Abstract
Small-conductance (KCa2) and intermediate-conductance (KCa3.1) calcium-activated K+ channels are voltage-independent and share a common calcium/calmodulin-mediated gating mechanism. Existing positive gating modulators like EBIO, NS309, or SKA-31 activate both KCa2 and KCa3.1 channels with similar potency or, as in the case of CyPPA and NS13001, selectively activate KCa2.2 and KCa2.3 channels. We performed a structure-activity relationship (SAR) study with the aim of optimizing the benzothiazole pharmacophore of SKA-31 toward KCa3.1 selectivity. We identified SKA-111 (5-methylnaphtho[1,2-d]thiazol-2-amine), which displays 123-fold selectivity for KCa3.1 (EC50 111 ± 27 nM) over KCa2.3 (EC50 13.7 ± 6.9 μM), and SKA-121 (5-methylnaphtho[2,1-d]oxazol-2-amine), which displays 41-fold selectivity for KCa3.1 (EC50 109 nM ± 14 nM) over KCa2.3 (EC50 4.4 ± 1.6 μM). Both compounds are 200- to 400-fold selective over representative KV (KV1.3, KV2.1, KV3.1, and KV11.1), NaV (NaV1.2, NaV1.4, NaV1.5, and NaV1.7), as well as CaV1.2 channels. SKA-121 is a typical positive-gating modulator and shifts the calcium-concentration response curve of KCa3.1 to the left. In blood pressure telemetry experiments, SKA-121 (100 mg/kg i.p.) significantly lowered mean arterial blood pressure in normotensive and hypertensive wild-type but not in KCa3.1−/− mice. SKA-111, which was found in pharmacokinetic experiments to have a much longer half-life and to be much more brain penetrant than SKA-121, not only lowered blood pressure but also drastically reduced heart rate, presumably through cardiac and neuronal KCa2 activation when dosed at 100 mg/kg. In conclusion, with SKA-121, we generated a KCa3.1-specific positive gating modulator suitable for further exploring the therapeutical potential of KCa3.1 activation.
Introduction
The human genome contains four voltage-independent Ca2+-activated K+ channels: the three small-conductance KCa2 channels: KCa2.1 (= KCNN1, SK1), KCa2.2 (= KCNN2, SK2), and KCa2.3 (= KCNN3, SK3), as well as the intermediate-conductance KCa3.1 (= KCNN4, IK1, SK4) (Kohler et al., 1996; Joiner et al., 1997; Wei et al., 2005). Their lack of voltage dependence enables these channels to remain open at negative membrane potentials and to hyperpolarize the membrane toward values near the K+ equilibrium potential of −89 mV. KCa3.1 and KCa2 channels are accordingly expressed in cells that need to be able to hyperpolarize to regulate Ca2+ influx through inward rectifier Ca2+ channels, pass on hyperpolarization through gap junctions, or regulate firing frequency by preventing an untimely or premature action potential initiation (Adelman et al., 2012; Wulff and Köhler, 2013). Pharmacologic activation of KCa channels has therefore been suggested for the treatment of various diseases. Whereas KCa2 activators can potentially reduce neuronal excitability in central nervous system disorders like epilepsy and ataxia, KCa3.1 activators could be useful as endothelial targeted antihypertensives and to enhance fluid secretion in the airways in cystic fibrosis (Wulff and Zhorov, 2008; Balut et al., 2012; Wulff and Köhler, 2013).
All four KCa2/3 channels are voltage-independent and share a Ca2+/calmodulin-mediated gating mechanism (Xia et al., 1998; Fanger et al., 1999). Calmodulin (CaM), which can be regarded as a β-subunit for these channels, is constitutively bound to a CaM-binding domain (CaMBD) in the intracellular C-terminus. On Ca2+ binding to CaM, the channels activate in a highly coordinated fashion with an extremely steep Hill equation and EC50 values in the range of 250–900 nM (Wei et al., 2005). KCa2/3 activators modulate this gating process and have therefore been termed positive gating modulators. The oldest positive modulator of KCa2/3 channels is the benzimidazolone EBIO (Devor et al., 1996), which activates KCa3.1 with an EC50 of ∼30 μM and all three KCa2 channels with EC50s around 300 μM (Wulff and Köhler, 2013). Two structurally similar, but more potent molecules, are the oxime NS309 (Strobaek et al., 2004) and the benzothiazole SKA-31 (Sankaranarayanan et al., 2009). Whereas NS309 is exquisitely potent (EC50 for KCa3.1 ∼20 nM; EC50 for KCa2 channels ∼600 nM), it unfortunately has an extremely short in vivo half-life and inhibits KV11.1 (hERG) at a concentration of 1 μM (Strobaek et al., 2004). SKA-31 is 10 times less potent than NS309 but has become a relatively widely used in vivo tool compound to activate both KCa3.1 and/or KCa2 channels because of its long half-life of 12 hours in rats (Sankaranarayanan et al., 2009). In contrast to these benzimidazole/benzothiazole-type KCa activators, all of which show only a modest 5- to 10-fold selectivity for KCa3.1 and do not distinguish at all between the three KCa2 channels, CyPPA, and its derivative NS13001, have quite different selectivity profiles. Both compounds activate KCa2.3 and KCa2.2 but are completely inactive on KCa2.1 and KCa3.1 (Hougaard et al., 2007; Kasumu et al., 2012). GW542573X and (-)-CM-TMPF, in contrast, are selective for KCa2.1 (Hougaard et al., 2009, 2012). So while there are some compounds that allow for the selective pharmacologic activation of KCa2 channels, there currently is no selective KCa3.1 activator.
