Abstract
Uterine leiomyomas (fibroids, myomas) are the most common benign tumors of female reproductive tract. They are highly prevalent, with 70–80% of women burdened by the end of their reproductive years. Fibroids are a leading cause of pelvic pain, abnormal vaginal bleeding, pressure on the bladder, miscarriage, and infertility. They are the leading indication for hysterectomy, and costs exceed 6 billion dollars annually in the United States. Unfortunately, no long-term medical treatments are available. Dysregulation of inflammatory processes are thought to be involved in the initiation of leiomyoma and extracellular matrix deposition, cell proliferation, and angiogenesis are the key cellular events implicated in leiomyoma growth. In modern pharmaceutical industries, dietary phytochemicals are used as source of new potential drugs for many kinds of tumors. Dietary phytochemicals may exert therapeutic effects by interfering with key cellular events of the tumorigenesis process. At present, a negligible number of phytochemicals have been tested as therapeutic agents against fibroids. In this context, our aim was to introduce some of the potential dietary phytochemicals that have shown anti-inflammatory, antiproliferative, antifibrotic, and antiangiogenic activities in different biological systems. This review could be useful to stimulate the evaluation of these phytochemicals as possible therapies for uterine fibroids.
Keywords: Antifibrotic, Antiproliferative, Dietary phytochemicals, Inflammation, Uterine fibroid
1 Introduction
Uterine leiomyomas (fibroids or myomas) are common benign smooth muscle tumors of the uterus [1–3]. They are highly prevalent, with 70–80% of women burdened by the end of their reproductive years [4]. Several studies have demonstrated that there are ethnic differences in fibroid burden [4–9]. African Americans have a higher (three times more) fibroid incidence [4, 5] and experience more severe symptoms with larger and more numerous leiomyomas compared with white women [6, 9]. The common symptoms associated with uterine leiomyomas are irregular and/or heavy menstrual bleeding, pain in the pelvic region and the back, bulk-related symptoms (pressure on bladder and bowel as well as increase in abdominal circumference), and subfertility [4, 10]. Uterine fibroids are the leading indication for hysterectomy in the United States [11], and fibroid associated costs $5.9– 34.4 billion annually [12]. This complicated disease process also exerts an enormous burden on health care resources in Australia [13] and European countries [14].
Despite the widespread prevalence of the disease, the pathogenesis of leiomyomas is not well understood. An increasingly popular view is that uterine leiomyoma arise as a consequence of a chronically active inflammatory immune system [15–17]. However, there is considerable evidence that estrogens and progestogens promote tumor growth [18, 19], as the fibroids rarely appear before menarche and tend to regress after menopause [20]. Besides growth factors, cytokines and chemokines may serve as mediators of sex steroids, and play an important role in the proliferation, fibrosis, and angiogenesis processes that are ultimately involved in the formation and growth of uterine fibroids [1, 2, 10, 17, 21].
Gonadotropin-releasing hormone agonist (GnRHa) is only medical therapy for leiomyoma treatment approved by US Food and Drug Administration. GnRHa was developed on the basis of the induction of a hypoestrogenic state. Although this treatment temporally (up to 6 months) is effective as preoperative therapy to reduce fibroid size and symptoms [22, 23], the benefits of GnRHa are tempered by significant side effects resulting from hypoestrogenism (e.g., hot flashes, vaginal dryness, bone demineralization) [24–26]. In addition to GnRHa, several potential therapies such as mifepristone (antiprogestin) [27], and selective progesterone receptor modulators such as asoprisnil [28], ulipristal acetate [29, 30], and proellex [31] have shown excellent therapeutic efficiency during the course of clinical trials. Additionally, aromatase inhibitors have shown therapeutic efficacy for uterine fibroids, but are not approved for that indication. Nonetheless, in comparison with the burden of disease to society, medical treatments for leiomyoma are still very limited and no preventative therapies have been developed.
Since ancient times, plants and plant-derived compounds have provided tremendous support in the traditional medicine systems, and have been used as source of new potential drugs in modern pharmaceutical industries. For example, from 1981 to 2010, natural products and their derivatives were the source of 41% of new drugs and 79.8% of all approved anticancer drugs [32]. In addition, the percentage of drugs from natural products without derivatives was greatly increased from 20.8% in 2009 to 50% in 2010 [32]. Recently, two systematic reviews assessed the efficacy of herbal preparations for uterine fibroids [33, 34]. Meta-analyses demonstrated that Guizhi Fuling Formula plus mifepristone were more effective than mifepristone alone in reducing the volume of fibroids [33]. In addition, the Guizhi Fuling Formula significantly improved symptoms of dysmenorrhea either when it was used alone or in combination with mifepristone [33].
At present, only few dietary phytochemicals such as epigallocatechin gallate (EGCG) [35, 36], curcumin [37], isoliquiritigenin [38], genistein [39], and resveratrol [40, 41] (Table 1) have been studied in myometrium and fibroids. A large number of phytochemicals remain to be tested for possible therapeutic effects against uterine leiomyoma. In this review, we introduce some of the more promising phytochemicals (Figs. 1 and 2) in the context of key features of leiomyoma development and growth: inflammation, fibrosis, cell proliferation, and angiogenesis.
Table 1. Therapeutic effects of dietary phytochemicals on uterine fibroids.
| Dietary phytochemicals | Dietary sources | Therapeutic effects on uterine fibroids |
|---|---|---|
| Epigallocatechin gallate | Green tea (Camellia sinensis) |
|
| Curcumin | Turmeric (Curcuma longa) | |
| Isoliquiritigenin | Licorice (Glycyrrhiza uralensis), shallot (Allium ascalonicum), soybean (Glycine max) | |
| Genistein | Soybeans (G. max), lupine (Lupinus spp.), fava bean, (Vicia faba), kudzu (Pueraria lobata), psoralea (Psoralea corylifolia) |
|
| Resveratrol | More than 70 species of plants, including mulberries and peanuts. Grapevines (Vitis vinifera) are the main sources. |
Figure 1.
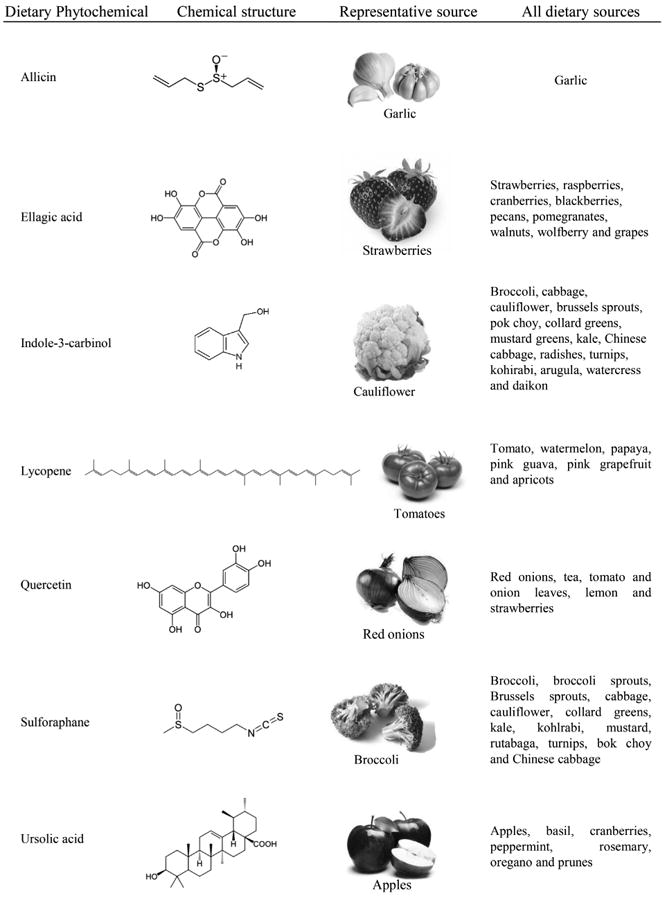
Dietary phytochemicals not yet studied in uterine fibroids, their chemical structure, and food sources.
Figure 2.
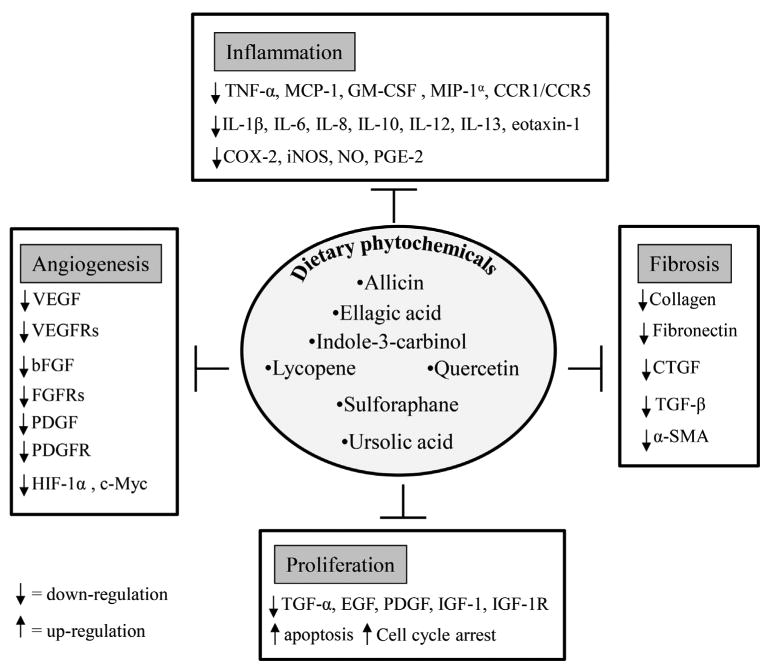
Regulation of major cellular events, inflammation, fibrosis, cell proliferation, and angiogenesis, by dietary phytochemicals.
2 Pathogenesis of uterine fibroids
2.1 Inflammatory mediators
Considerable evidence suggests that uterine leiomyoma development may be triggered, at least in part, by a chronically active inflammatory immune system [15–17]. The concept of inflammation actually fits into a theory of fibroid development based on an altered response to noxious stimuli; possibly tissue injury from extravasated menstrual blood into the myometrium, or hypoxia leading to altered tissue repair and fibrosis [15, 16]. Among major cytokines, the expression of IL-1, IL-6, IL-11, IL-13, IL-15, IL-33, tumor necrosis factor (TNF)-α, granulocyte-macrophage colony-stimulating factor have been implicated with their biological relevance to leiomyoma pathophysiology [42–47]. The expression profiles of many chemokines and chemokine receptors have also been characterized in leiomyomas and matched myometrium. These include monocyte chemoattractant protein (MCP)-1, IL-8, IL-8 receptor type A, macrophage inflammatory protein (MIP)-1α, MIP-1β, RANTES, eotaxin, eotaxin-2, IL-8, chemokine (cc-motif) receptor (CCR) 1, CCR3, CCR5, chemokine (cxc-motif) receptor (CXCR) 1, and CXCR2 mRNA [48–50]. Furthermore, inflammatory mediators such as cyclooxygenase-2 (COX-2) [51] and nitric oxide (NO) [52] have been implicated in myometrial pathophysiology. The involvement of nuclear factor kappa light chain enhancer of activated B cells (NF-κB) dependent inflammatory pathway has been documented in leiomyoma cells, as EGCG was reported to significantly decrease the expression of NF-κB-dependent pathway genes such as proliferating cell nuclear antigen (PCNA), cyclin-dependent kinase 4, and B-cell lymphoma 2 as well as increase the expression of the proapoptotic B-cell lymphoma 2 associated X in a dose-dependent manner [35]. The above information supports the tenet that the inflammatory response may play an important role to initiate the development of uterine fibroids. Therefore, anti-inflammatory agents could represent pharmacological targets for fibroids.
2.2 Fibrosis
Fibrosis is a pathological feature of many chronic inflammatory diseases. It is defined by the accumulation of excess extracellular matrix (ECM) components. Uterine leiomyomas are typically considered as a fibrotic disorder as they contain 50% more ECM than the corresponding myometrium [53]. The ECM of leiomyomas consists primarily of collagen, fibronectin, and proteoglycans [21, 54–57]. The abnormal ECM structure and orientation found in leiomyomas [21, 54], and alterations in ECM modifies mechanical stresses on resident cells, which leads to activation of internal mechanical signaling and may contribute to leiomyoma growth [58, 59]. The inhibition of fibrosis is a big challenge to control this tumor; therefore, the development of novel antifibrotic agents could represent a tractable approach for medical therapy. Two growth factors from the transforming growth factor-β (TGF-β) superfamily are known to be involved in the accumulation of ECM in leiomyoma. TGF-β increases fibronectin mRNA expression in both myometrial [60] and leiomyoma cells [55, 60]. TGF-β also increases collagen 1A1 [60] and versican [57] mRNA expression in myometrial and leiomyoma cells. Recently, our group demonstrated that activin-A increased fibronectin, collagen 1A1, and versican expression in leiomyoma cells [61]. Furthermore, platelet-derived growth factor (PDGF) also reported to increase collagen α1 (I) in both leiomyoma and myometrial cells [62]. The overproduced ECM itself may play a dynamic role in the metabolic processes leading to tumor growth, by influencing cellular proliferation and differentiation and by serving as a repository for biologically active growth factors, cytokines, chemokines, angiogenic and inflammatory response mediators, and proteases produced by tumor cells.
