefore, there appears to be a heightened level of metabolic activity, which may be ascribable to adaptive or compensatory mechanisms in this disease process.
In addition to the above well-characterized pathways and processes, we also discovered changes in genes thought to be physiologically dormant, which may not necessarily fit into the picture of the failing heart. These include the attenuation in functional expression of genes encoding growth factors, such as TGF-β, EGF and FGF, as well as numerous unclassified molecular functions on the one hand, and the up-regulation of those encoding homeobox transcription factors and processes, such as protein, nucleoside, nucleotide, nucleic acid metabolisms and cardiac development, on the other. This scenario points to a functional state in which regulatory processes that naturally occur in early stages of cardiac development are suppressed, while those that may be attributed to sustention of cellular integrity are elevated or possibly stimulated under these disease conditions. Our findings are consistent with similar microarray studies postulating specific role of various transcription factors in heart failure [62,63]. This implies therefore, that the dormancy of these entities in an adult heart serves definable functional purpose(s), which can be mobilized or may alternatively contribute to the shaping the disease pathway(s) to heart failure.
Interestingly, a large number of genes were similarly uncovered with currently undefined cardiac-related function. These include the up-regulated HTRA1, AIFM1, CRYM and PRDX2 genes and the down-regulated NR4A2, LGALS9, IFNA13, MX1, UNC5D, and HDAC2 in DCM. To date, the HTRA1 has been associated with, among others, Alzheimer's disease [64,65], various neoplasms [66,67] and regulation of several signaling pathways [68]. The AIFM1 is a mitochondrial oxidoreductase which has been implicated in neurological disorders, and may influence homeostasis possibly by interacting with apoptosis-related signaling protein [69–72]. The PRDX2 is a peroxidoreductase that catalyzes oxidation-reduction reactions and has been implicated in Down's syndrome [72], Alzheimer's disease [73] and various forms of neoplasms [74]. The protein may be involved in the regulation of apoptosis and response to oxidative stress [75], possibly by influencing oxidoreductase enzyme activities [67,69,76], among others. The CRYM has also been associated with various forms of cancer [77]. Of the down-regulated genes, the LGALS9 has been linked with various neoplasms and positive regulation of I-κB kinase/NF-κB cascade, Ras signalling and protein amino acid phosphorylation [78,79]. It probably inhibits cell growth in a fashion that is regulated by NF-κB [80]. The MIRHG2 [81], UNC5D [82] and HDAC2 [83,84] have similarly been associated with different types of cancer. However, the functional roles particularly with respect to cardiovascular function of their putative protein products remain largely ill-defined.
Thus, it appears that several altered pathways, processes and yet undefined genes described in the present study are also implicated in various non-cardiac disorders. These actions may be related to alterations in apoptotic signaling components, such as MAPKs, p90RSK, NF-κB, caspase-3 and Src, that have in turn been partly implicated in the pathogenesis of heart muscle disease [50,53,85]. The concomitant up-regulation and down-regulation of the apoptotic signaling components points to a dual role for this pathway, possibly contributing to both the progression of the disease to heart failure and to compensatory/adaptive mechanisms in response to the ventricular overload [80,86,87]. Besides, it has also been suggested that in HF, apoptosis may be interrupted and is therefore potentially reversible [20]. This might also explain in part some of these apparently incongruous signalling events under these disease conditions.
The fact that some of the above genes are similarly associated with neurodegenerative disorders, such as Alzheimer's disease, Hunting-ton's disease or Down's syndrome, attracts speculations with respect to the relevance of their existence in the myocardium. Interesting in this regard is also a recent finding of a mouse model of hypertension revealing the induction of Alzheimer's disease pathways [88]. It is therefore appealing to hypothesize common underlying mechanism (s) leading to or triggered by these biological processes with a missing link connecting cardiac disease pathways with these disorders. It is also noteworthy that the formation of amyloid plaques in Alzheimer's disease is associated partly with perturbations in Ca2+ metabolism, a pivotal second messenger in the regulation of cardiac contractility. Moreover, mitochondrial dysfunction and particularly oxidative stress are well-established major players in Alzheimer's disease [89,90] and possibly Down's syndrome [91], pointing to the likelihood of an inter-regulation of these disorders at the level of mitochondrial function or second messenger signal transduction. Hence, these observations necessitate more precisely focused studies to enhance our understanding of the missing links coupling such diverse forms of human disease with one another.