We previously used riluzole, a drug for the treatment of amyotrophic lateral sclerosis, as a template for the design of SKA-31. Riluzole is a “dirty” compound that exerts multiple pharmacological activities, the most prominent of which are inhibition of voltage-gated sodium (NaV) channels at concentrations of 1–50 μM (Debono et al., 1993; Duprat et al., 2000) and activation of KCa2/3 channels with EC50s of 10–20 μM (Grunnet et al., 2001). Through directed derivatization of riluzole, we managed to significantly reduce NaV channel blocking effects and increase activity on KCa2/3 channels. Whereas SKA-31 affects NaV channels only at concentrations of 25 μM or higher, it activates KCa2.3 with an EC50 of 3 μM and KCa3.1 with an EC50 of 260 nM. Since both KCa3.1 and KCa2.3 are expressed in vascular endothelium and have been shown to be involved in the so-called endothelium-derived hyperpolarization (EDH) response (Grgic et al., 2009; Dalsgaard et al., 2010; Edwards et al., 2010; Köhler et al., 2010), SKA-31 was used as a pharmacologic tool to explore the role of KCa channels in blood pressure regulation. While mice deficient in KCa3.1 and/or KCa2.3 exhibit impaired EDH responses and an increased mean arterial blood pressure (MAP) (Brahler et al., 2009), pharmacologic KCa channel activation with SKA-31 lowered blood pressure in both mice and dogs (Sankaranarayanan et al., 2009; Damkjaer et al., 2012; Radtke et al., 2013). In dogs, i.v. injection of 2 mg/kg SKA-31 produced an immediate and strong (−30 mmHg) but short-lived reduction in blood pressure (Damkjaer et al., 2012). In mice, SKA-31 doses of 10–30 mg/kg have been reported to lower blood pressure more prolonged (∼30 mmHg) for 60–90 minutes) (Sankaranarayanan et al., 2009; Köhler, 2012). Significant blood pressure–lowering effects with SKA-31 doses of 30 mg/kg have been further observed in models of hypertension like angiotensin II–infused (Sankaranarayanan et al., 2009) and connexin 40–deficient mice (Radtke et al., 2013), which exhibit severe chronic renin-dependent hypertension. However, the responses typically lasted only about 1 hour. Higher doses of SKA-31 (100 mg/kg) induced a stronger and longer-lasting response, accompanied by significant bradycardia. This reduction in heart rate (HR) was probably due to a centrally mediated decrease in sympathetic drive through activation of neuronal KCa2 channels by the brain penetrant SKA-31, as well as possible direct effects on KCa2 channels in cardiac pacemaker tissue (Radtke et al., 2013). Another side effect that might prohibit the use of KCa2 activators as antihypertensives is a possible impairment of learning and memory because of the role neuronal KCa2 channels play in in synaptic plasticity and long-term potentiation (Blank et al., 2003; Adelman et al., 2012). To avoid these KCa2 channel–mediated side effects, it therefore seems highly desirable to identify selective KCa3.1 activators that could be used as pharmacological tools to further dissect the in vivo role of KCa3.1 in blood pressure control and to help determine whether KCa3.1 activators could eventually be developed into a new class of endothelial-targeted antihypertensives. We therefore here explored whether we could further modify the benzothiazole SKA-31 and develop a KCa3.1-selective small-molecule activator. SKA-121, a compound generated through an isosteric replacement approach, activates KCa3.1 with an EC50 of 111 nM, exhibits 40- to 80-fold selectivity over the three KCa2 channels and lowers blood pressure in mice as determined by telemetry without exerting KCa2 channel–mediated effects on HR. We propose SKA-121 as a new KCa3.1 selective pharmacological tool compound despite its relatively short half-life in mice.
Materials and Methods
Commercially Available Compounds
2-Amino-4-(1-naphthyl)thiazole (SKA-75, CAS no. 56503-96-9), 2-amino-4-(2-naphthyl)thiazole (SKA-76), Chemical Abstracts Service (CAS) no. 21331-43-1), 2,3,3-trimethyl-3H-benzo[g]indole (SKA-92, CAS no. 74470-85-2), 2-methylnaphtho[2,3-d]oxazole (SKA-104, CAS no. 20686-66-2), and 2-methylnaphtho[2,1-d]oxazole (SKA-103, CAS no. 85-15-4) were purchased from Alfa Aesar (Pelham, NH); 2-methylnaphtho[1,2-d]thiazole (SKA-74, 2682-45-3) was purchased from Sigma (St. Louis, MO).
Chemical Synthesis
Compounds that were not commercially available were synthesized in our laboratory by the general methods described below. Compounds reported previously were characterized by melting point, proton nuclear magnetic resonance (1H NMR) and 13 carbon (13C) NMR to confirm their chemical identity. New chemical entities were additionally characterized by high-resolution mass spectrometry (HRMS) and a fully interpreted 13C NMR.
General Method I. Preparation of Thiazoles.
Thiourea (17 mmol) was added to a solution of substituted ketones (6 mmol) in 30 ml of absolute ethanol. The mixture was refluxed for 8 hours, which resulted in 2-aminothiazole hydrobromide salts. The 2-aminothiazole was obtained by treating the hydrobromide salt with 2M NaOH (5 ml) and extracting with ethyl acetate. The crude residue was concentrated, reconstituted in a methanol-water mixture (99:1), treated with charcoal, and recrystallized.
General Method II. Alternative Preparation of Benzothiazoles.
Thiourea (17 mmol) was added to a solution of substituted ketones (6 mmol) in 30 ml of absolute ethanol. The mixture was refluxed for 8 hours, which resulted in 2-aminothiazole hydrobromide salts. The free 2-aminothiazole was obtained by treating the hydrobromide salt with 2 M NaOH (5 ml) and extracting with ethyl acetate. The crude residue was concentrated, reconstituted in a methanol-water mixture (99:1), treated with charcoal, and recrystallized. The resulting 2-aminothiazole, 2-iodoxybenzoic acid and tetrabutylammonium tribromide were combined in ethyl acetate and stirred at room temperature (RT) for 10 hours. The reaction mixture was filtered through a pad of Celite, and the filtrate was diluted with saturated Na2S2O3 and extracted with ethyl acetate. The combined organic layers were dried with anhydrous sodium sulfate (anhydrous Na2SO4), concentrated, and then purified via flash chromatography (cyclohexane-EtOAc, 1:1).
General Method III. Preparation of Benzothiazoles.