2.3 Cell proliferation
At least one mechanism responsible for leiomyomas undergoing extensive enlargement is the increased rate of cell proliferation. Uterine cellular proliferation and differentiation are regulated by sex steroids, estrogen, and progesterone. Estrogen has traditionally been identified as the most important sex steroid for fibroid growth; however, progesterone seems to have the dominant steroidal influence on fibroids. This dominance is supported by the increased mitotic rates in fibroids during the secretory phase of the menstrual cycle [63]. There is growing evidence that signaling pathways are directly activated by estrogen and progesterone receptors, and these pathways can also interact with growth factors, cytokines, and chemokine signaling systems to promote proliferation of leiomyomas. Several growth factors such as epidermal growth factor (EGF), heparin-binding EGF, insulin-like growth factor (IGF), and PDGF have been identified, which are responsible for increasing myometrial and/or leiomyoma cell proliferation by activating various signaling pathways [1]. TGF-β exerts bimodal effects on cell proliferation and induces proliferation of cells at low concentrations by stimulating autocrine PDGF secretion, whereas it induces the opposite effect at higher concentrations via downregulation of the PDGF receptor (PDGFR) and by direct growth inhibition [64, 65]. Activin-A and myostatin have cytostatic effects in myometrial cells, but they do not have an antiproliferative effect in leiomyoma cells [61]. Oxidative stress has been shown to be an important player in uterine fibroids [66–68]. Fibroid cells are characterized by a unique nicotinamide adenine dinucleotide phosphate (NADPH) oxidase (NOX, a major source of superoxide and subsequent oxidative stress) profile. Expression of NOX4 increased in fibroid compared to myometrial tissues and cells [66]. In addition, fibroid cells are reported to have significantly lower antioxidant enzymes, superoxide dismutase, and catalase mRNA levels than normal myometrial cells [68]. Furthermore, NOX-derived reactive oxygen species (ROS) have been shown to be a critical component of the mitogen-activated protein kinase (MAPK) pathway of EGF and PDGF signaling in leiomyoma smooth muscle cell (SMC) proliferation [67]. A recent study reported that adipocytes can enhance the proliferation of human leiomyoma cells via TNF-α proinflammatory cytokine [69]. Therefore, an antiproliferative agent could also be useful for the treatment of this tumor.
2.4 Angiogenesis
Angiogenesis plays a critical role in physiological conditions such as embryonic development, reproduction, tissue repair, and bone remodeling. In contrast, angiogenesis is an important event for pathologic processes including primary tumor growth, invasion, and metastases [70, 71]. Angiogenesis is a multistep cellular process that involves endothelial cell (EC) proliferation, migration, tube formation, and ECM degradation [72]. It has been suggested that angiogenesis may play an important role in the regulation of leiomyoma growth [73, 74]. Multiple growth factors involved in angiogenesis are differentially expressed in leiomyoma compared with myometrium. These include vascular endothelial growth factor (VEGF), EGF, heparin-binding EGF, basic fibroblast growth factor, PDGF, activin-A, TGF-β, and adrenomedullin [74, 75]. Therefore, targeting angiogenic growth factors and growth factor receptors to block angiogenesis could represent an attractive therapeutic approach for fibroid treatment.
3 Dietary phytochemicals that have been studied in uterine fibroids
3.1 Epigallocatechin gallate
Dietary sources
EGCG is the ester of epigallocatechin and gallic acid, and is a type of catechin. Mostly, it is found in green tea [Camellia sinensis (L.) Kuntze] [76].
Therapeutic effects
EGCG inhibited the proliferation of human leiomyoma cells and induced apoptosis [35]. EGCG also effectively inhibited proliferation and induced apoptosis in rat ELT-3 (Eker rat-derived uterine leiomyoma cell lines) uterine leiomyoma cells in vitro and in vivo [77]. Interestingly, EGCG dramatically reduced the volume and weight of tumors of female mice (implanted with fibroid tumor cells) at 4 and 8 weeks after the treatment compared to control [77]. Furthermore, it has been reported that dietary supplementation with EGCG reduced the incidence and size of spontaneously occurring leiomyoma of the oviduct in Japanese quail [78]. Recently, a double-blinded, placebo-controlled randomized clinical trial reported that green tea extract (800 mg/day) treatment significantly reduced uterine fibroid volume, fibroid-specific symptom severity, and induced significant improvement in health-related quality of life in premenopausal women compared to the placebo group [36]. In addition, no adverse effects, endometrial hyperplasia, or other endometrial pathology were observed in both group [36].
3.2 Curcumin
Dietary sources
Curcumin is a polyphenol (bis-α, β-unsaturated β-diketone, commonly called diferuloyl-methane) derived from the rhizome of turmeric (Curcuma longa L.) [79].
Therapeutic effects
Curcumin has shown antiproliferative and antifibrotic effects on leiomyoma cells. Experimental data showed that curcumin inhibits uterine leiomyoma cell proliferation via regulation of apoptotic pathway [37]. Importantly, no statistically significant inhibition of growth was observed when patient-matched myometrial cells were exposed to equivalent concentrations of curcumin [37]. Furthermore, curcumin also inhibited expression of fibronectin in leiomyoma cells [37]. Tsuiji and colleagues demonstrated that curcumin significantly inhibited ELT-3 cell proliferation and the authors also found peroxisome proliferator-activated receptor gamma (PPARγ) was expressed in ELT-3 cells and that curcumin acted as a PPARγ ligand. The inhibitory effect of curcumin was attenuated by the treatment of cells with a PPARγ antagonist [80].
3.3 Isoliquiritigenin
Dietary sources
Isoliquiritigenin (4,2′,4′-trihydroxychalcone) is a calchone flavonoid found in licorice (Glycyrrhiza uralensis), shallot (Allium ascalonicum), and soybean (Glycine max) [81].
Therapeutic effects
Isoliquiritigenin has been reported to induce the growth inhibition and apoptosis in human uterine leiomyoma cells [38].
3.4 Genistein
Dietary sources
Genistein (5,7-dihydroxy-3-(4-hydroxyphenyl)chromen-4-one) is an isoflavone found in soybeans (G. max), lupine (Lupinus spp.), fava bean (Vicia faba), kudzu (Pueraria lobata), and psoralea (Psoralea corylifolia) [82].
Therapeutic effects
Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation have been reported [83, 84]. Lower concentrations (≤1 μg/mL) of genistein stimulated proliferation, increased PCNA labeling and the percentage of cells in the S-phase, but this did not occur in uterine SMCs [83]. The stimulatory effect of genistein was possibly mediated by interacting with estrogen receptor-α and IGF-IR [84]. On the other hand, higher concentrations (≥10 μg/mL) of genistein adversely affected the morphology, significantly inhibited proliferation, decreased PCNA labeling, and increased caspase activity and apoptosis in both myometrial and leiomyoma cells [83]. Later, Di and colleagues reported that genistein at more high concentration (50 μg/mL) also downregulated activin A, Smad3, and other TGF-β pathway genes in human uterine leiomyoma cells [84, 85]. Furthermore, it was reported that dietary supplementation (400 or 800 mg of genistein/kg) of genistein reduced the incidence and size of spontaneously occurring leiomyoma of the oviduct in the Japanese quail [86].
3.5 Resveratrol
Dietary sources
Resveratrol (RVS; trans-3,4′,5-trihydroxystilbene) is a polyphenolic phytoalexin produced in plants in response to environmental stress and infection by pathogenic microorganisms. It is found in more than 70 species of plants, including mulberries and peanuts. Grapevines (Vitis vinifera) are the main sources of resveratrol [87].
Therapeutic effects
Resveratrol has shown antiproliferative and antifibrotic effects on leiomyoma cells. Experimental data showed that resveratrol inhibits proliferation, induces apoptosis and cell cycle arrest in human uterine leiomyoma cells in vitro [40, 41]. In addition, resveratrol treatment reduced mRNA and protein expression of collagen types I and III in a dose-dependent manner in human uterine leiomyoma cells [40, 41].
4 Dietary phytochemicals of possible benefit for uterine fibroids
4.1 Allicin
Dietary sources
Allicin (diallylthiosulphinate) is an organosulfur compound obtained from garlic (Allium sativum L.), a species in the family Alliaceae [88].
Anti-inflammatory effect
Allicin has been shown to inhibit the TNF-α induced expression of NO and H2O2 in the human umbilical ECs [89]. Similarly, allicin inhibited spontaneous and TNF-α induced secretion of cytokines and chemokines IL-1β, IL-8 from intestinal epithelial cells [90]. Allicin alleviated inflammatory injury in the spine, possibly via a reduction in secretion of inflammatory factors (IL-6, IL-8, and TNF-α) in a murine model of ankylosing spondylitis [91].
Antifibrotic effect
Allicin protected against cardiac hypertrophy and fibrosis via attenuation of ROS-dependent signaling pathways [92], and through enhancement of Nrf2 antioxidant signaling pathways [93]. Allicin also protected against myocardial fibrosis in streptozotocin-induced diabetic rats by blocking the expression of connective tissue growth factor (CTGF) and TGF-β1 protein [94].
Antiproliferative effect
Allicin has been reported to induce caspase-mediated apoptosis in cervical cancer cells [95]. Allicin also induced apoptosis in gastric cancer cells [96], murine T-lymphocytes [97], colon cancer cells via nuclear factor erythroid 2 related factor 2 (Nrf2) [98], and in human glioblastoma cells through an extracellular signal-regulated kinase (ERK) dependent pathway [99]. Growth inhibition of breast cancer cells by allicin was accompanied by accumulation of cells in the G0/G1 and G2/M phases of the cell cycle [100].
Antiangiogenic effect
Allicin reduced angiogenesis in the aortic ring model as well as basic stages of vessel growth including ECs proliferation and tube formation. These effects were accompanied by downregulation of intracellular actin polymerization and protein kinase B (PKB/AKT) phosphorylation [101].
4.2 Ellagic acid
Dietary sources
Ellagic acid (EA; 2,3,7,8-tetrahydroxychromeno[5,4,3-cde]chromene-5,10-dione) is a polyphenol compound, found in many berries including strawberries, raspberries, cranberries, blackberries, pecans, pomegranates, walnuts, wolfberry, and grapes [102].
Anti-inflammatory effect
EA has been shown to downregulate inflammatory mediators such as IL-1β, IL-6, TNF-α, and MCP-1 mRNA expression in diabetic mice [103]. Additionally, EA decreased COX-2, inducible nitric oxide synthase (iNOS), TNF-α, IL-6, and NF-κB expression in 1,2-dimethylhydrazine-induced colon carcinogenesis [104]. EA also inhibited LPS-induced expression of enzymes COX-2, microsomal prostaglandin E (PGE) synthase-1, and cytosolic phospholipase A2α involved in the synthesis of PGE2 in human monocytes [105]. In the chronic ulcerative colitis model, EA reduced intestinal inflammation, and downregulated COX-2 and iNOS and blocked signaling pathways such as p38 MAPK, NF-κB, and signal transducer and activator of transcription 3 [106].
Antifibrotic effect
EA protected against carbon tetrachloride-induced liver fibrosis [107] and ischemia/reperfusion-induced gastric injury [108]. EA has also been reported to block transformation of pancreatic stellate cells (PSCs) to an activated, myofibroblast-like phenotype. EA inhibited expression of α-smooth muscle actin (α-SMA) and collagen genes, and activation of AP-1 and MAPKs [(ERK, c-Jun N-terminal kinase, and p38 MAPK] in PSCs [109]. Suzuki et al. also reported that EA attenuated pancreatic fibrosis by decreasing collagen content, TGF-β1 expression, and the number of α-SMA-positive cells (activated PSCs) [110].
Antiproliferative effect
EA inhibited PDGF-BB-induced PSCs proliferation [109] and proliferation of primary cultures of rat aortic SMCs [111]. The antiproliferative effect of EA was also mediated by the induction of cell cycle arrest and/or apoptosis in many cancer cell types, including cervical carcinoma cells [112], pancreatic cancer cells [113], ovarian carcinoma cells [114], colon, breast, and prostatic cancer cells [115], and oral carcinoma cells [116]. EA inhibited bladder cancer cell proliferation via p38-MAPK and/or c-Jun medicated caspase-3 activation [117].
Antiangiogenic effect
EA inhibited VEGF-induced phosphorylation of VEGF receptor (VEGFR)-2 in ECs as well as PDGF-induced phosphorylation of PDGFR in SMCs. EA also inhibited VEGF-induced migration of ECs as well as their differentiation into capillary-like tubular structures and abolished PDGF-dependent SMCs migration [118]. EA exerted antiangiogenesis effects via a VEGFR-2 signaling pathway in breast cancer [119]. EA inhibited a series of VEGF-induced angiogenesis processes including proliferation, migration, and tube formation of ECs, and directly inhibited VEGFR-2 tyrosine kinase activity and its downstream signaling pathways, including MAPK and phosphoinositide 3-kinase (PI3K)/AKT in ECs [119].
4.3 Indole-3-carbinol
Dietary sources
Indole-3-carbinol (I3C; 1H-indol-3-ylmethanol) is found in cruciferous vegetables such as broccoli, cabbage, cauliflower, brussels sprouts, bok choy, collard greens, mustard greens, kale, Chinese cabbage, radishes, turnips, kohirabi, arugula, watercress, and daikon [120].
Anti-inflammatory effect
I3C has been shown to be an inhibitor of NF-κB and nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IκBα) kinase activation [121]. I3C also suppressed the production of proinflammatory mediators including TNF-α, IL-1β, IL-6, IL-12, and NO, but increased IL-10 levels in LPS-activated dendritic cells [122]. In addition, I3C suppressed the production of proinflammatory mediators (such as IL-6, IL-1β, TNF-α, IL-10, iNOS, and NO) in macrophages [123–125].
Antifibrotic effect
I3C inhibited hepatic stellate cells proliferation (with or without PDGF-BB stimulation) by blocking the NADPH oxidase/ROS/p38 MAPK pathway. The expression of α-SMA, levels of type I collagen, NOX activity, and ROS were decreased by I3C in this cell type [126].