A further important question remains as to whether or not the observed alterations in gene expression are exclusive for DCM per se, heart muscle disease as a whole, or could be eventually ascribable to heart failure in general. Although the current study was not directed at addressing this issue, it is noteworthy that investigations involving global gene expression in DCM and heart failure thus far have yielded varying results. While some investigators purport disease-specific alterations in gene expression [25], others view these changes as ultimately describing the events associated with heart failure. It has also been argued that the alterations in gene expression in explanted DCM hearts are reflective of events pertaining to the progression of heart failure rather than the heart muscle disease per se [25,55,85,92]. However, while these studies commonly discuss the sharing of alterations in gene expression under different disease conditions, they do not necessarily preclude the possibility of yet undefined changes in signaling pathways exclusively related to DCM being discernible from events specifically pertinent to end-stage heart failure, in general. Indeed, differences have been described in gene expression between ischemic heart disease (IHD) and DCM [26,54,85,93]. On the other hand, it is believed that remodeling is a feature of both IHD and DCM, suggesting common mechanisms for their progression to cardiac dysfunction. However, it has also been asserted that, although heart failure emanating from these two diseases results in similar clinical endpoints, it progresses through different remodeling and molecular pathways [55]. Hence, further studies are necessary to ascertain the events determining various disease pathways to overt heart failure.
In summary, evaluation of global gene expression patterns provides a molecular depiction specific to DCM, yields insights into the pathophysiological aspects of heart muscle disease, and identifies novel genes and pathways whose cardiac-related functions have yet to be deciphered. The present study demonstrates not only concomitant activation of signaling components regulating partly counteractive mechanisms involved in cell death, survival and homeostasis, but also novel gene expression previously unknown to be related to cardiac function. The resemblance of DCM with disorders, such as cancer or neurodegenerative disorders, in the pattern of differential expression of several genes, molecular functions and pathways points to a link of these diseases at the level of apoptotic signaling, energy metabolism and maintenance of cellular structural integrity.
Supplementary Material
Acknowledgments
This work was supported by the Royal Cardiovascular Research Grant No 9900004 under the King Faisal Specialist Hospital and Research Centre. The authors express their gratitude for this financial support.
Glossary
- ANT(SLC25A4)
adenine nucleotide translocase
- β-AR
β-adrenoceptor
- BCAT2
mitochondrial branched chain aminotransferase 2
- MIRHG2
B-cell receptor-inducible gene
- BMP
bone morphogenic protein
- CRYM
crystalline-mu
- EGF
epidermal growth factor
- FDR
false discovery rate
- FGF
fibroblast growth factor
- HCM
hypertrophic cardiomyopathy
- HDAC2
histone deacetylase 2
- HTRA1
human high-temperature requirement factor A1
- IFNA13
interferon A13
- KCNQ2
potassium voltage-gated channel, member 2
- LGALS9
lectin, galactose binding, soluble 9
- MAPK
mitogen-activated protein kinase
- MLYCD
malonyl-CoA decarboxylase
- MX1
myxovirus subtype 1
- NF-κB
nuclear factor κB
- NR4A2
pyruvate dehydrogenase kinase, isoenzyme 4
- p90RSK
p90 Ribosomal S6 Kinase
- AIFM1
mitochondrial programmed cell-death protein 8
- PDK4
pyruvate dehydrogenase kinase, isozyme 4
- PRDX2
peroxiredoxin 2
- RASD1
dexamethasome-induced RAS encoding subtype 1 gene
- SERCA
sarco-endoplasmic reticulum Ca2+-ATPase
- TCA
tricarboxylic acid
- TGFR-β
transforming growth factor-β receptor
- TNF
tumor necrosis factor
- TNNI3
human troponin I subtype 3
- TNNI3K
TNNI3 interacting kinase
- UBB
ubiquitin B
- UNC5D
unc-5 homolog D
Appendix A
The original microarray data is available in the ArrayExpress database (Accession: E-TABM-480).
Appendix B. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ygeno.2009.03.003.
References