Benzoyl chloride was added drop wise to a stirred solution of NH4SCN in acetone and stirred at 50°C for 2 hours. Next, a solution of substituted naphthylamines in acetone was added drop wise, and the mixture was stirred at 50oC for 24 hours. The reaction mixture was diluted with water; the precipitated crystals collected by filtration and washed with water. The crystals were then suspended in 2M NaOH (50 ml), refluxed for 1 hour, and poured into cold water and filtered. The crude crystals of the resulting thiourea were dissolved in acetic acid, to which benzyl trimethylammonium tribromide was added and allowed to react overnight. Ethyl ether (Et2O) was added and the precipitate of the resulting product-HBr salt was collected by filtration and washed with Et2O. The salt was then treated with 1M NaOH to liberate the free base, which was recrystallized in methanol.
General Method IV. Preparation of 2-Aminonaphthooxazoles.
To procure the intermediate 2-hydroxy-naphthalenones, substituted ketones (1 g, 6.2 mmol) were added to a stirred mix of water (25 ml), acetonitrile (25 ml) trifluoroactic acid (6 ml, 7 mmol), iodobenzene (0.7 ml, 6 mmol), and oxone (11 g, 37 mmol). The resulting solution was refluxed for 1 hour, and the progress of the reaction was monitored by thin layer chromatography (TLC). The reaction was then allowed to cool to RT and filtered. The mixture was extracted with ethyl acetate (3 × 30 ml) and lastly neutralized with saturated NaHCO3 (3 × 30 ml). The combined organic phase was washed with brine (30 ml), dried with anhydrous Na2SO4, filtered, and concentrated. The residue was purified by flash chromatography over silica gel (cyclohexane-EtOAc, 3:1) to give substituted 2-hydroxy-naphthalenones (Rf = 0.20). The preparation of 2-aminonaphthooxazole began by adding cyanamide (2 mmol) to a stirred solution of substituted 2-hydroxy-naphthalenone (1.5 mmol), water (20 ml), and acetonitrile (10 ml). The resulting mixture was refluxed for 15 hours, and progression of the reaction was monitored by TLC. The reaction was allowed to cool to RT. The mixture was extracted with ethyl acetate (3 × 30 ml) to give a mixture of isomers. The combined organic phase was washed with brine (30 ml), dried with anhydrous Na2SO4, filtered, and concentrated. The isomers were purified and separated by flash chromatography over silica gel (cyclohexane-EtOAc, 1:1).
8H-Indeno[1,2-d]thiazol-2-amine (SKA-69)
SKA-69 was prepared from 1-indanone (1 g, 4.7 mmol) according to general method I. The product was isolated as brown crystals (563 mg, 63%); melting point (m.p.) = 212oC (CAS no. 85787-95-7). 1H NMR [500 MHz, dimethylsulfoxide (DMSO)-d6, δ]: 7.45 (doublet (d), J = 7.4 Hz, 1H, 4-H), 7.37 (d, J = 7.4 Hz, 1H, 7-H), 7.28 (t, J = 7.4 Hz, 1H, 5-H), 7.17–7.11 (m, 3H, 6-H, and NH2), 3.68 (s, 2H, CH2). 13C NMR (125 MHz: DMSO-d6, δ):173.74, 146.09, 138.04, 128.58, 126.68, 126.60, 125.47, 118.2, and 32.85.
4,5-Dihydronaphtho[1,2-d]thiazol-2-amine (SKA-70)
SKA-70 was prepared from 1-tetralone (1 g, 6.84 mmol) according to general method I. The product was isolated as white crystals (906 mg, 64%); m.p. = 135oC (CAS no. 34176-49-3). 1H NMR (500 MHz, DMSO-d6, δ): 7.52 (d, J = 7.3 Hz, 1H, 9-H), 7.23−7.14 (m, 2H, 7-H and 6-H), 7.10 (t, J = 7.3 Hz, 1H, 8-H), 6.93 (s, 2H, NH2), 2.93 (t, J = 7.8 Hz, 2H, 5-H), 2.76 (t, J = 7.8 Hz, 2H, 4-H). 13C NMR (125 MHz: DMSO-d6, δ): 167.28, 145.01, 134.99, 132.39, 128.37, 127.31, 126.87, 122.83, 118.25, 29.18, and 21.79.
6-Fluoro-8H-indeno[1,2-d]thiazol-2-amine (SKA-71)
SKA-71 was prepared from 5-fluoro-1-indanone (1.84 g, 12.4 mmol) according to general method I. The product was isolated as lavender crystals (934 mg, 40%); m.p. = 217oC dec (CAS no. 1025800-52-5). 1H NMR (500 MHz, DMSO-d6, δ): 7.33 (doublet of triplets (dt), J = 7.9, 3.8 Hz, 2H, 4-H, and 7-H), 7.16 (s, 2H, NH2), 7.10 (doublet of doublet of doublets (ddd), J = 10.2, 8.4, 2.5 Hz, 1H, 5-H), 3.70 (s, 2H, CH2). 13C NMR (125 MHz: DMSO-d6, δ): 182.98, 160.95, 139.84, 128.3, 126.68, 126.53, 124.01, 114.03, 112.77, and 32.72.
5-Chloronaphtho[1,2-d]thiazol-2-amine (SKA-72)
SKA-72 was prepared from 1-amino-4-chloronaphthalene (1.6 g, 8.8 mmol) according to general method III. The product was isolated as lavender crystals (1.27 g, 60%); m.p. = 253oC (CAS no. 1369250-74-7). 1H NMR (500 MHz, DMSO-d6, δ): 8.41 (m, 1H, 9-H), 8.14 (m, 1H, 6-H), 8.07 (s, 1H, 4-H), 7.75 (s, 2H, NH2), 7.63 (m, 2H, 8-H, and 7-H). 13C NMR (125 MHz: DMSO-d6, δ): 168.17, 147.65, 128.19, 126.46, 126.40, 126.22, 124.82, 124.14, 123.94, 122.07, and 119.66.