Antiproliferative effect
I3C inhibited PDGF-BB-induced proliferation of vascular SMCs (VSMCs) by inducing an arrest of cells in both the G0/G1 and S phases [127]. I3C was also reported to suppress the proliferation of a wide variety of tumor cells, including breast [128], prostate [129], colon [130], lung [131], and leukemia [121] by inducing apoptosis and cell cycle arrest.
Antiangiogenic effect
I3C suppressed angiogenesis by inhibiting tube formation and VEGF secretion in ECs [132] and, at least in part, via inactivation of ERK1/2 in human umbilical vein ECs (HUVECs) [133]. Antiangiogenic activity of I3C in ECs stimulated with activated macrophages has also been reported [134].
4.4 Lycopene
Dietary sources
Lycopene is a carotenoid compound naturally found in tomato, watermelon, papaya, pink guava, pink grapefruit, and apricots [135].
Anti-inflammatory effect
Lycopene attenuated LPS-induced TNF-α secretion in macrophages [136] and inhibited NF-κB-mediated IL-8 expression in cigarette smoke-stimulated macrophages [137]. Lycopene also inhibited proinflammatory cytokines (MCP-1, IL-6), and activation Toll-like receptor 4 and its downstream ERK and the NF-κB signaling pathway in HUVECs [138].
Antifibrotic effect
Lycopene inhibited bleomycin-induced pulmonary fibrosis in rats [139], oral submucous [140], and liver fibrosis [141]. It improved cardiac function and myocardial fibrosis after acute myocardial infarction in rats via the modulation of p38 and matrix metalloproteinase (MMP)-9 [142].
Antiproliferative effect
Lycopene has been found to inhibit proliferation of several types of cancer cells by modulating growth factor mediated signaling pathways, inducing apoptosis, and arresting cell cycle. Lycopene suppressed IGF-I-stimulated growth of mammary cancer cells [143]. Similarly, lycopene inhibited PDGF-BB-induced proliferation of SMCs, and markedly inhibited PDGF-BB-induced PDGFR-β, phospholipase C-γ, and ERK1/2 phosphorylation in rat SMCs and primary cultured aortic SMCs [144]. The antiproliferative effect of lycopene in several cancer cells such as human hepatoma Hep3B cells [145], breast and endometrial cancer cells [146], prostate carcinoma cells [147], and colon adenocarcinoma cells [148] are mediated by inducing cell cycle arrest and apoptosis.
Antiangiogenic effect
An inhibitory effect of lycopene on proangiogenic agents, VEGF and TNF-α in HUVEC and rat aortic rings has been reported [149]. Lycopene may inhibit angiogenesis by inhibiting MMP-2 and the urokinase plasminogen activator system through the inhibition of VEGFR2-mediated PI3K-AKT and ERK/p38 signaling pathways [150]. High doses of lycopene reduced tumor growth in nude mice xenotransplanted with the prostate carcinoma cells, partly by decreasing the circulating levels of VEGF [151].
4.5 Quercetin
Dietary sources
Quercetin (3,3′,4′,5,7-pentahydroxyflavone) is a flavonol present in tea, lemon, tomato [152], onion leaves [153], and strawberries [154].
Anti-inflammatory effect
Quercetin attenuated TNF-induced inflammation in hepatic cells by inhibition of the NF-κB signaling pathway [155]. The inhibitory action of quercetin on the MIP-1α-induced inflammatory responses of macrophages was mediated by downregulation of CCR1/CCR5, and inhibition of activation of c-Jun N-terminal kinase, p38 MAPK, and IkB kinase, as well as IκBα degradation [156]. Quercetin also inhibited LPS-induced NO, PGE2, iNOS, COX-2, TNF-α, IL-1β, IL-6, and granulocyte-macrophage colony-stimulating factor mRNA and protein expression in macrophage cells [157]. Quercetin has been shown to inhibit IL-1β-induced production of MMPs, COX-2, and PGE2 by rheumatoid synovial fibroblast [158], and IL-6 and IL-8 mRNA expression in Graves' orbitopathy orbital fibroblasts [159]. Quercetin was also effective in attenuating TNF-α-mediated inflammation and insulin resistance in primary human adipocytes. It attenuated TNF-α-induced expression of inflammatory genes, such as IL-6, IL-1β, IL-8, and MCP-1 and the secretion of IL-6, IL-8, and MCP-1 [160].
Antifibrotic effect
Quercetin possessed antifibrotic properties in hepatic fibrosis [161], pulmonary fibrosis [162], kidney fibroblasts [163]. It suppressed TGF-β-induced collagen production in lung fibroblasts by quercetin-induced heme oxygenase-1 [164]. Additionally, quercetin improved hepatic fibrosis through induction of hematopoietic stem cells apoptosis and downregulation of profibrotic molecules such as TGF-β, collagen 1α, and CTGF [165]. In isoproterenol-treated myocardial tissues, quercetin reduced the overexpression of TGF-β1, CTGF, and excessive deposition of ECM [166]. Quercetin exhibited strong inhibitory effects on collagen and fibronectin production in vitro [167, 168], and TGF-β/Smad-signaling pathway in keloid fibroblasts. Quercetin was shown to improve liver histology and reduce collagen content in rats with carbon tetrachloride-induced cirrhosis in vivo [169].
Antiproliferative effect
Quercetin has been shown to inhibit TGF-α and EGF-induced human prostate cancer cell proliferation [170]. Similarly, quercetin suppressed IGF-1-induced phosphorylation of IGF-1R, insulin receptor substrate-1, AKT, and S6K, and inhibited IGF-1-stimulated proliferation of mouse skin cancer cells [171]. The antiproliferative effect of quercetin was also mediated by induction of apoptosis and/or cell cycle arrest via modulation of multiple signaling pathways [AMP-activated protein kinase, NF-κB, and signal transducer and activator of transcription] in wide variety of cancer cells. These include lung cancer cells [172], breast cancer cells [173], colon cancer cells [174], melanoma cells [175], and chronic lymphocytic leukemia cells [176].
Antiangiogenic effect
Quercetin regulated angiogenesis by downregulating hypoxia-inducible factor 1α and VEGF expression in Dalton's lymphoma ascites induced solid tumors [177]. In HUVECs, quercetin inhibited the expression of VEGFR-2 and tube formation, and suppressed the ERK signaling pathway [178]. Quercetin also inhibited angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways [179]. Furthermore, quercetin was shown to reduce VEGF levels in leukemia cells [180], human ovarian cancer cells [181], and swine granulosa cells [182].
4.6 Sulforaphane
Dietary sources
Sulforaphone (SFN; 1-isothiocyanato-4-methylsulfinylbutane) is an isothiocyanate derived from cruciferous vegetables and is found in especially high levels in broccoli and broccoli sprouts [183]. It is also found in brussels sprouts, cabbage, cauliflower, collard greens, kale, kohlrabi, mustard, rutabaga, turnips, bok choy, and Chinese cabbage [184].
Anti-inflammatory effect
SFN exhibited anti-inflammatory activity by downregulation of iNOS, COX-2, TNF-α, and NF-κB expression in LPS-activated macrophages [185]. SFN suppressed LPS-induced inflammation via Nrf2-dependent pathway in mouse peritoneal macrophages [186]. Also, SFN attenuated inflammation in oxyhemoglobin-induced rat VSMCs by enhancing the activity of the Nrf2-ARE pathway [187].
Antifibrotic effect
SFN attenuated hepatic fibrosis through Nrf2-mediated inhibition of TGF-β/Smad signaling following suppression of hepatic stellate cell activation and fibrogenic gene expression, such as type-I collagen, fibronectin, tissue inhibitor of metalloproteinase-1, and plasminogen activator inhibitor 1 [188]. SFN also prevented diabetes-induced cardiac fibrosis by reducing accumulation of collagen and expression of both CTGF and TGF-β [189]. SFN induced dedifferentiation of human pulmonary fibroblast in vitro from idiopathic pulmonary fibrosis patients via Nrf2 activation, and inhibited TGF-β profibrotic effects in idiopathic pulmonary fibrosis and control fibroblasts [190].
Antiproliferative effect
SFN has been shown to inhibit PDGF-induced proliferation of rat aortic VSMCs via upregulation of p53 leading to G1/S cell cycle arrest [191]. SFN was also reported to induce cell cycle arrest and/or apoptosis in various human cancers cells including breast cancer [192], prostate cancer [193], hepatic cancer [194], colon cancer [195], and bladder cancer cells [196].
Antiangiogenic effect
SFN showed antiangiogenic properties by inhibition of hypoxia-induced mRNA expression of VEGF and two angiogenesis-associated transcription factors, HIP-1α and c-Myc, as well as the expression of the VEGFR-1/2 in HMEC-1 (an immortalized human microvascular EC line) [197]. SFN disrupted microtubule polymerization and prevented mitotic cell cycle progression in bovine aortic ECs and suppressed VEGF-stimulated angiogenesis within Matrigel implants in vivo [198]. Further, SNF inhibited angiogenesis through regulation of forkhead box O transcription factor induced by the inhibition MEK/ERK and PI3K/AKT pathways leading to suppression of cell migration and capillary tube formation in HUVECs [199].
4.7 Ursolic acid
Dietary sources
Ursolic acid (UA; 3-beta-3-hydroxy-urs-12-ene-28-oic-acid, 3-β-hydroxy-urs-12-en-28-oic acid) is a pentacyclic triterpene acid found in apples, basil, cranberries, peppermint, rosemary, oregano, and prunes [200].
Anti-inflammatory effect
UA has been shown to inhibit NF-κB activation [201]. In addition, UA attenuated d-galactose-induced inflammatory response in the mouse prefrontal cortex by downregulation of iNOS and COX-2 expression, and IL-1β, IL-6, and TNF-α level [202]. UA also attenuated LPS-induced cognitive deficits in the mouse by downregulation of proinflammatory markers including COX-2, iNOS, TNF-α, IL-1β, IL-2, and IL-6 production through suppression of p38/NF-κB-mediated inflammatory pathways [203]. Furthermore, UA suppressed ovalbumin-induced airway inflammation by downregulating IL-5, IL-13, and IL-17 in a murine model of allergic asthma [204].
Antifibrotic effect
UA reduced the development of fibrosis (collagen) in the myocardium of diabetic mice through partial inhibition of TGF-β1 expression [205]. UA ameliorated hepatic fibrosis, most likely through specific induction of apoptosis in activated hematopoietic stem cells [206].
Antiproliferative effect
UA has been shown to be an inhibitor of EGF receptor that eventually limits EGF-mediated breast cancer proliferation [207]. UA also suppressed proliferation and induced apoptosis and/or cell cycle arrest in wide variety of cancers such as colon cancer [208], ovarian cancer [209], prostate cancer [210], nonsmall cell lung cancer [211], gastric cancer, liver cancer [212], cervical cancer [213], pancreatic cancer [214], and bladder cancer [215], through modulating multiple signaling pathways (AKT/ERK, COX-2/PGE2, p300/NF-κB/CAMP response element-binding protein 2, and cytochrome c).
Antiangiogenic effect
UA inhibited key steps of angiogenesis in vitro, including EC proliferation, migration, and differentiation [216]. UA also inhibited tumor angiogenesis by the downregulation of VEGF in an Ehrlich ascites carcinoma tumor [217], VEGF-A and basic fibroblast growth factor in colorectal cancer [218], and VEGF in melanoma cells [219].
5 Concluding remarks
Uterine fibroids are extremely common benign tumors, and the condition exacts a significant morbidity on the health of women. Unfortunately, few medical treatments are available for this condition. In this context, dietary phytochemicals could play an important role in the new drug development for leiomyoma treatment. Currently, only EGCG [35], cur-cumin [37], isoliquiritigenin [38], genistein [39], and resveratrol [40, 41] (Table 1) have been tested for therapeutic efficacy for fibroids. Among these, EGCG has shown excellent efficiency to reduce leiomyoma cells proliferation in vitro [35], and to reduce the volume and weight of tumors of female mice (implanted with fibroid tumor cells in vivo [77]). A double-blinded, placebo-controlled randomized clinical trial has shown that green tea extract treatment significantly reduced uterine fibroid volume, fibroid-specific symptom severity, and induced significant improvement in health-related quality of life in premenopausal women compared to the placebo group [36]. In addition, in a case-control study of Italian women, it was shown that the risk of uterine leiomyoma was inversely associated with the intake of green vegetables and fruit [220]. Furthermore, in a prospective cohort study, investigators found that a high intake of fruit, particularly citrus fruit, was inversely associated with uterine fibroids risk among black women [221]. Therefore, considering the key characteristics of leiomyoma development and growth, inflammation, fibrosis, proliferation, and angiogenesis, our aim was to introduce some promising dietary phytochemicals (allicin, EA, I3C, lycopene, quercetin, sulforaphane, and UA) (Fig. 1) that have already shown multiple therapeutic effects in different biological conditions. Throughout this manuscript, we introduced and emphasized the enormous potential of dietary phytochemicals as possible effective therapeutic agents through (i) inhibition of inflammatory mediators; (ii) inhibition of fibrosis by decreasing ECM deposition, profibrotic growth factors expression, inactivation of activated cell types (responsible for myofibroblastic transformation); (iii) inhibition of cell proliferation via the activation of the apoptotic pathway and cell cycle arrest, as well as through inhibition of growth factors and/or their receptors; and (iv) inhibition of angiogenesis by reducing angiogenic growth factors/receptors and angiogenesis-related transcription factors. Based on the available evidence, these compounds modulate and regulate the key biological processes involved in leiomyoma development and growth (Fig. 2). Alone, these phytochemicals are promising, but it is also possible that in combination the therapeutic effects could be additive and the magnitude of the effect could translate to a significant clinical therapy. Lastly, further study of these promising compounds might lead to development of strategies to prevent the condition in women at risk for this extremely common, but debilitating disease.