- 1.Arimura T, Hayashi T, Kimura A. Molecular etiology of idiopathic cardiomyo-pathy. Acta Myol. 2007;26(3):153–158. [PMC free article] [PubMed] [Google Scholar]
- 2.Ross J., Jr. Dilated cardiomyopathy: concepts derived from gene deficient and transgenic animal models. Circ. J. 2002;66(3):219–224. doi: 10.1253/circj.66.219. [DOI] [PubMed] [Google Scholar]
- 3.Chang AN, Potter JD. Sarcomeric protein mutations in dilated cardiomyopathy. Heart Fail. Rev. 2005;10(3):225–235. doi: 10.1007/s10741-005-5252-6. [DOI] [PubMed] [Google Scholar]
- 4.Granzier H, Wu Y, Siegfried L, LeWinter M. Titin: physiological function and role in cardiomyopathy and failure. Heart Fail. Rev. 2005;10(3):211–223. doi: 10.1007/s10741-005-5251-7. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad F, Seidman JG, Seidman CE. The genetic basis for cardiac remodeling. Annu. Rev. Genomics Hum. Genet. 2005;6:185–216. doi: 10.1146/annurev.genom.6.080604.162132. [DOI] [PubMed] [Google Scholar]
- 6.Sutton MG, Sharpe N. Left ventricular remodeling after myocardial infarction: pathophysiology and therapy. Circulation. 2000;101(25):2981–2988. doi: 10.1161/01.cir.101.25.2981. [DOI] [PubMed] [Google Scholar]
- 7.Wolff MR, Buck SH, Stoker SW, Greaser ML, Mentzer RM. Myofibrillar calcium sensitivity of isometric tension is increased in human dilated cardiomyopathies: role of altered beta-adrenergically mediated protein phosphorylation. J. Clin. Invest. 1996;98(1):167–176. doi: 10.1172/JCI118762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dzimiri N. Regulation of beta-adrenoceptor signaling in cardiac function and disease. Pharmacol. Rev. 1999;51(3):465–501. [PubMed] [Google Scholar]
- 9.Mittmann C, Eschenhagen T, Scholz H. Cellular and molecular aspects of contractile dysfunction in heart failure. Cardiovasc. Res. 1998;39(2):267–275. doi: 10.1016/s0008-6363(98)00139-4. [DOI] [PubMed] [Google Scholar]
- 10.Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35(4):430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- 11.Solaro RJ, Rarick HM. Troponin and tropomyosin: proteins that switch on and tune in the activity of cardiac myofilaments. Circ. Res. 1998;83(5):471–480. doi: 10.1161/01.res.83.5.471. [DOI] [PubMed] [Google Scholar]
- 12.Palmer BM. Thick filament proteins and performance in human heart failure. Heart Fail. Rev. 2005;10(3):187–197. doi: 10.1007/s10741-005-5249-1. [DOI] [PubMed] [Google Scholar]
- 13.Layland J, Solaro RJ, Shah AM. Regulation of cardiac contractile function by troponin I phosphorylation. Cardiovasc. Res. 2005;66(1):12–21. doi: 10.1016/j.cardiores.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 14.Sivakumar P, Gupta S, Sarkar S, Sen S. Upregulation of lysyl oxidase and MMPs during cardiac remodeling in human dilated cardiomyopathy. Mol. Cell. Biochem. 2008;307(1–2):159–167. doi: 10.1007/s11010-007-9595-2. [DOI] [PubMed] [Google Scholar]
- 15.Escobales N, Crespo MJ. Early pathophysiological alterations in experimental cardiomyopathy: the Syrian cardiomyopathic hamster. P. R. Health Sci. J. 2008;27(4):307–314. [PubMed] [Google Scholar]
- 16.McCloskey DT, Turcato S, Wang GY, Turnbull L, Zhu BQ, Bambino T, Nguyen AP, Lovett DH, Nissenson RA, Karliner JS, Baker AJ. Expression of a Gicoupled receptor in the heart causes impaired Ca2+ handling, myofilament injury, and dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2008;294(1):H205–H212. doi: 10.1152/ajpheart.00829.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mahmoudabady M, Mathieu M, Dewachter L, Hadad I, Ray L, Jespers P, Brimioulle S, Naeije R, McEntee K. Activin-A, transforming growth factor-beta, and myostatin signaling pathway in experimental dilated cardiomyopathy. J. Card. Fail. 2008;14(8):703–709. doi: 10.1016/j.cardfail.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 18.Princen F, Bard E, Sheikh F, Zhang SS, Wang J, Zago WM, Wu D, Trelles RD, Bailly-Maitre B, Kahn CR, Chen Y, Reed JC, Tong GG, Mercola M, Chen J, Feng GS. Deletion of Shp2 tyrosine phosphatase in muscle leads to dilated cardiomyopathy insulin resistance, and premature death. Mol. Cell. Biol. 2009;29(2):378–388. doi: 10.1128/MCB.01661-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zheng M, Cheng H, Li X, Zhang J, Cui L, Ouyang K, Han L, Zhao T, Gu Y, Dalton ND, Bang ML, Peterson KL, Chen J. Cardiac-specific ablation of cypher leads to a severe form of dilated cardiomyopathy with premature death. Hum. Mol. Genet. 2008 doi: 10.1093/hmg/ddn400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, Yacoub MH, Evans PC. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc. Res. 2008;79(3):472–480. doi: 10.1093/cvr/cvn083. [DOI] [PubMed] [Google Scholar]