4,5-Dihydroacenaphtho[5,4-d]thiazol-8-amine (SKA-73)
SKA-73 was prepared from 1,2-dihydroacenaphthylen-5-amine (0.4 g, 2 mmol) according to general method III. The product was isolated a brown solid (400 mg, 30%); m.p. = 257°C (CAS. 108954-84-3). 1H NMR (500 MHz, DMSO-d6, δ): 7.87 (d, J = 8.2 Hz, 1H, 9-H), 7.56 (singlet (s), 1H, 4-H), 7.45 (t, J = 7.5 Hz, 1H, 8-H), 7.30 – 7.20 (m, 3H, 7-H and NH2), 3.37 (d, J = 12.2 Hz, 4H, 4-H, and 5-H). 13C NMR (125 MHz: DMSO-d6, δ): 167.17, 146.65, 139.05, 128.19, 128.46, 124.40, 119.66, 119,24, 113.08, 31.25, and 29.92. (Note: We are following the NMR numbering designation of 2-aminobenzothiazoles, not dihydroacenaphthothiazoles.)
7,8-Dihydro-6H-indeno[4,5-d]thiazol-2-amine (SKA-81)
SKA-81 was prepared from 4-aminoindan (500 mg, 3.7 mmol) according to general method III. The product was isolated as a white solid (200 mg, 30%); m.p. = 195°C. 1H NMR (500 MHz, DMSO-d6, δ): 7.38–7.37 (d, J = 7.75 Hz, 1H, 5-H), 7.25 (s, 2H, NH2), 6.90 (d, J = 7.8 Hz, 1H, 4-H), 3.00 (t, J = 7.3, 2H, 8-H), 2.91 (t, J = 7.3 Hz, 2H, 6-H), 2.10–2.04 (q, J = 7.3 Hz, 2H, 7-H). 13C NMR (125 MHz, DMSO-d6, δ): 167.37 (2-C), 149.90 (3-C), 141.97 (4-C), 133.02 (6-C), 129.00 (8-C), 119.08 (5-C), 117.58 (4-C), 33.42 (6-C), 31.55 (8-C), 25.62 (7-C). HRMS (ESI): calculated: 191.0637; found: 191.0638.
5-Bromonaphtho[1,2-d]thiazol-2-amine (SKA-87)
SKA-87 was prepared from 1-amino-4-bromonaphthalene (1.6 g, 8.8 mmol) according to general method III. The product was isolated as silver crystalline rods (500 mg, 45%); m.p. = 253°C (CAS. 412312-09-5). 1H NMR (800 MHz, DMSO-d6, δ): 8.40 (d, J = 8.6 Hz, 1H, 9-H), 8.24 (s, 1H, 4-H), 8.10 (d, J = 8.1 Hz, 1H, 6-H), 7.78 (s, 2H, NH2), 7.65–7.60 (multiplet (m), 2H, 7-H, and 6-H). 13C NMR (125 MHz, DMSO-d6, δ): 168.67, 148.68, 129.71, 127.10, 127.00, 126.89, 126.88, 125.90, 124.61, 123.50, and 112.73.
Naphtho[1,2-d]oxazol-2-amine (SKA-102)
Solid 1-amino-2-naphthol hydrochloride (0.80 g, 4 mmol) was suspended in 20 ml of dichloromethane, treated with triethylamine (0.6 ml, 20 mmol) and cyanogen bromide (3M BrCN in dichloromethane; 3 ml, 6.2 mmol), and allowed to react overnight yielding naphtho[1,2-d]oxazol-2-amine HBr. To isolate the free amine, the HBr salt was suspended in ethyl acetate and free-based with ammonium hydroxide (NH4OH). The solid residue was dissolved in a diethyl ether-ethyl acetate, treated with charcoal, and recrystallized from diethyl ether-ethyl acetate (10:1), resulting in a purple solid (100 mg, 45%); m.p. = 194°C (CAS. 858432-45-8). 1H NMR (800 MHz, DMSO-d6, δ): 8.27 (d, J = 8.3 Hz, 1H, 9-H), 7.9 (d, 1H, J = 8.16 Hz, 6-H), 7.60 (d, J = 8.88 Hz, 1H, 5-H), 7.58 (triplet (t), J = 7.92 Hz, 1H, 8-H), 7.53 (d, J = 8.72 Hz, 1H, 4-H), 7.47 (t, J = 8.01 Hz, 1H, 8-H). 13C NMR (200 MHz, DMSO-d6, δ): 163.25, 144.14, 138.53, 130.95, 128.80, 125.97, 124.72, 124.49, 121.99, 120.29, and 110.32.
5-Fluoronaphtho[1,2-d]thiazol-2-amine (SKA-106)
SKA-106 was prepared from 4-fluoronaphthalen-1-amine (1 g, 6 mmol) according to general method III. The product was isolated as a clear oil (210 mg, 20%). 1H NMR (500 MHz, acetone-d6, δ): 8.48 (d, J = 8.1 Hz, 1H, 9-H), 8.06 (d, J = 8.2 Hz, 1H, 6-H), 7.62 (m, 3H, 8-H, 7-H, and 4-H), 6.91 (s, 2H, NH2). 13C NMR (125 MHz, acetone-d6, δ): 167.23 (2-C), 157.95 (5-C), 145.12 (3′-C), 128.33 (6-C), 125.17 (1′-C), 126.94 (7-C), 125.80 (9-C), 125.01 (8-C) 124.29 (9′-H), 116.06 (6′-C), 103.59 (4-C). HRMS (ESI): calculated: 219.0387; found: 219.0383.
2-Aminonaphtho[1,2-d]thiazole-5-carbonitrile (SKA-107)
SKA-107 was prepared from 4-amino-1-naphthalenecarbonitrile (1 g, 6 mmol) according to general method III. The product was isolated as a brown solid (121 mg, 45%); m.p. = 265°C. 1H NMR (500 MHz, acetone-d6, δ): 8.62 (d, J = 8.22 Hz, 1H, 9-H), 8.42 (s, 1H, 4-H), 8.20 (d, J = 8.34 Hz, 1H, 6-H), 7.78 (t, J = 7.04 Hz, 1H, 7-H), 7.73 (t, J = 7.25 Hz, 1H, 8-H), 7.48 (s, 2H, NH2).13C NMR (200 MHz, DMSO-d6, δ): 171.82 (2-C), 153.14 (3′-C), 131.15 (6-C), 128.32 (4-C), 127.55 (9-C), 127.39 (8-C), 125.25 (7-C), 124.96 (6′-C), 124.84 (1′-C), 124.69 (9′-C), 118.94 (CN), and 99.96 (5-C). HRMS (ESI): calculated: 226.0433; found: 226.0432.