In this review, dietary phytochemicals have been shown as tumor-fighting weapons. However, greater attention is needed to clarify the following important issues. (i) Poor potency and bioavailability of dietary phytochemicals creates challenges to scientists. However, introducing synthetic analogs of dietary phytochemicals could be a solution for these potency and bioavailability limitations. For example, the potency of synthetic curcumin analog EF24 was shown to be approximately tenfold greater than that of natural curcumin [222]. (ii) Instability of dietary phytochemicals is often associated with pH and/or enzyme-mediated degradation in the upper gut. These can be overcome by formulation approaches such as enteric coating. A major stability factor that is often overlooked is the effect of microbiota in the gastrointestinal tract. The stability of a drug to the microbiota is clinically relevant as drug metabolism can render a drug pharmacologically active, inactive, or toxic. An important example of the significance of metabolism was seen in Japan in 1993 when sorivudine, a promising antiviral drug was introduced into the Japanese market. This was later discovered to be transformed by gut microbiota into (E)-5-(2-bromovinyl)uracil, which can inhibit the metabolism of the anticancer drug 5-fluorouracil leading to toxic levels of this drug [223]. (iii) Although most studies have suggested that dietary phytochemicals kill tumor cells selectively, phytochemicals may have similar effect on normal cells as well [224]. (iv) In many cases, the chemopreventive effects of dietary phytochemicals in cultured cells or tissues are only achievable at supraphysiological concentrations. Such concentrations might not be attained when the phytochemicals are administered as part of diet. (v) The efficacy of most dietary phytochemicals has been tested only in preclinical conditions, either in vitro or in vivo. However, the beneficial effects of dietary phytochemicals in humans are largely unknown. Based on the above facts, it is clear that there is huge need to better understand the efficacy of dietary phytochemicals in the prevention and treatment of uterine fibroids. Future studies should focus on careful and accurate characterization of dietary phytochemicals, better elucidation of the molecular mechanisms involved in their actions, determination of their efficacy by in vivo studies using proper animal models of uterine fibroids, and demonstration of their safety and effectiveness in clinical trials.
Acknowledgments
This work was supported by a grant from the “Fondazione Cassa di Risparmio di Fabriano e Cupramontana” (to M.C. and P.C.) and by Italian Ministry of the University and Research (PRIN 2010–2011, No. 20102CHST5_007, to S.R.G.).
Abbreviations
- AKT/PKB
protein kinase B
- α-SMA
α-smooth muscle actin
- CCR
chemokine (cc-motif) receptor
- COX-2
cyclooxygenase-2
- CTGF
connective tissue growth factor
- CXCR
chemokine (cxc-motif) receptor
- EA
ellagic acid
- ECM
extracellular matrix
- ECs
endothelial cells
- EGCG
epigallocatechin gallate
- EGF
epidermal growth factor
- ERK
extracellular signal-regulated kinase
- GnRHa
gonadotropin-releasing hormone agonist
- HUVECs
human umbilical vein ECs
- I3C
indole-3-carbinol
- IGF
insulin-like growth factor
- IκBα
nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha
- iNOS
inducible nitric oxide synthase
- MAPK
mitogen-activated protein kinase
- MCP
monocyte chemoattractant protein
- MIP
macrophage inflammatory protein
- MMP
matrix metalloproteinase
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor kappa light chain enhancer of activated B cells
- NO
nitric oxide
- NOX
NADPH oxidase
- Nrf2
nuclear factor erythroid 2 related factor 2
- PDGF
platelet-derived growth factor
- PDGFR
PDGF receptor
- PGE
prostaglandin E
- PI3K
phosphoinositide 3-kinase
- PPARγ
peroxisome proliferator-activated receptor gamma
- PSCs
pancreatic stellate cells
- PCNA
proliferating cell nuclear antigen
- ROS
reactive oxygen species
- SFN
sulforaphone
- SMCs
smooth muscle cells
- TGF-β
transforming growth factor-β
- TNF-α
tumor necrosis factor-alpha
- UA
ursolic acid
- VEGF
vascular endothelial growth factor
- VEGFR
vascular endothelial growth factor receptor
- VSMCs
vascular SMCs
Footnotes
The authors have declared no conflict of interest.
References
- 1.Ciarmela P, Islam MS, Reis FM, Gray PC, et al. Growth factors and myometrium: biological effects in uterine fibroid and possible clinical implications. Hum Reprod Update. 2011;17:772–790. doi: 10.1093/humupd/dmr031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walker CL, Stewart EA. Uterine fibroids: the elephant in the room. Science. 2005;308:1589–1592. doi: 10.1126/science.1112063. [DOI] [PubMed] [Google Scholar]
- 3.Islam MS, Protic O, Toti P, Giannubilo SR, et al. Uterine leiomyoma: available medical treatments and new possible therapeutic options. J Clin Endocrinol Metab. 2013;98:921–934. doi: 10.1210/jc.2012-3237. [DOI] [PubMed] [Google Scholar]
- 4.Day Baird D, Dunson DB, Hill MC, Cousins D, et al. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol. 2003;188:100–107. doi: 10.1067/mob.2003.99. [DOI] [PubMed] [Google Scholar]
- 5.Marshall LM, Spiegelman D, Barbieri RL, Goldman MB, et al. Variation in the incidence of uterine leiomyoma among premenopausal women by age and race. Obstet Gynecol. 1997;90:967–973. doi: 10.1016/s0029-7844(97)00534-6. [DOI] [PubMed] [Google Scholar]
- 6.Kjerulff KH, Langenberg P, Seidman JD, Stolley PD, et al. Uterine leiomyomas. Racial differences in severity, symptoms and age at diagnosis. J Reprod Med. 1996;41:483–490. [PubMed] [Google Scholar]
- 7.Moore AB, Flake GP, Swartz CD, Heartwell G, et al. Association of race, age and body mass index with gross pathology of uterine fibroids. J Reprod Med. 2008;53:90–96. [PubMed] [Google Scholar]
- 8.Peddada SD, Laughlin SK, Miner K, Guyon JP, et al. Growth of uterine leiomyomata among premenopausal black and white women. Proc Natl Acad Sci USA. 2008;105:19887–19892. doi: 10.1073/pnas.0808188105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weiss G, Noorhasan D, Schott LL, Powell L, et al. Racial differences in women who have a hysterectomy for benign conditions. Women's Health Issues. 2009;19:202–210. doi: 10.1016/j.whi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stewart EA. Uterine fibroids. Lancet. 2001;357:293–298. doi: 10.1016/S0140-6736(00)03622-9. [DOI] [PubMed] [Google Scholar]
- 11.Farquhar CM, Steiner CA. Hysterectomy rates in the United States 1990–1997. Obstet Gynecol. 2002;99:229–234. doi: 10.1016/s0029-7844(01)01723-9. [DOI] [PubMed] [Google Scholar]
- 12.Cardozo ER, Clark AD, Banks NK, Henne MB, et al. The estimated annual cost of uterine leiomyomata in the United States. Am J Obstet Gynecol. 2012;206(211):e211–e219. doi: 10.1016/j.ajog.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Treloar SA, Do KA, O'Connor VM, O'Connor DT, et al. Predictors of hysterectomy: an Australian study. Am J Obstet Gynecol. 1999;180:945–954. doi: 10.1016/s0002-9378(99)70666-6. [DOI] [PubMed] [Google Scholar]
- 14.Downes E, Sikirica V, Gilabert-Estelles J, Bolge SC, et al. The burden of uterine fibroids in five European countries. Eur J Obstet Gynecol Reprod Biol. 2010;152:96–102. doi: 10.1016/j.ejogrb.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Wegienka G. Are uterine leiomyoma a consequence of a chronically inflammatory immune system? Med Hypotheses. 2012;79:226–231. doi: 10.1016/j.mehy.2012.04.046. [DOI] [PubMed] [Google Scholar]
- 16.Leppert P, Fouany M, Segars JH. In: Understanding Uterine Fibroids—Fibroids. Segars JH, editor. John Wiley & Sons, Ltd; Oxford: 2013. pp. 1–10. [Google Scholar]
- 17.Chegini N. Proinflammatory and profibrotic mediators: principal effectors of leiomyoma development as a fibrotic disorder. Semin Reprod Med. 2010;28:180–203. doi: 10.1055/s-0030-1251476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marsh EE, Bulun SE. Steroid hormones and leiomyomas. Obstet Gynecol Clin North Am. 2006;33:59–67. doi: 10.1016/j.ogc.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 19.Rein MS, Barbieri RL, Friedman AJ. Progesterone: a critical role in the pathogenesis of uterine myomas. Am J Obstet Gynecol. 1995;172:14–18. doi: 10.1016/0002-9378(95)90077-2. [DOI] [PubMed] [Google Scholar]
- 20.Englund K, Blanck A, Gustavsson I, Lundkvist U, et al. Sex steroid receptors in human myometrium and fibroids: changes during the menstrual cycle and gonadotropin-releasing hormone treatment. J Clin Endocrinol Metab. 1998;83:4092–4096. doi: 10.1210/jcem.83.11.5287. [DOI] [PubMed] [Google Scholar]
- 21.Islam MS, Protic O, Stortoni P, Grechi G, et al. Complex networks of multiple factors in the pathogenesis of uterine leiomyoma. Fertil Steril. 2013;100:178–193. doi: 10.1016/j.fertnstert.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 22.Friedman AJ, Rein MS, Harrison-Atlas D, Garfield JM, et al. A randomized, placebo-controlled, double-blind study evaluating leuprolide acetate depot treatment before myomectomy. Fertil Steril. 1989;52:728–733. [PubMed] [Google Scholar]
- 23.Andreyko JL, Blumenfeld Z, Marshall LA, Monroe SE, et al. Use of an agonistic analog of gonadotropin-releasing hormone (nafarelin) to treat leiomyomas: assessment by magnetic resonance imaging. Am J Obstet Gynecol. 1988;158:903–910. doi: 10.1016/0002-9378(88)90092-0. [DOI] [PubMed] [Google Scholar]
- 24.Leather AT, Studd JWW, Watson NR, Holland EFN. The prevention of bone loss in young women treated with GnRH analogues with “add-back” estrogen therapy. Obstet Gynecol. 1993;81:104–107. [PubMed] [Google Scholar]
- 25.Friedman AJ, Hoffman DI, Comite F, Browneller RW, et al. Treatment of leiomyomata uteri with leuprolide acetate depot: a double-blind, placebo-controlled, multicenter study. The Leuprolide Study Group. Obstet Gynecol. 1991;77:720–725. [PubMed] [Google Scholar]
- 26.Stovall TG, Muneyyirci-Delale O, Summitt RL, Jr, Scialli AR. GnRH agonist and iron versus placebo and iron in the anemic patient before surgery for leiomyomas: a randomized controlled trial. The Leuprolide Study Group. Obstet Gynecol. 1995;86:65–71. doi: 10.1016/0029-7844(95)00102-w. [DOI] [PubMed] [Google Scholar]
- 27.Carbonell Esteve JL, Acosta R, Heredia B, Pérez Y, et al. Mifepristone for the treatment of uterine leiomyomas: a randomized controlled trial. Obstet Gynecol. 2008;112:1029–1036. doi: 10.1097/AOG.0b013e31818aa930. [DOI] [PubMed] [Google Scholar]
- 28.Chwalisz K, Larsen L, Mattia-Goldberg C, Edmonds A, et al. A randomized, controlled trial of asoprisnil, a novel selective progesterone receptor modulator, in women with uterine leiomyomata. Fertil Steril. 2007;87:1399–1412. doi: 10.1016/j.fertnstert.2006.11.094. [DOI] [PubMed] [Google Scholar]
- 29.Donnez J, Tomaszewski J, Vázquez F, Bouchard P, et al. Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366:421–432. doi: 10.1056/NEJMoa1103180. [DOI] [PubMed] [Google Scholar]
- 30.Donnez J, Tatarchuk TF, Bouchard P, Puscasiu L, et al. Ulipristal acetate versus placebo for fibroid treatment before surgery. N Engl J Med. 2012;366:409–420. doi: 10.1056/NEJMoa1103182. [DOI] [PubMed] [Google Scholar]
- 31.Wiehle RD, Goldberg J, Brodniewicz T, Jarus-Dziedzic K, et al. Effects of a new progesterone receptor modulator, CDB-4124, on fibroid size and uterine bleeding. US Obstet Gynecol. 2008;3:17–20. [Google Scholar]