- 21.Tavi P, Laine M, Weckstrom M, Ruskoaho H. Cardiac mechanotransduction: from sensing to disease and treatment. Trends Pharmacol. Sci. 2001;22(5):254–260. doi: 10.1016/s0165-6147(00)01679-5. [DOI] [PubMed] [Google Scholar]
- 22.Barth AS, Kuner R, Buness A, Ruschhaupt M, Merk S, Zwermann L, Kaab S, Kreuzer E, Steinbeck G, Mansmann U, Poustka A, Nabauer M, Sultmann H. Identification of a common gene expression signature in dilated cardiomyopathy across independent microarray studies. J. Am. Coll. Cardiol. 2006;48(8):1610–1617. doi: 10.1016/j.jacc.2006.07.026. [DOI] [PubMed] [Google Scholar]
- 23.Yung CK, Halperin VL, Tomaselli GF, Winslow RL. Gene expression profiles in end-stage human idiopathic dilated cardiomyopathy: altered expression of apoptotic and cytoskeletal genes. Genomics. 2004;83(2):281–297. doi: 10.1016/j.ygeno.2003.08.007. [DOI] [PubMed] [Google Scholar]
- 24.Barrans JD, Allen PD, Stamatiou D, Dzau VJ, Liew CC. Global gene expression profiling of end-stage dilated cardiomyopathy using a human cardiovascular-based cDNA microarray. Am. J. Pathol. 2002;160(6):2035–2043. doi: 10.1016/S0002-9440(10)61153-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaab S, Barth AS, Margerie D, Dugas M, Gebauer M, Zwermann L, Merk S, Pfeufer A, Steinmeyer K, Bleich M, Kreuzer E, Steinbeck G, Nabauer M. Global gene expression in human myocardium-oligonucleotide microarray analysis of regional diversity and transcriptional regulation in heart failure. J. Mol. Med. 2004;82(5):308–316. doi: 10.1007/s00109-004-0527-2. [DOI] [PubMed] [Google Scholar]
- 26.Kittleson MM, Minhas KM, Irizarry RA, Ye SQ, Edness G, Breton E, Conte JV, Tomaselli G, Garcia JG, Hare JM. Gene expression analysis of ischemic and nonischemic cardiomyopathy: shared and distinct genes in the development of heart failure. Physiol. Genomics. 2005;21(3):299–307. doi: 10.1152/physiolgenomics.00255.2004. [DOI] [PubMed] [Google Scholar]
- 27.Shi L, Reid LH, Jones WD, Shippy R, Warrington JA, Baker SC, Collins PJ, de Longueville F, Kawasaki ES, Lee KY, Luo Y, Sun YA, Willey JC, Setterquist RA, Fischer GM, Tong W, Dragan YP, Dix DJ, Frueh FW, Goodsaid FM, Herman D, Jensen RV, Johnson CD, Lobenhofer EK, Puri RK, Schrf U, Thierry-Mieg J, Wang C, Wilson M, Wolber PK, Zhang L, Amur S, Bao W, Barbacioru CC, Lucas AB, Bertholet V, Boysen C, Bromley B, Brown D, Brunner A, Canales R, Cao XM, Cebula TA, Chen JJ, Cheng J, Chu TM, Chudin E, Corson J, Corton JC, Croner LJ, Davies C, Davison TS, Delenstarr G, Deng X, Dorris D, Eklund AC, Fan XH, Fang H, Fulmer-Smentek S, Fuscoe JC, Gallagher K, Ge W, Guo L, Guo X, Hager J, Haje PK, Han J, Han T, Harbottle HC, Harris SC, Hatchwell E, Hauser CA, Hester S, Hong H, Hurban P, Jackson SA, Ji H, Knight CR, Kuo WP, LeClerc JE, Levy S, Li QZ, Liu C, Liu Y, Lombardi MJ, Ma Y, Magnuson SR, Maqsodi B, McDaniel T, Mei N, Myklebost O, Ning B, Novoradovskaya N, Orr MS, Osborn TW, Papallo A, Patterson TA, Perkins RG, Peters EH, Peterson R, Philips KL, Pine PS, Pusztai L, Qian F, Ren H, Rosen M, Rosenzweig BA, Samaha RR, Schena M, Schroth GP, Shchegrova S, Smith DD, Staedtler F, Su Z, Sun H, Szallasi Z, Tezak Z, Thierry-Mieg D, Thompson KL, Tikhonova I, Turpaz Y, Vallanat B, Van C, Walker SJ, Wang SJ, Wang Y, Wolfinger R, Wong A, Wu J, Xiao C, Xie Q, Xu J, Yang W, Zhang L, Zhong S, Zong Y, Slikker W., Jr. The MicroArray Quality Control (MAQC) project shows inter- and intraplatform reproducibility of gene expression measurements. Nat. Biotechnol. 2006;24(9):1151–1116. doi: 10.1038/nbt1239. 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo L, Lobenhofer EK, Wang C, Shippy R, Harris SC, Zhang L, Mei N, Chen T, Herman D, Goodsaid FM, Hurban P, Phillips KL, Xu J, Deng X, Sun YA, Tong W, Dragan YP, Shi L. Rat toxicogenomic study reveals analytical consistency across microarray platforms. Nat. Biotechnol. 2006;24(9):1162–1169. doi: 10.1038/nbt1238. [DOI] [PubMed] [Google Scholar]