6,8-Dimethyl-4,5-dihydronaphtho[1,2-d]thiazol-2-amine (SKA-108)
SKA-108 was prepared from 5,7-dimethyl-1-tetralone (2 g, 11 mmol) according to general method I. The product was isolated as pink crystals (300 mg, 16%); m.p. = 159°C. 1H NMR (500 MHz, acetone-d6, δ): 7.40 (s, 1H, 9-H), 6.83 (s, 1H, 7-H), 6.17 (s, 2H, NH2), 2.91 (t, J = 7.87 Hz, 2H, 4-H), 2.81 (t, J = 7.56 Hz, 2H, 5-H), 2.27 (s, 3H, 8-CH3), 2.25 (s, 3H, 6-CH3). 13C NMR (200 MHz, DMSO-d6, δ): 166.86 (2-C), 145.13 (3′-C), 135.11 (8-C), 135.09 (6-C), 131.86 (6′-H), 129.81 (7-H), 129.41 (9′-C), 121.69 (9-C), 117.36 (1′-C), 28.8 (4-CH2), 24.57 (5-CH2), 21.37 (8-CH3), and 19.88 (6-CH3). HRMS (ESI): calculated: 231.0950; found: 231.0949.
6,8-Dimethylnaphtho[1,2-d]thiazol-2-amine (SKA-109)
SKA-109 was prepared from 5,7-dimethyl-1-tetralone (2 g, 11 mmol) according to general method II. The product was isolated as pink crystals (15 mg, 0.6%); m.p. = 157°C. 1H NMR (500 MHz, DMSO-d6, δ): 8.02 (s, 1H, 9-H), 7.73 (d, J = 8.81 Hz, 1H, 4-H), 7.59 (d, J = 8.84 Hz, 1H, 5-H), 7.53 (s, 2H, NH2), 7.17 (s, 1H, 7-H), 2.61 (s, 3H, 8-CH3), 2.45 (s, 3H, 6-CH3). 13C NMR (125 MHz, DMSO-d6, δ): 167.84 (2-C), 148.80 (3′-C), 135.61 (8-C), 134.92 (6-C), 128.70 (7-C), 126.90 (9-C), 121.68 (6′-C), 118.78 (1′-C), 117.57 (4-C), 100.61 (9′-C), 99.85 (5-C), 22.21 (8-CH3), and 20.14 (6-CH3). HRMS (ESI): calculated: 222.0794; found: 222.0794.
Thieno[2′,3′:5,6]benzo[1,2-d]thiazol-2-amine (SKA-110)
SKA-110 was prepared from 6,7-dihydro-4-benzo[b]thiophenone (0.5 g, 3 mmol) according to general procedure II. The product was isolated as a white solid (26 mg, 3.8%), m.p. = 161°C (CAS. 35711-03-6). 1H NMR (800 MHz, DMSO-d6, δ): 7.71 (d, J = 5.36 Hz, 1H, 7-H), 7.68 – 7.62 (m, 4H, 4-H, 5-H, and NH2), 7.58 (d, J = 5.41 Hz, 1H, 6-H). 13C NMR (125 MHz, DMSO-d6, δ): 168.26, 147.81, 137.83, 131.22, 126.97, 125.72, 121.95, 117.97, and 115.51.
5-Methylnaphtho[1,2-d]thiazol-2-amine (SKA-111)
SKA-111 was prepared from 4-methyl-1-tetralone (1 g, 11 mmol) according to general procedure II. The product was isolated as yellow crystals (100 mg, 16%); m.p. = 209°C (CAS. 1369170-24-0). 1H NMR (800 MHz, DMSO-d6, δ): 8.96 (d, J = 7.86 Hz, 1H, 9-H), 8.46 (d, J = 7.99 Hz, 1H, 6-H), 8.06 (s, 1H, 4-H), 7.98 (dt, J = 6.83, 13.83 Hz, 2H, 7-H and 8-H), 7.16 (s, 2H, NH2), 3.14 (s, 3H, CH3).13C NMR (125 MHz, DMSO-d6, δ): 166.73, 147.60, 131.54, 127.88, 127.33, 126.02, 125.58, 125.27, 124.75, 124.70, 119.39, and 19.01.
8-Fluoronaphtho[1,2-d]thiazol-2-amine (SKA-112)
SKA-112 was prepared from 7-fluoro-1-tetralone (0.5 g, 3 mmol) according to general procedure II. The product was isolated a clear oil (10 mg, 3%). 1H NMR (500 MHz, DMSO-d6, δ): 8.00 (dd, J = 5.74, 9.02 Hz, 1H, 7-H), 7.90 (dd, J = 2.67, 10.46 Hz, 1H, 9-H), 7.79 (d, J = 8.61 Hz, 1H, 5-H), 7.67 (s, 2H, NH2), 7.60 (d, J = 8.64 Hz, 1H, 4-H), 7.37 (triplet of doublets (td), J = 2.73, 8.83 Hz, 1H, 6-H). 13C NMR (125 MHz, DMSO, δ): 168.40 (2-C), 161.60 (8-CF), 159.67 (3′-C), 148.183 (6-C), 131.73 (6′-C), 129.52 (1′-C), 126.84 (9′-C), 121.27 (7-C), 119.43 (4-C), 115.54 (5-C), and 107.29 (9-C). HRMS (ESI): calculated: 219.0387; found: 219.0383.