- 32.Newman DJ, Cragg GM. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J Nat Prod. 2012;75:311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen NN, Han M, Yang H, Yang GY, et al. Chinese herbal medicine Guizhi Fuling Formula for treatment of uterine fibroids: a systematic review of randomised clinical trials. BMC Complement Altern Med. 2014;14:2. doi: 10.1186/1472-6882-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu JP, Yang H, Xia Y, Cardini F. Herbal preparations for uterine fibroids. Cochrane Database Syst Rev. 2009;4:CD005292. doi: 10.1002/14651858.CD005292.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang D, Al-Hendy M, Richard-Davis G, Montgomery-Rice V, et al. Antiproliferative and proapoptotic effects of epigallocatechin gallate on human leiomyoma cells. Fertil Steril. 2010;94:1887–1893. doi: 10.1016/j.fertnstert.2009.08.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roshdy E, Rajaratnam V, Maitra S, Sabry M, et al. Treatment of symptomatic uterine fibroids with green tea extract: a pilot randomized controlled clinical study. Int J Women's Health. 2013;5:477–486. doi: 10.2147/IJWH.S41021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Malik M, Mendoza M, Payson M, Catherino WH. Cur-cumin, a nutritional supplement with antineoplastic activity, enhances leiomyoma cell apoptosis and decreases fibronectin expression. Fertil Steril. 2009;91:2177–2184. doi: 10.1016/j.fertnstert.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 38.Kim D, Ramachandran S, Baek S, Kwon SH, et al. Induction of growth inhibition and apoptosis in human uterine leiomyoma cells by isoliquiritigenin. Reprod Sci. 2008;15:552. doi: 10.1177/1933719107312681. [DOI] [PubMed] [Google Scholar]
- 39.Shushan A, Ben-Bassat H, Mishani E, Laufer N, et al. Inhibition of leiomyoma cell proliferation in vitro by genistein and the protein tyrosine kinase inhibitor TKS050. Fertil Steril. 2007;87:127–135. doi: 10.1016/j.fertnstert.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 40.Catherino WH, Parrott E, Segars J. Proceedings from the National Institute of Child Health and Human Development Conference on the Uterine Fibroid Research Update Workshop. Fertil Steril. 2011;95:9–12. doi: 10.1016/j.fertnstert.2010.08.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Christman GM, Marsh CA, Campbell EJ. In: Counseling the Patient with Uterine Fibroids— Fibroids. Segars JH, editor. John Wiley & Sons; Oxford: 2012. pp. 134–144. [Google Scholar]
- 42.Hatthachote P, Gillespie JI. Complex interactions between sex steroids and cytokines in the human pregnant myometrium: evidence for an autocrine signaling system at term. Endocrinology. 1999;140:2533–2540. doi: 10.1210/endo.140.6.6785. [DOI] [PubMed] [Google Scholar]
- 43.Litovkin KV, Domenyuk VP, Bubnov VV, Zaporozhan VN. Interleukin-6-174G/C polymorphism in breast cancer and uterine leiomyoma patients: a population-based case control study. Exp Oncol. 2007;29:295–298. [PubMed] [Google Scholar]
- 44.Luo X, Ding L, Xu J, Chegini N. Gene expression profiling of leiomyoma and myometrial smooth muscle cells in response to transforming growth factor-beta. Endocrinology. 2005;146:1097–1118. doi: 10.1210/en.2004-1377. [DOI] [PubMed] [Google Scholar]
- 45.Kurachi O, Matsuo H, Samoto T, Maruo T. Tumor necrosis factor-α expression in human uterine leiomyoma and its down-regulation by progesterone. J Clin Endocrinol Metab. 2001;86:2275–2280. doi: 10.1210/jcem.86.5.7469. [DOI] [PubMed] [Google Scholar]
- 46.Chegini N, Tang XM, Ma C. Regulation of transforming growth factor-beta1 expression by granulocyte macrophage-colony-stimulating factor in leiomyoma and myometrial smooth muscle cells. J Clin Endocrinol Metab. 1999;84:4138–4143. doi: 10.1210/jcem.84.11.6147. [DOI] [PubMed] [Google Scholar]
- 47.Santulli P, Even M, Chouzenoux S, Millischer AE, et al. Profibrotic interleukin-33 is correlated with uterine leiomyoma tumour burden. Hum Reprod. 2013;28:2126–2133. doi: 10.1093/humrep/det238. [DOI] [PubMed] [Google Scholar]
- 48.Sozen I, Olive DL, Arici A. Expression and hormonal regulation of monocyte chemotactic protein-1 in myometrium and leiomyomata. Fertil Steril. 1998;69:1095–1102. doi: 10.1016/s0015-0282(98)00072-7. [DOI] [PubMed] [Google Scholar]
- 49.Senturk LM, Sozen I, Gutierrez L, Arici A. Interleukin 8 production and interleukin 8 receptor expression in human myometrium and leiomyoma. Am J Obstet Gynecol. 2001;184:559–566. doi: 10.1067/mob.2001.111160. [DOI] [PubMed] [Google Scholar]
- 50.Syssoev KA, Kulagina NV, Chukhlovin AB, Morozova EB, et al. Expression of mRNA for chemokines and chemokine receptors in tissues of the myometrium and uterine leiomyoma. Bull Exp Biol Med. 2008;145:84–89. doi: 10.1007/s10517-008-0038-1. [DOI] [PubMed] [Google Scholar]
- 51.Cesen-Cummings K, Houston KD, Copland JA, Moorman VJ, et al. Uterine leiomyomas express myometrial contractile-associated proteins involved in pregnancy-related hormone signaling. J Soc Gynecol Investig. 2003;10:11–20. [PubMed] [Google Scholar]
- 52.Khorram O, Garthwaite M, Magness RR. Endometrial and myometrial expression of nitric oxide synthase isoforms in pre-and postmenopausal women. J Clin Endocrinol Metab. 1999;84:2226–2232. doi: 10.1210/jcem.84.6.5759. [DOI] [PubMed] [Google Scholar]
- 53.Fujita M. Histological and biochemical studies of collagen in human uterine leiomyomas. Hokkaido Igaku Zasshi. 1985;60:602–615. [PubMed] [Google Scholar]
- 54.Leppert PC, Baginski T, Prupas C, Catherino WH, et al. Comparative ultrastructure of collagen fibrils in uterine leiomyomas and normal myometrium. Fertil Steril. 2004;82(Suppl 3):1182–1187. doi: 10.1016/j.fertnstert.2004.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Arici A, Sozen I. Transforming growth factor-beta3 is expressed at high levels in leiomyoma where it stimulates fibronectin expression and cell proliferation. Fertil Steril. 2000;73:1006–1011. doi: 10.1016/s0015-0282(00)00418-0. [DOI] [PubMed] [Google Scholar]
- 56.Stewart EA, Friedman AJ, Peck K, Nowak RA. Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle. J Clin Endocrinol Metab. 1994;79:900–906. doi: 10.1210/jcem.79.3.8077380. [DOI] [PubMed] [Google Scholar]
- 57.Norian JM, Malik M, Parker CY, Joseph D, et al. Transforming growth factor beta3 regulates the versican variants in the extracellular matrix-rich uterine leiomyomas. Reprod Sci. 2009;16:1153–1164. doi: 10.1177/1933719109343310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rogers R, Norian J, Malik M, Christman G, et al. Mechanical homeostasis is altered in uterine leiomyoma. Am J Obstet Gynecol. 2008;198:474.e471–474.e411. doi: 10.1016/j.ajog.2007.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Norian JM, Owen CM, Taboas J, Korecki C, et al. Characterization of tissue biomechanics and mechanical signaling in uterine leiomyoma. Matrix Biol. 2012;31:57–65. doi: 10.1016/j.matbio.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joseph DS, Malik M, Nurudeen S, Catherino WH. Myometrial cells undergo fibrotic transformation under the influence of transforming growth factor beta-3. Fertil Steril. 2010;93:1500–1508. doi: 10.1016/j.fertnstert.2009.01.081. [DOI] [PubMed] [Google Scholar]
- 61.Islam MS, Catherino WH, Protic O, Janjusevic M, et al. Role of activin-A and myostatin and their signaling pathway in human myometrial and leiomyoma cell function. J Clin Endocrinol Metab. 2014;99:775–785. doi: 10.1210/jc.2013-2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liang M, Wang H, Zhang Y, Lu S, Wang Z. Expression and functional analysis of platelet-derived growth factor in uterine leiomyomata. Cancer Biol Ther. 2006;5:28–33. doi: 10.4161/cbt.5.1.2234. [DOI] [PubMed] [Google Scholar]
- 63.Kawaguchi K, Fujii S, Konishi I, Nanbu Y, et al. Mitotic activity in uterine leiomyomas during the menstrual cycle. Am J Obstet Gynecol. 1989;160:637–641. doi: 10.1016/s0002-9378(89)80046-8. [DOI] [PubMed] [Google Scholar]
- 64.Wolanska M, Bankowski E. Transforming growth factor beta and platelet-derived growth factor in human myometrium and in uterine leiomyomas at various stages of tumour growth. Eur J Obstet Gynecol Reprod Biol. 2007;130:238–244. doi: 10.1016/j.ejogrb.2006.01.034. [DOI] [PubMed] [Google Scholar]
- 65.Arici A, Sozen I. Expression, menstrual cycle-dependent activation, and bimodal mitogenic effect of transforming growth factor-beta1 in human myometrium and leiomyoma. Am J Obstet Gynecol. 2003;188:76–83. doi: 10.1067/mob.2003.118. [DOI] [PubMed] [Google Scholar]
- 66.Fletcher NM, Saed MG, Abuanzeh S, Abu-Soud HM, et al. Nicotinamide adenine dinucleotide phosphate oxidase is differentially regulated in normal myometrium versus leiomyoma. Reprod Sci. 2014 doi: 10.1177/1933719114522552. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mesquita FS, Dyer SN, Heinrich DA, Bulun SE, et al. Reactive oxygen species mediate mitogenic growth factor signaling pathways in human leiomyoma smooth muscle cells. Biol Reprod. 2010;82:341–351. doi: 10.1095/biolreprod.108.075887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fletcher NM, Saed MG, Abu-Soud HM, Al-Hendy A, et al. Uterine fibroids are characterized by an impaired antioxidant cellular system: potential role of hypoxia in the pathophysiology of uterine fibroids. J Assist Reprod Genet. 2014;30:969–974. doi: 10.1007/s10815-013-0029-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nair S, Al-Hendy A. Adipocytes enhance the proliferation of human leiomyoma cells via TNF-α proinflammatory cytokine. Reprod Sci. 2011;18:1186–1192. doi: 10.1177/1933719111408111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 71.Imhof BA, Aurrand-Lions M. Angiogenesis and inflammation face off. Nat Med. 2006;12:171–172. doi: 10.1038/nm0206-171. [DOI] [PubMed] [Google Scholar]
- 72.Chung AS, Lee J, Ferrara N. Targeting the tumour vasculature: insights from physiological angiogenesis. Nat Rev Cancer. 2010;10:505–514. doi: 10.1038/nrc2868. [DOI] [PubMed] [Google Scholar]
- 73.Fleischer R, Weston GC, Vollenhoven BJ, Rogers PAW. Pathophysiology of fibroid disease: angiogenesis and regulation of smooth muscle proliferation. Best Pract Res Clin Obstet Gynaecol. 2008;22:603–614. doi: 10.1016/j.bpobgyn.2008.01.005. [DOI] [PubMed] [Google Scholar]
- 74.Tal R, Segars JH. The role of angiogenic factors in fibroid pathogenesis: potential implications for future therapy. Hum Reprod Update. 2013;20:194–216. doi: 10.1093/humupd/dmt042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ciarmela P, Carrarelli P, Islam MS, Janjusevic M, et al. Effect of ulipristal acetate on activin A expression and functions in myometrial and leiomyoma cells. Reprod Sci. 2014 doi: 10.1177/1933719114542019. [DOI] [PubMed] [Google Scholar]
- 76.Singh BN, Shankar S, Srivastava RK. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem Pharmacol. 2011;82:1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang D, Al-Hendy M, Richard-Davis G, Montgomery-Rice V, et al. Green tea extract inhibits proliferation of uterine leiomyoma cells in vitro and in nude mice. Am J Obstet Gynecol. 2010;202:289.e281–289.e289. doi: 10.1016/j.ajog.2009.10.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozercan IH, Sahin N, Akdemir F, Onderci M, et al. Chemoprevention of fibroid tumors by [-]-epigallocatechin-3-gallate in quail. Nutr Res. 2008;28:92–97. doi: 10.1016/j.nutres.2007.11.009. [DOI] [PubMed] [Google Scholar]
- 79.Wilken R, Veena MS, Wang MB, Srivatsan ES. Curcumin: a review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tsuiji K, Takeda T, Li B, Wakabayashi A, et al. Inhibitory effect of curcumin on uterine leiomyoma cell proliferation. Gynecol Endocrinol. 2011;27:512–517. doi: 10.3109/09513590.2010.507287. [DOI] [PubMed] [Google Scholar]
- 81.Cuendet M, Guo J, Luo Y, Chen S, et al. Cancer chemo-preventive activity and metabolism of isoliquiritigenin, a compound found in licorice. Cancer Prev Res. 2010;3:221–232. doi: 10.1158/1940-6207.CAPR-09-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kaufman PB, Duke JA, Brielmann H, Boik J, et al. A comparative survey of leguminous plants as sources of the isoflavones, genistein and daidzein: implications for human nutrition and health. J Altern Complement Med. 1997;3:7–12. doi: 10.1089/acm.1997.3.7. [DOI] [PubMed] [Google Scholar]