- 29.Ali-Seyed M, Laycock N, Karanam S, Xiao W, Blair ET, Moreno CS. Cross-platform expression profiling demonstrates that SV40 small tumor antigen activates Notch, Hedgehog, and Wnt signaling in human cells. BMC Cancer. 2006;6:54. doi: 10.1186/1471-2407-6-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker SJ, Wang Y, Grant KA, Chan F, Hellmann GM. Long versus short oligonucleotide microarrays for the study of gene expression in nonhuman primates. J. Neurosci. Methods. 2006;152(1–2):179–189. doi: 10.1016/j.jneumeth.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Wang Y, Barbacioru C, Hyland F, Xiao W, Hunkapiller KL, Blake J, Chan F, Gonzalez C, Zhang L, Samaha RR. Large scale real-time PCR validation on gene expression measurements from two commercial long-oligonucleotide micro-arrays. BMC Genomics. 2006;7:59. doi: 10.1186/1471-2164-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using realtime quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Gentleman RC, Carey VJ, Bates DM, Bolstad B, Dettling M, Dudoit S, Ellis B, Gautier L, Ge Y, Gentry J, Hornik K, Hothorn T, Huber W, Iacus S, Irizarry R, Leisch F, Li C, Maechler M, Rossini AJ, Sawitzki G, Smith C, Smyth G, Tierney L, Yang JY, Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5(10):R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57:289–300. [Google Scholar]
- 35.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc. Natl. Acad. Sci. U. S. A. 2001;98(9):5116–5121. doi: 10.1073/pnas.091062498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li C, Hung Wong W. Model-based analysis of oligonucleotide arrays: model validation, design issues and standard error application. Genome Biol. 2001;2(8) doi: 10.1186/gb-2001-2-8-research0032. RESEARCH0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Z, Irizarry RA. Stochastic models inspired by hybridization theory for short oligonucleotide arrays. J. Comput. Biol. 2005;12(6):882–893. doi: 10.1089/cmb.2005.12.882. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z, Irizarry RA. Preprocessing of oligonucleotide array data. Nat. Biotechnol. 2004;22(6):656–658. doi: 10.1038/nbt0604-656b. author reply 658. [DOI] [PubMed] [Google Scholar]
- 39.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Currier T, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Liu Z, Vinsavich A, Trush V, Quackenbush J. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34(2):374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 40.Sherman BT, Huang DW, Tan Q, Guo Y, Bour S, Liu D, Stephens R, Baseler MW, Lane HC, Lempicki RA. DAVID Knowledgebase: a gene-centered database integrating heterogeneous gene annotation resources to facilitate high-throughput gene functional analysis. BMC Bioinformatics. 2007;8(1):426. doi: 10.1186/1471-2105-8-426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hosack DA, Dennis G, Jr., Sherman BT, Lane HC, Lempicki RA. Identifying biological themes within lists of genes with EASE. Genome Biol. 2003;4(10):R70. doi: 10.1186/gb-2003-4-10-r70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thomas PD, Kejariwal A, Campbell MJ, Mi H, Diemer K, Guo N, Ladunga I, Ulitsky-Lazareva B, Muruganujan A, Rabkin S, Vandergriff JA, Doremieux O. PANTHER: a browsable database of gene products organized by biological function using curated protein family and subfamily classification. Nucleic. Acids Res. 2003;31(1):334–341. doi: 10.1093/nar/gkg115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas PD, Kejariwal A, Guo N, Mi H, Campbell MJ, Muruganujan A, Lazareva-Ulitsky B. Applications for protein sequence-function evolution data: mRNA/protein expression analysis and coding SNP scoring tools. Nucleic. Acids Res. 2006;34:W645–W650. doi: 10.1093/nar/gkl229. (Web Server issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298(5593):601–604. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 45.Dudoit SSP, Boldrick JC. Multiple hypothesis testing in microarray experiments. Statist. Sci. 2003;18(1):71–103. [Google Scholar]
- 46.Pedotti P, t Hoen PA, Vreugdenhil E, Schenk GJ, Vossen RH, Ariyurek Y, de Hollander M, Kuiper R, van Ommen GJ, den Dunnen JT, Boer JM, de Menezes RX. Can subtle changes in gene expression be consistently detected with different microarray platforms? BMC Genomics. 2008;9:124. doi: 10.1186/1471-2164-9-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chuang LY, Ke CH, Chang HW, Yang CH. A two-stage feature selection method for gene expression data. Omics. 2009 doi: 10.1089/omi.2008.0083. [DOI] [PubMed] [Google Scholar]