5-Methyl-4,5-dihydronaphtho[1,2-d]thiazol-2-amine (SKA-113)
SKA-113 was prepared from 4-methyl-1-tetralone (2 g, 11 mmol) according to general procedure I. The product was isolated as white crystals (250 mg, 20%); m.p. = 109°C dec (CAS no. 896156-31-3). 1H NMR (500 MHz, DMSO-d6, δ): 7.56 (d, J = 6.4 Hz, 1H, 9-H), 7.40 (s, 2H, NH2), 7.21 (m, 3H, 6-H, 7-H, and 8-H), 3.12 (h, J = 6.8 Hz, 1H, 5-H), 2.76 (ddd, J = 175.4, 16.2, 6.6 Hz, 2H, 4-CH2), 1.23 (d, J = 6.9 Hz, 3H, 5-CH3). 13C NMR (125 MHz, DMSO-d6, δ): 167.85, 152.42, 140.02, 127.69, 127.37, 127.29, 122.97, 116.67, 99.85, 33.48, 29.20, and 21.45.
6-Methoxy-4,5-dihydronaphtho[1,2-d]thiazol-2-amine (SKA-114)
SKA-114 was prepared from 5-methoxy-1-tetralone (2 g, 11 mmol) according to general procedure I. The product was isolated as brown crystals (1.5 g 57%); m.p. = 200°C (CAS no. 489430-53-7). 1H NMR (500 MHz, DMSO-d6, δ): 7.23–7.13 (m, 2H, 9-H, and 8-H), 6.91 (s, 2H, NH2), 6.84 (d, J = 7.59 Hz, 1H, 7-H), 3.79 (s, 3H, OCH3), 2.89 (t, J = 8.09 Hz, 2H, 4-CH2), 2.73 (t, J = 8.07 Hz, 2H, 5-CH2). 13C NMR (125 MHz, DMSO-d6, δ): 167.04, 156.69, 144.90, 133.25, 127.75, 122.02, 118.20, 115.94, 110.01, 56.10, 21.50, and 21.18.
5-Methoxynaphtho[1,2-d]thiazol-2-amine (SKA-117)
SKA-117 was prepared from 1-amino-4-methoxynaphthalene (0.1 g, 0.51 mmol) according to general method III. The product was isolated as lavender crystals (16 mg, 14%); m.p. = 213°C (CAS. 1368289-59-1). 1H NMR (500 MHz, DMSO-d6, δ): 8.87 (d, J = 8.25 Hz, 1H, 9-H), 8.68 (d, J = 8.3 Hz, 1H, 6-H), 8.02 (t, J = 7.4 Hz, 1H, 7-H), 7.95 (t, J = 7.4 Hz, 1H, 8-H), 7.56 (s, 1H, 4-H), 7.11 (s, 2H, NH2), 4.49 (s, 3H, OCH3). 13C NMR (125 MHz, DMSO-d6, δ): 168.11, 155.81, 148.63, 127.48, 126.6, 126.56, 123.94, 119.16, 116.51, 114.98, 104.55, and 56.26.
5-Methylnaphtho[1,2-d]oxazol-2-amine (SKA-120)
SKA-120 was prepared from 4-methyl-1-tetralone according to general procedure IV. The product was isolated as light brown crystals (50 mg, 4%); m.p. = 209°C; Rf = 0.38 (cyclohexane-EtOAc, 1:1). 1H NMR (800 MHz, CDCl3, δ): 8.28 (d, J = 8.3 Hz, 1H, 9-H), 8.03 (d, J = 8.4 Hz, 1H, 6-H), 7.58 (t, J = 7.4 Hz, 1H, 8-H), 7.52 (t, J = 7.5 Hz, 1H, 7-H), 7.38 (s, 1H, 4-H), 5.39 (bs, 2H, NH2), and 2.74 (s, 3H, CH3). 13C NMR (200 MHz, CDCl3, δ): 160.37 (2-C), 152.05 (1′-C), 135.20 (3′-C), 130.07 (9′-C), 129.14 (6′-C), 126.12 (8-C), 125.33 (5-C), 124.91 (6-C), 124.77 (7′-C), 122.38 (9-C), 110.56 (4-C), 19.93 (5-CH3). 1H,13C-HSQC (800 MHz, CDCl3, cross-peaks δ): 8.28/122.38, 8.03/124.91, 7.58/126.12, 7.51/124.77, 7.38/110.57, and 2.80/19.93. HRMS (ESI): calculated: 199.0866; found: 199.0864.
5-Methylnaphtho[2,1-d]oxazol-2-amine (SKA-121)
SKA-121 was prepared from 4-methyl-1-tetralone according to general procedure IV. The product was isolated as brown crystals (50 mg, 4%); m.p. = 186°C dec; Rf = 0.28 (cyclohexane-EtOAc, 1:1). 1H NMR (800 MHz, CDCl3, δ): 8.03 (d, J = 8.5 Hz, 1H, 9-H), 8.01 (d, J = 8.3 Hz, 1H, 6-H), 7.56 (t, J = 7.5 Hz, 1H, 7-H), 7.45 (t, J = 6.0 Hz, 1H, 8-H), 7.43 (s, 1H, 4-H), 5.33 (bs, 2H, NH2), 2.73 (s, 3H, CH3). 13C NMR (200 MHz, CDCl3, δ): 160.80 (2-C), 141.72 (1'-C), 137.24 (3′-C), 128.85 (5-C), 119.28 (6-C), 125.21 (9-C), 126.39 (7-C), 123.92 (8-C), 131.34 (6′-C), 119.77 (9′-C), 117.17 (4-C), 19.58 (5-CH3). 1H,13C-HSQC (800 MHz, CDCl3, cross-peaks δ): 8.03/125.21, 8.01/119.28, 7.56/126.39, 7.45/123.92, 7.43/117.17, and 2.73/19.58. HRMS (ESI): calculated: 199.0866; found: 199.0864.
Crystal Structure Determinations
The SKA-120 and SKA-121 crystals selected for data collection were mounted in the 90-K nitrogen cold stream provided by a CRYO Industries of America (Manchester, NH) low-temperature apparatus on the goniometer head of a Bruker D8 diffractometer equipped with an ApexII CCD detector (Bruker, Fremont, CA). Data were collected with the use of Mo Kα radiation (λ = 0.71073 Å). The structures were solved by direct methods (SHELXS-97) and refined by full-matrix least-squares on F2 (SHELXL-2013). All nonhydrogen atoms were refined with anisotropic displacement parameters. For a description of the method, see Sheldrick (2008).