- 83.Moore AB, Castro L, Yu L, Zheng X, et al. Stimulatory and inhibitory effects of genistein on human uterine leiomyoma cell proliferation are influenced by the concentration. Hum Reprod. 2007;22:2623–2631. doi: 10.1093/humrep/dem185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Di X, Yu L, Moore AB, Castro L, et al. A low concentration of genistein induces estrogen receptor-alpha and insulin-like growth factor-I receptor interactions and proliferation in uterine leiomyoma cells. Hum Reprod. 2008;23:1873–1883. doi: 10.1093/humrep/den087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Di X, Andrews DMK, Tucker CJ, Yu L, et al. A high concentration of genistein down-regulates activin A, Smad3 and other TGF-β pathway genes in human uterine leiomyoma cells. Exp Mol Med. 2012;44:281–292. doi: 10.3858/emm.2012.44.4.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sahin K, Akdemir F, Tuzcu M, Sahin N, et al. Genistein suppresses spontaneous oviduct tumorigenesis in Quail. Nutr Cancer. 2009;61:799–806. doi: 10.1080/01635580903285163. [DOI] [PubMed] [Google Scholar]
- 87.Labinskyy N, Csiszar A, Veress G, Stef G, et al. Vascular dysfunction in aging: potential effects of resveratrol, an anti-inflammatory phytoestrogen. Curr Med Chem. 2006;13:989. doi: 10.2174/092986706776360987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Block E. The chemistry of garlic and onions. Sci Am. 1985;252:114–119. doi: 10.1038/scientificamerican0385-114. [DOI] [PubMed] [Google Scholar]
- 89.Mo SJ, Son EW, Rhee DK, Pyo S. Modulation of TNF-α-induced ICAM-1 expression, NO and H2O2 production by alginate, allicin and ascorbic acid in human endothelial cells. Arch Pharm Res. 2003;26:244–251. doi: 10.1007/BF02976837. [DOI] [PubMed] [Google Scholar]
- 90.Lang A, Lahav M, Sakhnini E, Barshack I, et al. Allicin inhibits spontaneous and TNF-alpha induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clin Nutr. 2004;23:1199–1208. doi: 10.1016/j.clnu.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 91.Gu X, Wu H, Fu P. Allicin attenuates inflammation and suppresses HLA-B27 protein expression in ankylosing spondylitis mice. J Biomed Biotechnol. 2013;2013:171573. doi: 10.1155/2013/171573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Liu C, Cao F, Tang QZ, Yan L, et al. Allicin protects against cardiac hypertrophy and fibrosis via attenuating reactive oxygen species-dependent signaling pathways. J Nutr Biochem. 2010;21:1238–1250. doi: 10.1016/j.jnutbio.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 93.Li XH, Li CY, Xiang ZG, Hu JJ, et al. Allicin ameliorates cardiac hypertrophy and fibrosis through enhancing of Nrf2 antioxidant signaling pathways. Cardiovasc Drugs Ther. 2012;26:457–465. doi: 10.1007/s10557-012-6415-z. [DOI] [PubMed] [Google Scholar]
- 94.Liu Y, Qi H, Wang Y, Wu M, et al. Allicin protects against myocardial apoptosis and fibrosis in streptozotocin-induced diabetic rats. Phytomedicine. 2012;19:693–698. doi: 10.1016/j.phymed.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 95.Oommen S, Anto RJ, Srinivas G, Karunagaran D. Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. Eur J Pharmacol. 2004;485:97–103. doi: 10.1016/j.ejphar.2003.11.059. [DOI] [PubMed] [Google Scholar]
- 96.Zhang W, Ha M, Gong Y, Xu Y, et al. Allicin induces apoptosis in gastric cancer cells through activation of both extrinsic and intrinsic pathways. Oncol Rep. 2010;24:1585–1592. doi: 10.3892/or_00001021. [DOI] [PubMed] [Google Scholar]
- 97.Wang Z, Liu Z, Cao Z, Li L. Allicin induces apoptosis in EL-4 cells in vitro by activation of expression of caspase-3 and-12 and up-regulation of the ratio of Bax/Bcl-2. Nat Prod Res. 2012;26:1033–1037. doi: 10.1080/14786419.2010.550894. [DOI] [PubMed] [Google Scholar]
- 98.Bat-Chen W, Golan T, Peri I, Ludmer Z, et al. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr Cancer. 2010;62:947–957. doi: 10.1080/01635581.2010.509837. [DOI] [PubMed] [Google Scholar]
- 99.Cha JH, Choi YJ, Cha SH, Choi CH, et al. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway. Oncol Rep. 2012;28:41–48. doi: 10.3892/or.2012.1772. [DOI] [PubMed] [Google Scholar]
- 100.Hirsch K, Danilenko M, Giat J, Miron T, et al. Effect of purified allicin, the major ingredient of freshly crushed garlic, on cancer cell proliferation. Nutr Cancer. 2000;38:245–254. doi: 10.1207/S15327914NC382_14. [DOI] [PubMed] [Google Scholar]
- 101.Sela U, Brill A, Kalchenko V, Dashevsky O, et al. Allicin inhibits blood vessel growth and downregulates Akt phosphorylation and actin polymerization. Nutr Cancer. 2008;60:412–420. doi: 10.1080/01635580701733083. [DOI] [PubMed] [Google Scholar]
- 102.Vattem DA, Shetty K. Biological functionality of ellagic acid: a review. J Food Biochem. 2005;29:234–266. [Google Scholar]
- 103.Chao PC, Hsu CC, Yin MC. Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice. Nutr Metab. 2009;6:33. doi: 10.1186/1743-7075-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Umesalma S, Sudhandiran G. Differential inhibitory effects of the polyphenol ellagic acid on inflammatory mediators NF-kappaB, iNOS, COX-2, TNF-alpha, IL-6 in 1,2-dimethylhydrazine-induced rat colon carcinogenesis. Basic Clin Pharmacol Toxicol. 2010;107:650–655. doi: 10.1111/j.1742-7843.2010.00565.x. [DOI] [PubMed] [Google Scholar]
- 105.Karlsson S, Nånberg E, Fjaeraa C, Wijkander J. Ellagic acid inhibits lipopolysaccharide-induced expression of enzymes involved in the synthesis of prostaglandin E2 in human monocytes. Br J Nutr. 2010;103:1102. doi: 10.1017/S0007114509992935. [DOI] [PubMed] [Google Scholar]
- 106.Marín M, María Giner R, Ríos JL, Carmen Recio M. Intestinal anti-inflammatory activity of ellagic acid in the acute and chronic dextrane sulfate sodium models of mice colitis. J Ethnopharmacol. 2013;150:925–934. doi: 10.1016/j.jep.2013.09.030. [DOI] [PubMed] [Google Scholar]
- 107.Thresiamma KC, Kuttan R. Inhibition of liver fibrosis by ellagic acid. Indian J Physiol Pharmacol. 1996;40:363–366. [PubMed] [Google Scholar]
- 108.Iino T, Tashima K, Umeda M, Ogawa Y, et al. Effect of ellagic acid on gastric damage induced in ischemic rat stomachs following ammonia or reperfusion. Life Sci. 2002;70:1139–1150. doi: 10.1016/s0024-3205(01)01493-x. [DOI] [PubMed] [Google Scholar]
- 109.Masamune A, Satoh M, Kikuta K, Suzuki N, et al. Ellagic acid blocks activation of pancreatic stellate cells. Biochem Pharmacol. 2005;70:869–878. doi: 10.1016/j.bcp.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 110.Suzuki N, Masamune A, Kikuta K, Watanabe T, et al. Ellagic acid inhibits pancreatic fibrosis in male Wistar Bonn/Kobori rats. Dig Dis Sci. 2009;54:802–810. doi: 10.1007/s10620-008-0423-7. [DOI] [PubMed] [Google Scholar]
- 111.Rani PU, Kesavan R, Ganugula R, Kumar PU, et al. Ellagic acid inhibits PDGF-BB-induced vascular smooth muscle cell proliferation and prevents atheroma formation in streptozotocin-induced diabetic rats. J Nutr Biochem. 2013;24:1830–1839. doi: 10.1016/j.jnutbio.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 112.Narayanan BA, Geoffroy O, Willingham MC, Re GG, et al. p53/p21 (WAF1/CIP1) expression and its possible role in G1 arrest and apoptosis in ellagic acid treated cancer cells. Cancer Lett. 1999;136:215–221. doi: 10.1016/s0304-3835(98)00323-1. [DOI] [PubMed] [Google Scholar]
- 113.Edderkaoui M, Lugea A, Hui H, Eibl G, et al. Ellagic acid and embelin affect key cellular components of pancreatic adenocarcinoma, cancer, stellate cells. Nutr Cancer. 2013;65:1232–1244. doi: 10.1080/01635581.2013.832779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chung YC, Lu LC, Tsai MH, Chen YJ, et al. The inhibitory effect of ellagic acid on cell growth of ovarian carcinoma cells. Evid Based Complement Alternat Med. 2013;2013:306705. doi: 10.1155/2013/306705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Losso JN, Bansode RR, Trappey Ii A, Bawadi HA, et al. In vitro anti-proliferative activities of ellagic acid. J Nutr Biochem. 2004;15:672–678. doi: 10.1016/j.jnutbio.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 116.Weisburg JH, Schuck AG, Reiss SE, Wolf BJ, et al. Ellagic acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells. Anticancer Res. 2013;33:1829–1836. [PubMed] [Google Scholar]
- 117.Qiu Z, Zhou B, Jin L, Yu H, et al. In vitro antioxidant and antiproliferative effects of ellagic acid and its colonic metabolite, urolithins, on human bladder cancer T24 cells. Food Chem Toxicol. 2013;59:428–437. doi: 10.1016/j.fct.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 118.Labrecque L, Lamy S, Chapus Al, Mihoubi S, et al. Combined inhibition of PDGF and VEGF receptors by ellagic acid, a dietary-derived phenolic compound. Carcinogenesis. 2005;26:821–826. doi: 10.1093/carcin/bgi024. [DOI] [PubMed] [Google Scholar]
- 119.Wang N, Wang ZY, Mo SL, Loo TY, et al. Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat. 2012;134:943–955. doi: 10.1007/s10549-012-1977-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Aggarwal BB, Ichikawa H. Molecular targets and anticancer potential of indole-3-carbinol and its derivatives. Cell Cycle. 2005;4:1201–1215. doi: 10.4161/cc.4.9.1993. [DOI] [PubMed] [Google Scholar]
- 121.Takada Y, Andreeff M, Aggarwal BB. Indole-3-carbinol suppresses NF-κB and κBα kinase activation, causing inhibition of expression of NF-κB-regulated antiapoptotic and metastatic gene products and enhancement of apoptosis in myeloid and leukemia cells. Blood. 2005;106:641–649. doi: 10.1182/blood-2004-12-4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Benson JM, Shepherd DM. Dietary ligands of the aryl hydrocarbon receptor induce anti-inflammatory and immunoregulatory effects on murine dendritic cells. Toxicol Sci. 2013;124:327–338. doi: 10.1093/toxsci/kfr249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Chen YH, Dai HJ, Chang HP. Suppression of inducible nitric oxide production by indole and isothiocyanate derivatives from Brassica plants in stimulated macrophages. Planta Med. 2003;69:696–700. doi: 10.1055/s-2003-42790. [DOI] [PubMed] [Google Scholar]
- 124.Tsai JT, Liu HC, Chen YH. Suppression of inflammatory mediators by cruciferous vegetable-derived indole-3-carbinol and phenylethyl isothiocyanate in lipopolysaccharide-activated macrophages. Mediators Inflamm. 2010;2010:293642. doi: 10.1155/2010/293642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jiang J, Kang TB, Shim DW, Oh NH, et al. Indole-3-carbinol inhibits LPS-induced inflammatory response by blocking TRIF-dependent signaling pathway in macrophages. Food Chem Toxicol. 2013;57:256–261. doi: 10.1016/j.fct.2013.03.040. [DOI] [PubMed] [Google Scholar]
- 126.Ping J, Li Jt, Liao Zx, Shang L, et al. Indole-3-carbinol inhibits hepatic stellate cells proliferation by blocking NADPH oxidase/reactive oxygen species/p38 MAPK pathway. Eur J Pharmacol. 2011;650:656–662. doi: 10.1016/j.ejphar.2010.10.057. [DOI] [PubMed] [Google Scholar]
- 127.Guan H, Chen C, Zhu L, Cui C, et al. Indole-3-carbinol blocks platelet-derived growth factor-stimulated vascular smooth muscle cell function and reduces neointima formation in vivo. J Nutr Biochem. 2012;24:62–69. doi: 10.1016/j.jnutbio.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 128.Marconett CN, Singhal AK, Sundar SN, Firestone GL. Indole-3-carbinol disrupts estrogen receptor-alpha dependent expression of insulin-like growth factor-1 receptor and insulin receptor substrate-1 and proliferation of human breast cancer cells. Mol Cell Endocrinol. 2012;363:74–84. doi: 10.1016/j.mce.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhang J, Hsu BA, Jocelyn C, Kinseth BA, et al. Indole 3 carbinol induces a G1 cell cycle arrest and inhibits prostate specific antigen production in human LNCaP prostate carcinoma cells. Cancer. 2003;98:2511–2520. doi: 10.1002/cncr.11844. [DOI] [PubMed] [Google Scholar]
- 130.Suzui M, Inamine M, Kaneshiro T, Morioka T, et al. Indole-3-carbinol inhibits the growth of human colon carcinoma cells but enhances the tumor multiplicity and volume of azoxymethane-induced rat colon carcinogenesis. Int J Oncol. 2005;27:1391–1399. [PubMed] [Google Scholar]
- 131.Choi HS, Cho MC, Lee HG, Yoon DY. Indole-3-carbinol induces apoptosis through p53 and activation of caspase-8 pathway in lung cancer A549 cells. Food Chem Toxcol. 2010;48:883–890. doi: 10.1016/j.fct.2009.12.028. [DOI] [PubMed] [Google Scholar]
- 132.Wu HT, Lin SH, Chen YH. Inhibition of cell proliferation and in vitro markers of angiogenesis by indole-3-carbinol, a major indole metabolite present in cruciferous vegetables. J Agric Food Chem. 2005;53:5164–5169. doi: 10.1021/jf050034w. [DOI] [PubMed] [Google Scholar]