- 48.Barrett AB, Phan JH, Wang MD. Combining multiple microarray studies using bootstrap meta-analysis. Conf. Proc. IEEE. Eng. Med. Biol. Soc. 2008;1:5660–5663. doi: 10.1109/IEMBS.2008.4650498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Francois P, Garzoni C, Bento M, Schrenzel J. Comparison of amplification methods for transcriptomic analyses of low abundance prokaryotic RNA sources. J. Microbiol. Methods. 2007;68(2):385–391. doi: 10.1016/j.mimet.2006.09.022. [DOI] [PubMed] [Google Scholar]
- 50.Dzimiri N, Afrane B, Canver CC. Preferential existence of death-inducing proteins in the human cardiomyopathic left ventricle. J. Surg. Res. 2007 doi: 10.1016/j.jss.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Dzimiri N, Muiya P, Andes E, Afrane B. Differential regulation of myocyte death/ survival signalling in human dilated cardiomyopathy. FASEB J. 2006;20:164. [Google Scholar]
- 52.Steenbergen C, Afshari CA, Petranka JG, Collins J, Martin K, Bennett L, Haugen A, Bushel P, Murphy E. Alterations in apoptotic signaling in human idiopathic cardiomyopathic hearts in failure. Am. J. Physiol. Heart Circ. Physiol. 2003;284(1):H268–H276. doi: 10.1152/ajpheart.00707.2002. [DOI] [PubMed] [Google Scholar]
- 53.Takeishi Y, Huang Q, Abe J, Che W, Lee JD, Kawakatsu H, Hoit BD, Berk BC, Walsh RA. Activation of mitogen-activated protein kinases and p90 ribosomal S6 kinase in failing human hearts with dilated cardiomyopathy. Cardiovasc. Res. 2002;53(1):131–137. doi: 10.1016/s0008-6363(01)00438-2. [DOI] [PubMed] [Google Scholar]
- 54.Plenz G, Song ZF, Reichenberg S, Tjan TD, Robenek H, Deng MC. Left-ventricular expression of interleukin-6 messenger-RNA higher in idiopathic dilated than in ischemic cardiomyopathy. Thorac. Cardiovasc. Surg. 1998;46(4):213–216. doi: 10.1055/s-2007-1010227. [DOI] [PubMed] [Google Scholar]
- 55.Hwang JJ, Allen PD, Tseng GC, Lam CW, Fananapazir L, Dzau VJ, Liew CC. Microarray gene expression profiles in dilated and hypertrophic cardiomyopathic end-stage heart failure. Physiol. Genomics. 2002;10(1):31–44. doi: 10.1152/physiolgenomics.00122.2001. [DOI] [PubMed] [Google Scholar]
- 56.Grzeskowiak R, Witt H, Drungowski M, Thermann R, Hennig S, Perrot A, Osterziel KJ, Klingbiel D, Scheid S, Spang R, Lehrach H, Ruiz P. Expression profiling of human idiopathic dilated cardiomyopathy. Cardiovasc. Res. 2003;59(2):400–411. doi: 10.1016/s0008-6363(03)00426-7. [DOI] [PubMed] [Google Scholar]
- 57.Xu J, Gong NL, Bodi I, Aronow BJ, Backx PH, Molkentin JD. Myocyte enhancer factors 2A and 2C induce dilated cardiomyopathy in transgenic mice. J. Biol. Chem. 2006;281(14):9152–9162. doi: 10.1074/jbc.M510217200. [DOI] [PubMed] [Google Scholar]
- 58.LeWinter MM. Functional consequences of sarcomeric protein abnormalities in failing myocardium. Heart Fail. Rev. 2005;10(3):249–257. doi: 10.1007/s10741-005-5254-4. [DOI] [PubMed] [Google Scholar]
- 59.Lopes R, Solter PF, Sisson DD, Oyama MA, Prosek R. Characterization of canine mitochondrial protein expression in natural and induced forms of idiopathic dilated cardiomyopathy. Am. J. Vet. Res. 2006;67(6):963–970. doi: 10.2460/ajvr.67.6.963. [DOI] [PubMed] [Google Scholar]
- 60.Portman MA. The adenine nucleotide translocator: regulation and function during myocardial development and hypertrophy. Clin. Exp. Pharmacol. Physiol. 2002;29(4):334–338. doi: 10.1046/j.1440-1681.2002.03654.x. [DOI] [PubMed] [Google Scholar]
- 61.Dorner A, Giessen S, Gaub R, Grosse Siestrup H, Schwimmbeck PL, Hetzer R, Poller W, Schultheiss HP. An isoform shift in the cardiac adenine nucleotide translocase expression alters the kinetic properties of the carrier in dilated cardiomyopathy. Eur. J. Heart Fail. 2006;8(1):81–89. doi: 10.1016/j.ejheart.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 62.Margulies KB, Matiwala S, Cornejo C, Olsen H, Craven WA, Bednarik D. Mixed messages: transcription patterns in failing and recovering human myocardium. Circ. Res. 2005;96(5):592–599. doi: 10.1161/01.RES.0000159390.03503.c3. [DOI] [PubMed] [Google Scholar]
- 63.Hannenhalli S, Putt ME, Gilmore JM, Wang J, Parmacek MS, Epstein JA, Morrisey EE, Margulies KB, Cappola TP. Transcriptional genomics associates FOX transcription factors with human heart failure. Circulation. 2006;114(12):1269–1276. doi: 10.1161/CIRCULATIONAHA.106.632430. [DOI] [PubMed] [Google Scholar]