Crystal Data SKA-120
C12H10N2O, F.w. = 198.22, brown plate, dimensions 0.18 × 0.34 × 0.60 mm, monoclinic, P21/n, a = 14.2110(9) Å, b = 3.8854(3) Å, c = 17.5101(11) Å, β = 107.537(2)°, V = 921.89(11) Å3, Z = 4, R1 [1518 reflections with I > 2σ(I)] = 0.0307, wR2 (all 1671 data) = 0.0900, 176 parameters, 0 restraints.
Crystal Data SKA-121
C12H10N2O, F.w. = 198.22, brown plate, monoclinic, P21/n, a = 8.0532(12) Å, b = 21.377(3) Å, c = 11.5094(17) Å, β = 107.823(2)°, V = 1886.3(5) Å3, Z = 8, R1 [2571 reflections with I > 2σ(I)] = 0.0376, wR2 (all 3413 data) = 0.0949, 335 parameters, 0 restraints.
CCDC 9966657 and 996658 contains the supplemental crystallographic data for this article. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Cells, Cell Lines, and Clones
Human embryonic kidney cells (HEK-293) stably expressing hKCa2.1, rKCa2.2, and hKCa3.1 were obtained from Khaled Houamed (University of Chicago, IL) in 2002 and have been maintained in the Wulff Laboratory at the University of California since then. The cloning of hKCa2.3 (19 CAG repeats) and hKCa3.1 was previously described (Wulff et al., 2000). The hKCa2.3 clone was later stably expressed in COS-7 cells at Aurora Biosciences Corp. (San Diego, CA). Cell lines stably expressing other mammalian ion channels were gifts from several sources: hKCa1.1 in HEK-293 cells (Andrew Tinker, University College London); hKV2.1 in HEK293 cells (James Trimmer, University of California Davis, Davis, CA); KV11.1 (HERG) in HEK-293 cells (Craig January, University of Wisconsin, Madison); hNaV1.4 in HEK-293 cells (Frank Lehmann-Horn, University of Ulm, Germany); hNaV1.5 in HEK-293 cells (Christopher Lossin, University of California Davis); and hCaV1.2 in HEK-293 cells (Franz Hofmann, Munich, Germany). L929 cells stably expressing mKV1.3 and mKV3.1 have been previously described (Grissmer et al., 1994); N1E-115 neuroblastoma cells (expressing mNaV1.2) were obtained from ATCC; division arrested CHO cells expressing hNaV1.7 were purchased from ChanTest (Cleveland, OH).
Electrophysiology
Experiments were conducted either manually with an EPC-10 amplifier (HEKA, Lambrecht/Pfalz, Germany) or on a QPatch-16 automated electrophysiology platform (Sophion Biosciences, Ballerup, Denmark). For manual experiments, COS-7, HEK-293, or L929 cells were trypsinized, plated onto poly-l-lysine–coated coverslips, and typically recorded from between 20 minutes and 4 hours after plating. Patch pipettes were pulled from soda lime glass (micro-hematocrit tubes, Kimble Chase, Rochester, NY) and had resistances of 2-3 MΩ. For measurements of KCa channels expressed in HEK-293 cells (KCa2.1, KCa2.2, and KCa3.1), we used normal Ringer solution as external with an internal pipette solution containing (in mM): 140 KCl, 1.75 MgCl2, 10 HEPES, 10 EGTA, and 7.4 CaCl2 (500 nM free Ca2+) or 6 CaCl2 (250 nM free Ca2+), pH 7.2, 290–310 mOsm. Free Ca2+ concentrations were calculated with MaxChelator assuming a temperature of 25°C, a pH of 7.2, and an ionic strength of 160 mM. To reduce contaminating currents from native chloride channels in COS-7 cells, KCa2.3 currents were recorded with an internal pipette solution containing (in mM) the following: 145 K+ aspartate, 2 MgCl2, 10 HEPES, 10 EGTA, and 7.4 CaCl2 (500 nM free Ca2+), pH 7.2, 290–310 mOsm. Na+ aspartate Ringer was used as an external solution (in mM): 160 Na+ aspartate, 4.5 KCl, 2 CaCl2, 1 MgCl2, 5 HEPES, pH 7.4, 290–310 mOsm. Both KCa2 and KCa3.1 currents were elicited by 200-ms voltage ramps from −120 mV to 40 mV applied every 10 seconds, and the -fold increase of slope conductance at −80 mV by drug taken as a measure of channel activation. KV2.1, KV1.3, and KV3.1 currents were recorded in normal Ringer solution with a Ca2+-free KF-based pipette solution as previously described (Schmitz et al., 2005). HERG (KV11.1) currents were recorded with a two-step pulse from −80 mV first, to 20 mV for 1 second, and then to −50 mV for a 1-second reduction of both peak and tail current by the drug. NaV1.7 currents were recorded with 30-millisecond pulses from −90 mV to 10 mV every 10 seconds with a CsF-based pipette solution and normal Ringer as an external solution. CaV1.2 currents were elicited by 100-millisecond depolarizing pulses from −80 to 20 mV every 10 seconds with a CsCl-based pipette solution and an external solution containing 30 mM BaCl2. Blockade of both Na+ and Ca2+ currents was determined as reduction of the current minimum.