- 133.Kunimasa K, Kobayashi T, Kaji K, Ohta T. Antiangiogenic effects of indole-3-carbinol and 3, 3′-diindolylmethane are associated with their differential regulation of ERK1/2 and Akt in tube-forming HUVEC. J Nutr. 2010;140:1–6. doi: 10.3945/jn.109.112359. [DOI] [PubMed] [Google Scholar]
- 134.Wang ML, Shih CK, Chang HP, Chen YH. Antiangiogenic activity of indole-3-carbinol in endothelial cells stimulated with activated macrophages. Food Chem. 2012;134:811–820. doi: 10.1016/j.foodchem.2012.02.185. [DOI] [PubMed] [Google Scholar]
- 135.Holzapfel NP, Holzapfel BM, Champ S, Feldthusen J, et al. The potential role of lycopene for the prevention and therapy of prostate cancer: from molecular mechanisms to clinical evidence. Int J Mol Sci. 2013;14:14620–14646. doi: 10.3390/ijms140714620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Marcotorchino J, Romier B, Gouranton E, Riollet C, et al. Lycopene attenuates LPS-induced TNF-α secretion in macrophages and inflammatory markers in adipocytes exposed to macrophage-conditioned media. Mol Nutr Food Res. 2010;56:725–732. doi: 10.1002/mnfr.201100623. [DOI] [PubMed] [Google Scholar]
- 137.Simone RE, Russo M, Catalano A, Monego G, et al. Lycopene inhibits NF-kB-mediated IL-8 expression and changes redox and PPARγ signalling in cigarette smoke– stimulated macrophages. PLoS One. 2011;6:e19652. doi: 10.1371/journal.pone.0019652. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Wang Y, Gao Y, Yu W, Jiang Z, et al. Lycopene protects against LPS-induced proinflammatory cytokine cascade in HUVECs. Pharmazie. 2013;68:681–684. [PubMed] [Google Scholar]
- 139.Zhou C, Han W, Zhang P, Cai M, et al. Lycopene from tomatoes partially alleviates the bleomycin-induced experimental pulmonary fibrosis in rats. Nutr Res. 2008;28:122–130. doi: 10.1016/j.nutres.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 140.Kumar A, Bagewadi A, Keluskar V, Singh M. Efficacy of lycopene in the management of oral submucous fibrosis. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2007;103:207–213. doi: 10.1016/j.tripleo.2006.07.011. [DOI] [PubMed] [Google Scholar]
- 141.Kitade Y, Watanabe S, Masaki T, Nishioka M, et al. Inhibition of liver fibrosis in LEC rats by a carotenoid, lycopene, or a herbal medicine, Sho-saiko-to. Hepatol Res. 2002;22:196–205. doi: 10.1016/s1386-6346(01)00132-2. [DOI] [PubMed] [Google Scholar]
- 142.Palozza P, E Simone R, Catalano A, Saraceni F, et al. Modulation of MMP-9 pathway by lycopene in macrophages and fibroblasts exposed to cigarette smoke. Inflamm Allergy Drug Targets. 2012;11:36–47. doi: 10.2174/187152812798889376. [DOI] [PubMed] [Google Scholar]
- 143.Karas M, Amir H, Fishman D, Danilenko M, et al. Lycopene interferes with cell cycle progression and insulinlike growth factor I signaling in mammary cancer cells. Nutr Cancer. 2000;36:101–111. doi: 10.1207/S15327914NC3601_14. [DOI] [PubMed] [Google Scholar]
- 144.Lo HM, Hung CF, Tseng YL, Chen BH, et al. Lycopene binds PDGF-BB and inhibits PDGF-BB-induced intracellular signaling transduction pathway in rat smooth muscle cells. Biochem Pharmacol. 2007;74:54–63. doi: 10.1016/j.bcp.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 145.Park YO, Hwang ES, Moon TW. The effect of lycopene on cell growth and oxidative DNA damage of Hep3B human hepatoma cells. Biofactors. 2005;23:129–139. doi: 10.1002/biof.5520230302. [DOI] [PubMed] [Google Scholar]
- 146.Nahum A, Hirsch K, Danilenko M, Watts CK, et al. Lycopene inhibition of cell cycle progression in breast and endometrial cancer cells is associated with reduction in cyclin D levels and retention of p27 (Kip1) in the cyclin E-cdk2 complexes. Oncogene. 2001;20:3428–3436. doi: 10.1038/sj.onc.1204452. [DOI] [PubMed] [Google Scholar]
- 147.Palozza P, Colangelo M, Simone R, Catalano A, et al. Lycopene induces cell growth inhibition by altering mevalonate pathway and Ras signaling in cancer cell lines. Carcinogenesis. 2010;31:1813–1821. doi: 10.1093/carcin/bgq157. [DOI] [PubMed] [Google Scholar]
- 148.Palozza P, Serini S, Boninsegna A, Bellovino D, et al. The growth-inhibitory effects of tomatoes digested in vitro in colon adenocarcinoma cells occur through down regulation of cyclin D1, Bcl-2 and Bcl-xL. Br J Nutr. 2007;98:789–795. doi: 10.1017/S0007114507746883. [DOI] [PubMed] [Google Scholar]
- 149.Elgass S, Cooper A, Chopra M. Lycopene inhibits angiogenesis in human umbilical vein endothelial cells and rat aortic rings. Br J Nutr. 2012;108:431–439. doi: 10.1017/S0007114511005800. [DOI] [PubMed] [Google Scholar]
- 150.Chen ML, Lin YH, Yang CM, Hu ML. Lycopene inhibits angiogenesis both in vitro and in vivo by inhibiting MMP-2/uPA system through VEGFR2-mediated PI3K-Akt and ERK/p38 signaling pathways. Mol Nutr Food Res. 2012;56:889–899. doi: 10.1002/mnfr.201100683. [DOI] [PubMed] [Google Scholar]
- 151.Yang CM, Yen YT, Huang CS, Hu ML. Growth inhibitory efficacy of lycopene and β-carotene against androgen-independent prostate tumor cells xenografted in nude mice. Mol Nutr Food Res. 2011;55:606–612. doi: 10.1002/mnfr.201000308. [DOI] [PubMed] [Google Scholar]
- 152.Hertog MGL, Hollman PCH, Van de Putte B. Content of potentially anticarcinogenic flavonoids of tea infusions, wines, fruit juices. J Agric Food Chem. 1993;41:1242–1246. [Google Scholar]
- 153.Miean KH, Mohamed S. Flavonoid (myricetin, quercetin, kaempferol, luteolin, apigenin) content of edible tropical plants. J Agric Food Chem. 2001;49:3106–3112. doi: 10.1021/jf000892m. [DOI] [PubMed] [Google Scholar]
- 154.Seeram NP, Lee R, Scheuller HS, Heber D. Identification of phenolic compounds in strawberries by liquid chromatography electrospray ionization mass spectroscopy. Food Chem. 2006;97:1–11. [Google Scholar]
- 155.Granado-Serrano AB, Martin MA, Bravo L, Goya L, et al. Quercetin attenuates TNF-induced inflammation in hepatic cells by inhibiting the NF-kB pathway. Nutr Cancer. 2012;64:588–598. doi: 10.1080/01635581.2012.661513. [DOI] [PubMed] [Google Scholar]
- 156.Noh HJ, Kim CS, Kang JH, Park JY, et al. Quercetin suppresses MIP-1α-induced adipose inflammation by downregulating its receptors CCR1/CCR5 and inhibiting inflammatory signaling. J Med Food. 2013;17:550–557. doi: 10.1089/jmf.2013.2912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Endale M, Park SC, Kim S, Kim SH, et al. Quercetin disrupts tyrosine-phosphorylated phosphatidylinositol 3-kinase and myeloid differentiation factor-88 association, inhibits MAPK/AP-1 and IKK/NF-κB-induced inflammatory mediators production in RAW 264.7 cells. Immunobiology. 2013;218:1452–1467. doi: 10.1016/j.imbio.2013.04.019. [DOI] [PubMed] [Google Scholar]
- 158.Sung MS, Lee EG, Jeon HS, Chae HJ, et al. Quercetin inhibits IL-1β-induced proliferation and production of MMPs, COX-2, PGE2 by rheumatoid synovial fibroblast. Inflammation. 2012;35:1585–1594. doi: 10.1007/s10753-012-9473-2. [DOI] [PubMed] [Google Scholar]
- 159.Yoon JS, Lee HJ, Choi SH, Chang EJ, et al. Quercetin inhibits IL-1 β-induced inflammation, hyaluronan production and adipogenesis in orbital fibroblasts from Graves' orbitopathy. PLoS One. 2011;6:e26261. doi: 10.1371/journal.pone.0026261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Chuang CC, Martinez K, Xie G, Kennedy A, et al. Quercetin is equally or more effective than resveratrol in attenuating tumor necrosis factor-α mediated inflammation and insulin resistance in primary human adipocytes. Am J Clin Nutr. 2011;92:1511–1521. doi: 10.3945/ajcn.2010.29807. [DOI] [PubMed] [Google Scholar]
- 161.Lee ES, Lee HE, Shin JY, et al. The flavonoid quercetin inhibits dimethylnitrosamine-induced liver damage in rats. J Pharm Pharmacol. 2003;55:1169–1174. doi: 10.1211/0022357021396. [DOI] [PubMed] [Google Scholar]
- 162.Baowen Q, Yulin Z, Xin W, Wenjing X, et al. A further investigation concerning correlation between anti-fibrotic effect of liposomal quercetin and inflammatory cytokines in pulmonary fibrosis. Eur J Pharmacol. 2010;642:134–139. doi: 10.1016/j.ejphar.2010.05.019. [DOI] [PubMed] [Google Scholar]
- 163.Hu Q, Noor M, Wong YF, Hylands PJ, et al. In vitro anti-fibrotic activities of herbal compounds and herbs. Nephrol Dial Transplant. 2009;24:3033–3041. doi: 10.1093/ndt/gfp245. [DOI] [PubMed] [Google Scholar]
- 164.Nakamura T, Matsushima M, Hayashi Y, Shibasaki M, et al. Attenuation of transforming growth factor-beta-stimulated collagen production in fibroblasts by quercetin-induced heme oxygenase-1. Am J Respir Cell Mol Biol. 2011;44:614–620. doi: 10.1165/rcmb.2010-0338OC. [DOI] [PubMed] [Google Scholar]
- 165.Hernandez-Ortega LD, Alcantar-Díaz BE, Ruiz-Corro LA, Sandoval-Rodriguez A, et al. Quercetin improves hepatic fibrosis reducing hepatic stellate cells and regulating pro-fibrogenic/anti-fibrogenic molecules balance. J Gastroenterol Hepatol. 2012;27:1865–1872. doi: 10.1111/j.1440-1746.2012.07262.x. [DOI] [PubMed] [Google Scholar]
- 166.Li M, Jiang Y, Jing W, Sun B, et al. Quercetin provides greater cardioprotective effect than its glycoside derivative rutin on isoproterenol-induced cardiac fibrosis in the rat. Can J Physiol Pharmacol. 2013;91:951–959. doi: 10.1139/cjpp-2012-0432. [DOI] [PubMed] [Google Scholar]
- 167.Phan TT, See P, Tran E, Nguyen TTT, et al. Suppression of insulin-like growth factor signalling pathway and collagen expression in keloid-derived fibroblasts by quercetin: its therapeutic potential use in the treatment and/or prevention of keloids. Br J Dermatol. 2003;148:544–552. doi: 10.1046/j.1365-2133.2003.05174.x. [DOI] [PubMed] [Google Scholar]
- 168.Phan TT, Lim IJ, Sun L, Chan SY, et al. Quercetin inhibits fibronectin production by keloid-derived fibroblasts. Implication for the treatment of excessive scars. J Dermatol Sci. 2003;33:192–194. doi: 10.1016/j.jdermsci.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 169.Pavanato A, Tunón MJ, Sanchez-Campos S, Marroni CA, et al. Effects of quercetin on liver damage in rats with carbon tetrachloride-induced cirrhosis. Dig Dis Sci. 2003;48:824–829. doi: 10.1023/a:1022869716643. [DOI] [PubMed] [Google Scholar]
- 170.Huynh H, Nguyen TTT, Chan E, Tran E. Inhibition of ErbB-2 and ErbB-3 expression by quercetin prevents transforming growth factor alpha (TGF-α)- and epidermal growth factor (EGF)-induced human PC-3 prostate cancer cell proliferation. Int J Oncol. 2003;23:821–829. [PubMed] [Google Scholar]
- 171.Jung M, Bu SY, Tak KH, Park JE, et al. Anticarcinogenic effect of quercetin by inhibition of insulin-like growth factor (IGF)-1 signaling in mouse skin cancer. Nutr Res Pract. 2013;7:439–445. doi: 10.4162/nrp.2013.7.6.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Yang JH, Hsia TC, Kuo HM, Chao PDL, et al. Inhibition of lung cancer cell growth by quercetin glucuronides via G2/M arrest and induction of apoptosis. Drug Metab Dispos. 2006;34:296–304. doi: 10.1124/dmd.105.005280. [DOI] [PubMed] [Google Scholar]
- 173.Choi JA, Kim JY, Lee JY, Kang CM, et al. Induction of cell cycle arrest and apoptosis in human breast cancer cells by quercetin. Int J Oncol. 2001;19:837–844. doi: 10.3892/ijo.19.4.837. [DOI] [PubMed] [Google Scholar]
- 174.Kim HJ, Kim SK, Kim BS, Lee SH, et al. Apoptotic effect of quercetin on HT-29 colon cancer cells via the AMPK signaling pathway. J Agric Food Chem. 2010;58:8643–8650. doi: 10.1021/jf101510z. [DOI] [PubMed] [Google Scholar]
- 175.Cao HH, Tse AKW, Kwan HY, Yu H, et al. Quercetin exerts anti-melanoma activities and inhibits STAT3 signaling. Biochem Pharmacol. 2013;87:424–434. doi: 10.1016/j.bcp.2013.11.008. [DOI] [PubMed] [Google Scholar]
- 176.Gokbulut AA, Apohan E, Baran Y. Resveratrol and quercetin-induced apoptosis of human 232B4 chronic lymphocytic leukemia cells by activation of caspase-3 and cell cycle arrest. Hematology. 2013;18:144–150. doi: 10.1179/1607845412Y.0000000042. [DOI] [PubMed] [Google Scholar]
- 177.Anand K, Asthana P, Kumar A, Ambasta RK, et al. Quercetin mediated reduction of angiogenic markers and chaperones in DLA-induced solid tumours. Asian Pac J Cancer Prev. 2011;12:2829–2835. [PubMed] [Google Scholar]