- 64.Grau S, Baldi A, Bussani R, Tian X, Stefanescu R, Przybylski M, Richards P, Jones SA, Shridhar V, Clausen T, Ehrmann M. Implications of the serine protease HtrA1 in amyloid precursor protein processing. Proc. Natl. Acad. Sci. U. S. A. 2005;102(17):6021–6026. doi: 10.1073/pnas.0501823102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang Z, Camp NJ, Sun H, Tong Z, Gibbs D, Cameron DJ, Chen H, Zhao Y, Pearson E, Li X, Chien J, Dewan A, Harmon J, Bernstein PS, Shridhar V, Zabriskie NA, Hoh J, Howes K, Zhang K. A variant of the HTRA1 gene increases susceptibility to age-related macular degeneration. Science. 2006;314(5801):992–993. doi: 10.1126/science.1133811. [DOI] [PubMed] [Google Scholar]
- 66.Carta F, Demuro PP, Zanini C, Santona A, Castiglia D, D'Atri S, Ascierto PA, Napolitano M, Cossu A, Tadolini B, Turrini F, Manca A, Sini MC, Palmieri G, Rozzo AC. Analysis of candidate genes through a proteomics-based approach in primary cell lines from malignant melanomas and their metastases. Melanoma Res. 2005;15(4):235–244. doi: 10.1097/00008390-200508000-00002. [DOI] [PubMed] [Google Scholar]
- 67.Furuta J, Nobeyama Y, Umebayashi Y, Otsuka F, Kikuchi K, Ushijima T. Silencing of Peroxiredoxin 2 and aberrant methylation of 33 CpG islands in putative promoter regions in human malignant melanomas. Cancer Res. 2006;66(12):6080–6086. doi: 10.1158/0008-5472.CAN-06-0157. [DOI] [PubMed] [Google Scholar]
- 68.Oka C, Tsujimoto R, Kajikawa M, Koshiba-Takeuchi K, Ina J, Yano M, Tsuchiya A, Ueta Y, Soma A, Kanda H, Matsumoto M, Kawaichi M. HtrA1 serine protease inhibits signaling mediated by Tgfbeta family proteins. Development. 2004;131(5):1041–1053. doi: 10.1242/dev.00999. [DOI] [PubMed] [Google Scholar]
- 69.Vahsen N, Cande C, Briere JJ, Benit P, Joza N, Larochette N, Mastrobe-rardino PG, Pequignot MO, Casares N, Lazar V, Feraud O, Debili N, Wissing S, Engelhardt S, Madeo F, Piacentini M, Penninger JM, Schagger H, Rustin P, Kroemer G. AIF deficiency compromises oxidative phosphorylation. EMBO J. 2004;23(23):4679–4689. doi: 10.1038/sj.emboj.7600461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urbano A, Lakshmanan U, Choo PH, Kwan JC, Ng PY, Guo K, Dhakshina-moorthy S, Porter A. AIF suppresses chemical stress-induced apoptosis and maintains the transformed state of tumor cells. EMBO J. 2005;24(15):2815–2826. doi: 10.1038/sj.emboj.7600746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Delettre C, Yuste VJ, Moubarak RS, Bras M, Robert N, Susin SA. Identification and characterization of AIFsh2, a mitochondrial apoptosis-inducing factor (AIF) isoform with NADH oxidase activity. J. Biol. Chem. 2006;281(27):18507–18518. doi: 10.1074/jbc.M601751200. [DOI] [PubMed] [Google Scholar]
- 72.Sanchez-Font MF, Sebastia J, Sanfeliu C, Cristofol R, Marfany G, Gonzalez-Duarte R. Peroxiredoxin 2 (PRDX2), an antioxidant enzyme, is under-expressed in Down syndrome fetal brains. Cell. Mol. Life Sci. 2003;60(7):1513–1523. doi: 10.1007/s00018-003-3048-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yao J, Taylor M, Davey F, Ren Y, Aiton J, Coote P, Fang F, Chen JX, Yan SD, Gunn-Moore FJ. Interaction of amyloid binding alcohol dehydrogenase/Abeta mediates up-regulation of peroxiredoxin II in the brains of Alzheimer's disease patients and a transgenic Alzheimer's disease mouse model. Mol. Cell. Neurosci. 2007;35(2):377–382. doi: 10.1016/j.mcn.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 74.Huang E, Cheng SH, Dressman H, Pittman J, Tsou MH, Horng CF, Bild A, Iversen ES, Liao M, Chen CM, West M, Nevins JR, Huang AT. Gene expression predictors of breast cancer outcomes. Lancet. 2003;361(9369):1590–1596. doi: 10.1016/S0140-6736(03)13308-9. [DOI] [PubMed] [Google Scholar]
- 75.Klein JA, Longo-Guess CM, Rossmann MP, Seburn KL, Hurd RE, Frankel WN, Bronson RT, Ackerman SL. The harlequin mouse mutation downregulates apoptosis-inducing factor. Nature. 2002;419(6905):367–374. doi: 10.1038/nature01034. [DOI] [PubMed] [Google Scholar]
- 76.Chikamori K, Hill JE, Grabowski DR, Zarkhin E, Grozav AG, Vaziri SA, Wang J, Gudkov AV, Rybicki LR, Bukowski RM, Yen A, Tanimoto M, Ganapathi MK, Ganapathi R. Downregulation of topoisomerase IIbeta in myeloid leukemia cell lines leads to activation of apoptosis following all-trans retinoic acid-induced differentiation/growth arrest. Leukemia. 2006;20(10):1809–1818. doi: 10.1038/sj.leu.2404351. [DOI] [PubMed] [Google Scholar]