- 178.Zhao D, Qin C, Fan X, Li Y, et al. Inhibitory effects of quercetin on angiogenesis in larval zebrafish and human umbilical vein endothelial cells. Eur J Pharmacol. 2014;723:360–367. doi: 10.1016/j.ejphar.2013.10.069. [DOI] [PubMed] [Google Scholar]
- 179.Pratheeshkumar P, Budhraja A, Son YO, Wang X, et al. Quercetin inhibits angiogenesis mediated human prostate tumor growth by targeting VEGFR-2 regulated AKT/mTOR/P70S6K signaling pathways. PLoS One. 2012;7:e47516. doi: 10.1371/journal.pone.0047516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Zhong L, Chen FY, Wang HR, Ten Y, et al. Effects of quercetin on morphology and VEGF secretion of leukemia cells NB4 in vitro] Zhonghua Zhong Liu Za Zhi. 2006;28:25. [PubMed] [Google Scholar]
- 181.Luo H, Jiang BH, King SM, Chen YC. Inhibition of cell growth and VEGF expression in ovarian cancer cells by flavonoids. Nutr Cancer. 2008;60:800–809. doi: 10.1080/01635580802100851. [DOI] [PubMed] [Google Scholar]
- 182.Santini SE, Basini G, Bussolati S, Grasselli F. The phytoestrogen quercetin impairs steroidogenesis and angiogenesis in swine granulosa cells in vitro. J Biomed Biotechnol. 2009;2009:419891. doi: 10.1155/2009/419891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Zhang Y, Talalay P, Cho CG, Posner GH. A major inducer of anticarcinogenic protective enzymes from broccoli: isolation and elucidation of structure. Proc Natl Acad Sci USA. 1992;89:2399–2403. doi: 10.1073/pnas.89.6.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Kristal AR, Lampe JW. Brassica vegetables and prostate cancer risk: a review of the epidemiological evidence. Nutr Cancer. 2002;42:1–9. doi: 10.1207/S15327914NC421_1. [DOI] [PubMed] [Google Scholar]
- 185.Heiss E, Herhaus C, Klimo K, Bartsch H, et al. Nuclear factor κB is a molecular target for sulforaphane-mediated anti-inflammatory mechanisms. J Biol Chem. 2001;276:32008–32015. doi: 10.1074/jbc.M104794200. [DOI] [PubMed] [Google Scholar]
- 186.Lin W, Wu RT, Wu T, Khor TO, et al. Sulforaphane suppressed LPS-induced inflammation in mouse peritoneal macrophages through Nrf2 dependent pathway. Biochem Pharmacol. 2008;76:967–973. doi: 10.1016/j.bcp.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao XD, Zhou YT, Lu XJ. Sulforaphane enhances the activity of the Nrf2-ARE pathway and attenuates inflammation in OxyHb-induced rat vascular smooth muscle cells. Inflamm Res. 2013;62:857–863. doi: 10.1007/s00011-013-0641-0. [DOI] [PubMed] [Google Scholar]
- 188.Challa AA, Vukmirovic M, Blackmon J, Stefanovic B. Withaferin-a reduces type I collagen expression in vitro and inhibits development of myocardial fibrosis in vivo. PLoS One. 2012;7:e42989. doi: 10.1371/journal.pone.0042989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bai Y, Cui W, Xin Y, Miao X, et al. Prevention by sulforaphane of diabetic cardiomyopathy is associated with up-regulation of Nrf2 expression and transcription activation. J Mol Cell Cardiol. 2013;57:82–95. doi: 10.1016/j.yjmcc.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 190.Artaud-Macari E, Goven D, Brayer S, Hamimi A, et al. Nuclear factor erythroid 2-related factor 2 nuclear translocation induces myofibroblastic dedifferentiation in idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2013;18:66–79. doi: 10.1089/ars.2011.4240. [DOI] [PubMed] [Google Scholar]
- 191.Yoo SH, Lim Y, Kim SJ, Yoo KD, et al. Sulforaphane inhibits PDGF-induced proliferation of rat aortic vascular smooth muscle cell by up-regulation of p53 leading to G1/S cell cycle arrest. Vasc Pharmacol. 2013;59:44–51. doi: 10.1016/j.vph.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 192.Kanematsu S, Uehara N, Miki H, Yoshizawa K, et al. Autophagy inhibition enhances sulforaphane-induced apoptosis in human breast cancer cells. Anticancer Res. 2010;30:3381–3390. [PubMed] [Google Scholar]
- 193.Clarke JD, Hsu A, Yu Z, Dashwood RH, et al. Differential effects of sulforaphane on histone deacetylases, cell cycle arrest and apoptosis in normal prostate cells versus hyperplastic and cancerous prostate cells. Mol Nutr Food Res. 2011;55:999–1009. doi: 10.1002/mnfr.201000547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Jeon YK, Yoo DR, Jang YH, Jang SY, et al. Sulforaphane induces apoptosis in human hepatic cancer cells through inhibition of 6-phosphofructo-2-kinase/fructose-2, 6-biphosphatase4, mediated by hypoxia inducible factor-1-dependent pathway. Biochim Biophys Acta. 2011;1814:1340–1348. doi: 10.1016/j.bbapap.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 195.Wang M, Chen S, Wang S, Sun D, et al. Effects of phytochemicals sulforaphane on uridine diphosphateglucuronosyltransferase expression as well as cell-cycle arrest and apoptosis in human colon cancer Caco-2 cells. Chin J Physiol. 2012;55:134–144. doi: 10.4077/CJP.2012.BAA085. [DOI] [PubMed] [Google Scholar]
- 196.Park HS, Han MH, Kim GY, Moon SK, et al. Sulforaphane induces reactive oxygen species-mediated mitotic arrest and subsequent apoptosis in human bladder cancer 5637 cells. Food Chem Toxicol. 2014;64:157–165. doi: 10.1016/j.fct.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 197.Bertl E, Bartsch H, Gerhuser C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol Cancer Ther. 2006;5:575–585. doi: 10.1158/1535-7163.MCT-05-0324. [DOI] [PubMed] [Google Scholar]
- 198.Jackson SJT, Singletary KW, Venema RC. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vasc Pharmacol. 2007;46:77–84. doi: 10.1016/j.vph.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 199.Davis R, Singh KP, Kurzrock R, Shankar S. Sulforaphane inhibits angiogenesis through activation of FOXO transcription factors. Oncol Rep. 2009;22:1473–1478. doi: 10.3892/or_00000589. [DOI] [PubMed] [Google Scholar]
- 200.Checker R, Sandur SK, Sharma D, Patwardhan RS, et al. Potent anti-inflammatory activity of ursolic acid, a triterpenoid antioxidant, is mediated through suppression of NF-kB, AP-1 and NF-AT. PLoS One. 2012;7:e31318. doi: 10.1371/journal.pone.0031318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Shishodia S, Majumdar S, Banerjee S, Aggarwal BB. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase. Cancer Res. 2003;63:4375–4383. [PubMed] [Google Scholar]
- 202.Lu J, Wu DM, Zheng Yl, Hu B, et al. Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kB pathway activation. Cereb Cortex. 2010;20:2540–2548. doi: 10.1093/cercor/bhq002. [DOI] [PubMed] [Google Scholar]
- 203.Wang YJ, Lu J, Wu DM, Zheng ZH, et al. Ursolic acid attenuates lipopolysaccharide-induced cognitive deficits in mouse brain through suppressing p38/NF-kB mediated inflammatory pathways. Neurobiol Learn Mem. 2011;96:156–165. doi: 10.1016/j.nlm.2011.03.010. [DOI] [PubMed] [Google Scholar]
- 204.Kim SH, Hong JH, Lee YC. Ursolic acid, a potential PPARg agonist, suppresses ovalbumin-induced airway inflammation and Penh by down-regulating IL-5, IL-13, IL-17 in a mouse model of allergic asthma. Eur J Pharmacol. 2013;701:131–143. doi: 10.1016/j.ejphar.2012.11.033. [DOI] [PubMed] [Google Scholar]
- 205.Yang JJ, Gong Y, Shi J, Qi MY. [Study on the effect of ursolic acid (UA) on the myocardial fibrosis of experimental diabetic mice] Zhongguo Ying Yong Sheng Li Xue Za Zhi. 2013;29:353–356. [PubMed] [Google Scholar]
- 206.Wang X, Ikejima K, Kon K, Arai K, et al. Ursolic acid ameliorates hepatic fibrosis in the rat by specific induction of apoptosis in hepatic stellate cells. J Hepatol. 2011;55:379–387. doi: 10.1016/j.jhep.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 207.Sathya S, Sudhagar S, Sarathkumar B, Lakshmi BS. EGFR inhibition by pentacyclic triterpenes exhibit cell cycle and growth arrest in breast cancer cells. Life Sci. 2013;95:53–62. doi: 10.1016/j.lfs.2013.11.019. [DOI] [PubMed] [Google Scholar]
- 208.Wang J, Liu L, Qiu H, Zhang X, et al. Ursolic acid simultaneously targets multiple signaling pathways to suppress proliferation and induce apoptosis in colon cancer cells. PLoS One. 2013;8:e63872. doi: 10.1371/journal.pone.0063872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Wang X, Li L, Wang B, Xiang J. Effects of ursolic acid on the proliferation and apoptosis of human ovarian cancer cells. J Huazhong Univ Sci Technolog Med Sci. 2009;29:761–764. doi: 10.1007/s11596-009-0618-y. [DOI] [PubMed] [Google Scholar]
- 210.Zhang Y, Kong C, Zeng Y, Wang L, et al. Ursolic acid induces PC 3 cell apoptosis via activation of JNK and inhibition of Akt pathways in vitro. Mol Carcinog. 2010;49:374–385. doi: 10.1002/mc.20610. [DOI] [PubMed] [Google Scholar]
- 211.Hsu YL, Kuo PL, Lin CC. Proliferative inhibition, cell-cycle dysregulation, induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004;75:2303–2316. doi: 10.1016/j.lfs.2004.04.027. [DOI] [PubMed] [Google Scholar]
- 212.Wang X, Zhang F, Yang L, Mei Y, et al. Ursolic acid inhibits proliferation and induces apoptosis of cancer cells in vitro and in vivo. J Biomed Biotechnol. 2011;2011:419343. doi: 10.1155/2011/419343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Yim EK, Lee MJ, Lee KH, Um SJ, et al. Antiproliferative and antiviral mechanisms of ursolic acid and dexamethasone in cervical carcinoma cell lines. Int J Gynecol Cancer. 2006;16:2023–2031. doi: 10.1111/j.1525-1438.2006.00726.x. [DOI] [PubMed] [Google Scholar]
- 214.Chadalapaka G, Jutooru I, McAlees A, Stefanac T, et al. Structure-dependent inhibition of bladder and pancreatic cancer cell growth by 2-substituted glycyrrhetinic and ursolic acid derivatives. Bioorg Med Chem Lett. 2008;18:2633–2639. doi: 10.1016/j.bmcl.2008.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Gai L, Cai N, Wang L, Xu X, et al. Ursolic acid induces apoptosis via Akt/NF-κB signaling suppression in T24 human bladder cancer cells. Mol Med Rep. 2013;7:1673–1677. doi: 10.3892/mmr.2013.1364. [DOI] [PubMed] [Google Scholar]
- 216.Cardenas C, Quesada AR, Medina MA. Effects of ursolic acid on different steps of the angiogenic process. Biochem Biophys Res Commun. 2004;320:402–408. doi: 10.1016/j.bbrc.2004.05.183. [DOI] [PubMed] [Google Scholar]
- 217.Saraswati S, Agrawal SS, Alhaider AA. Ursolic acid inhibits tumor angiogenesis and induces apoptosis through mitochondrial-dependent pathway in Ehrlich ascites carcinoma tumor. Chem Biol Interact. 2013;206:153–165. doi: 10.1016/j.cbi.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 218.Lin J, Chen Y, Wei L, Hong Z, et al. Ursolic acid inhibits colorectal cancer angiogenesis through suppression of multiple signaling pathways. Int J Oncol. 2013;43:1666–1674. doi: 10.3892/ijo.2013.2101. [DOI] [PubMed] [Google Scholar]
- 219.Kanjoormana M, Kuttan G. Antiangiogenic activity of ursolic acid. Integr Cancer Ther. 2010;9:224–235. doi: 10.1177/1534735410367647. [DOI] [PubMed] [Google Scholar]
- 220.Chiaffarino F, Parazzini F, La Vecchia C, Chatenoud L, et al. Diet and uterine myomas. Obstet Gynecol. 1999;94:395–398. doi: 10.1016/s0029-7844(99)00305-1. [DOI] [PubMed] [Google Scholar]
- 221.Wise LA, Radin RG, Palmer JR, Kumanyika SK, et al. Intake of fruit, vegetables, carotenoids in relation to risk of uterine leiomyomata. Am J Clin Nutr. 2011;94:1620–1631. doi: 10.3945/ajcn.111.016600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kasinski AL, Du Y, Thomas SL, Zhao J, et al. Inhibition of IkappaB kinase-nuclear factor-kappaB signaling pathway by 3,5-bis(2-flurobenzylidene) piperidin-4-one (EF24), a novel monoketone analog of curcumin. Mol Pharmacol. 2008;74:654–661. doi: 10.1124/mol.108.046201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Okuda H, Ogura K, Kato A, Takubo H, et al. A possible mechanism of eighteen patient deaths caused by interactions of sorivudine, a new antiviral drug, with oral 5-fluorouracil prodrugs. J Pharmacol Exp Ther. 1998;287:791–799. [PubMed] [Google Scholar]
- 224.Gupta SC, Kim JH, Prasad S, Aggarwal BB. Regulation of survival, proliferation, invasion, angiogenesis, metastasis of tumor cells through modulation of inflammatory pathways by nutraceuticals. Cancer Metastasis Rev. 2010;29:405–434. doi: 10.1007/s10555-010-9235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]