- 77.Mousses S, Bubendorf L, Wagner U, Hostetter G, Kononen J, Cornelison R, Goldberger N, Elkahloun AG, Willi N, Koivisto P, Ferhle W, Raffeld M, Sauter G, Kallioniemi OP. Clinical validation of candidate genes associated with prostate cancer progression in the CWR22 model system using tissue microarrays. Cancer Res. 2002;62(5):1256–1260. [PubMed] [Google Scholar]
- 78.Abedin MJ, Kashio Y, Seki M, Nakamura K, Hirashima M. Potential roles of galectins in myeloid differentiation into three different lineages. J. Leukoc. Biol. 2003;73(5):650–656. doi: 10.1189/jlb.0402163. [DOI] [PubMed] [Google Scholar]
- 79.Kasamatsu A, Uzawa K, Nakashima D, Koike H, Shiiba M, Bukawa H, Yokoe H, Tanzawa H. Galectin-9 as a regulator of cellular adhesion in human oral squamous cell carcinoma cell lines. Int. J. Mol. Med. 2005;16(2):269–273. [PubMed] [Google Scholar]
- 80.Okudaira T, Hirashima M, Ishikawa C, Makishi S, Tomita M, Matsuda T, Kawakami H, Taira N, Ohshiro K, Masuda M, Takasu N, Mori N. A modified version of galectin-9 suppresses cell growth and induces apoptosis of human T-cell leukemia virus type I-infected T-cell lines. Int. J. Cancer. 2007;120(10):2251–2261. doi: 10.1002/ijc.22534. [DOI] [PubMed] [Google Scholar]
- 81.Eis PS, Tam W, Sun L, Chadburn A, Li Z, Gomez MF, Lund E, Dahlberg JE. Accumulation of miR-155 and BIC RNA in human B cell lymphomas. Proc. Natl. Acad. Sci. U. S. A. 2005;102(10):3627–3632. doi: 10.1073/pnas.0500613102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Koed K, Wiuf C, Christensen LL, Wikman FP, Zieger K, Moller K, von der Maase H, Orntoft TF. High-density single nucleotide polymorphism array defines novel stage and location-dependent allelic imbalances in human bladder tumors. Cancer Res. 2005;65(1):34–45. [PubMed] [Google Scholar]
- 83.Zimmermann S, Kiefer F, Prudenziati M, Spiller C, Hansen J, Floss T, Wurst W, Minucci S, Gottlicher M. Reduced body size and decreased intestinal tumor rates in HDAC2-mutant mice. Cancer Res. 2007;67(19):9047–9054. doi: 10.1158/0008-5472.CAN-07-0312. [DOI] [PubMed] [Google Scholar]
- 84.Tateno T, Izumiyama H, Doi M, Yoshimoto T, Shichiri M, Inoshita N, Oyama K, Yamada S, Hirata Y. Differential gene expression in ACTH-secreting and non-functioning pituitary tumors. Eur. J. Endocrinol. 2007;157(6):717–724. doi: 10.1530/EJE-07-0428. [DOI] [PubMed] [Google Scholar]
- 85.Steenman M, Chen YW, Le Cunff M, Lamirault G, Varro A, Hoffman E, Leger JJ. Transcriptomal analysis of failing and nonfailing human hearts. Physiol. Genomics. 2003;12(2):97–111. doi: 10.1152/physiolgenomics.00148.2002. 2. [DOI] [PubMed] [Google Scholar]
- 86.Lou H, Danelisen I, Singal PK. Involvement of mitogen-activated protein kinases in adriamycin-induced cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2005;288(4):H1925–H1930. doi: 10.1152/ajpheart.01054.2004. [DOI] [PubMed] [Google Scholar]
- 87.Bueno OF, Molkentin JD. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ. Res. 2002;91(9):776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 88.Larkin JE, Frank BC, Gaspard RM, Duka I, Gavras H, Quackenbush J. Cardiac transcriptional response to acute and chronic angiotensin II treatments. Physiol. Genomics. 2004;18(2):152–166. doi: 10.1152/physiolgenomics.00057.2004. [DOI] [PubMed] [Google Scholar]
- 89.Su B, Wang X, Nunomura A, Moreira PI, Lee HG, Perry G, Smith MA, Zhu X. Oxidative stress signaling in Alzheimer's disease. Curr. Alzheimer Res. 2008;5(6):525–532. doi: 10.2174/156720508786898451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Fukui H, Moraes CT. The mitochondrial impairment. oxidative stress and neurodegeneration connection: reality or just an attractive hypothesis? Trends Neurosci. 2008;31(5):251–256. doi: 10.1016/j.tins.2008.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arbuzova S, Hutchin T, Cuckle H. Mitochondrial dysfunction and Down's syndrome. Bioessays. 2002;24(8):681–684. doi: 10.1002/bies.10138. [DOI] [PubMed] [Google Scholar]
- 92.Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc. Natl. Acad. Sci. U. S. A. 2002;99(17):11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ikeda S, Kong SW, Lu J, Bisping E, Zhang H, Allen PD, Golub TR, Pieske B, Pu WT. Altered microRNA expression in human heart disease. Physiol. Genomics. 2007 doi: 10.1152/physiolgenomics.00144.2007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.