For automated electrophysiology experiments, cells were grown to ∼70% confluency, rinsed in sterile phosphate-buffered saline containing 0.02% EDTA, and lifted with 2 ml of TrypLE Express (Gibco, Grand Island, NY) for ∼2 minutes. When cells were rounded but not detached, they were dislodged by gentle tapping, suspended in Dulbecco’s modified Eagle’s medium, centrifuged, and resuspended in 1 ml of external solution; placed into the Qfuge tube; and resuspended in 150–200 μl of extracellular solution after one additional spin on the QPatch. Whole-cell patch-clamp experiments were then carried out using disposable 16-channel planar patch chip plates (QPlates; patch-hole diameter approximately 1 μm, resistance 2.00 ± 0.02 MΩ). Cell positioning and sealing parameters were set as follows: positioning pressure −70 mbar, resistance increase for success 750%, minimum-seal resistance 0.1 GΩ, holding potential −80 mV, and holding pressure −20 mbar. To avoid the rejection of cells with large KCa3.1 currents, the minimum seal resistance for whole-cell requirement was lowered to 0.001 GΩ. Access was obtained with the following sequence: 1) suction pulses in 29-mbar increments from −250 mbar to −453 mbar; 2) a suction ramp of an amplitude of −450 mbar; 3) 400-mV voltage zaps of 1-milliseconds’ duration (10×). After establishment of the whole-cell configuration, cells were held at −80 mV and KCa3.1, KCa2.1, or KCa2.2 currents elicited by a voltage protocol that held at −80 mV for 20 ms, stepped to −120 mV for 20 milliseconds, ramped from −120 to 40 mV in 200 milliseconds, and then stepped back to −120 mV for 20 milliseconds. This pulse protocol was applied every 10 seconds. KCa1.1 currents were elicited by 160-ms voltage ramps from −80 to 80 mV applied every 10 seconds (500 nM free Ca2+), and channel modulation was measured as a change in mean current amplitude. NaV1.2 currents from N1E-115 cells, NaV1.4, and NaV1.5 currents from stably transfected HEK cells were recorded with 20-millisecond pulses from −90 mV to 0 mV every 10 seconds with a KF-based internal solution and normal Ringer as an external solution. Current slopes (in ampere per sec) were measured using the Sophion QPatch software and exported to Microsoft Excel and Origin 7.0 (OriginLab Corp., Northampton, MA) for analysis. Increases or decreases of slopes between −85 and −65 mV were used to calculate KCa2/3 activation. Data fitting to the Hill equation to obtain EC50 and IC50 values were performed with Origin 7.0. Data are expressed as mean ± S.D.
The inside-out experiments shown in Fig. 4 were performed on the KCa3.1-stable HEK 293 cell line. Symmetrical K+ was used to obtain larger currents. The extracellular solutions contained (in mM) the following: 154 KCl, 10 HEPES (pH = 7.4), 2 CaCl2, and 1 MgCl2. Solutions on the intracellular side contained (in mM) the following: 154 KCl, 10 HEPES (pH = 7.2), 10 EGTA, 1.75 MgCl2, and CaCl2 to yield calculated free Ca2+-concentrations of 0.05, 0.1, 0.25, 0.3, 0.5, 1.0, and 10.0 μM. Cells were clamped to a holding potential of at 0 mV, and KCa currents were elicited by 200-millisecond voltage ramps from −80 to 80 mV applied every 10 seconds.
Fig. 4.
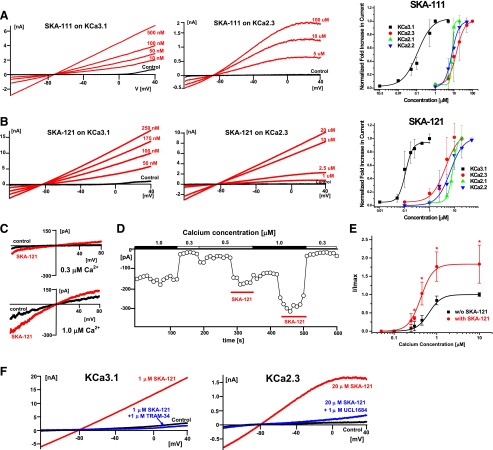
SKA-111 and SKA-121 are potent and selective KCa3.1 activators. (A) Example traces of KCa3.1 and KCa2.3 activation by SKA-111 and concentration-response curves for KCa3.1 (EC50 111 ± 27 nM, nH 1.5), KCa2.3 (EC50 13.7 ± 6.9 μM, nH 1.9), KCa2.1 (EC50 8.1 ± 0.4 μM, nH 4.5), and KCa2.2 (EC50 7.7 ± 1.9 μM, nH 2.3). (B) Example traces of KCa3.1 and KCa2.3 activation by SKA-121 and concentration-response curves for KCa3.1 (EC50 109 ± 14 nM, nH 3.0), KCa2.3 (EC50 4.4 ± 2.3 μM, nH 1.6), KCa2.1 (EC50 8.7 ± 1.6 μM, nH 4.1), and KCa2.2 (EC50 6.8 ± 2.2 μM, nH 1.7). All data points are mean ± S.D. (C) Representative currents from inside-out patches in the presence of 0.3 μM (top) and 1 μM (bottom) Ca2+ before and after application of 1 μM SKA-121. (D) KCa3.1 current at −75 mV in an inside-out patch exposed to varying Ca2+ concentrations as a function of time. (Note: SKA-121 applied with 500 nM Ca2+ was washed out with 1 μM Ca2+). (E) Ca2+ concentration-response curve for KCa3.1 activation measured from inside-out patches in the absence or presence of 1 μM SKA-121. Currents from individual patches were normalized to the effect of 10 μM Ca2+ in the absence of SKA-121. Data are mean ± S.D. (n = 3–5/data point; *P < 0.05, unpaired Student t test). An extra sum-of-squares F test (GraphPad Prism5) to compare the two curves rendered a P < 0.0001 for comparison of the EC50 values in the presence and absence of SKA-121. (F) Blockade of KCa3.1 and KCa2.3 currents activated by SKA-121 by TRAM-34 and UCL1684.
For all electrophysiology experiments, solutions of benzothiazoles and benzooxazoles were always freshly prepared from 1 mM or 10 mM stock solutions in DMSO during the experiment. The final DMSO concentration never exceeded 1%. For automated assays, glass vial inserts (Sophion Biosciences) were filled with 350–400 μl of compound solution and placed into the glass insert base plate for use in the QPatch assay right before starting the QPatch.
Isometric Myography on Porcine Coronary Arteries
Porcine coronary arteries (PCAs) were carefully dissected from hearts kindly provided by the local abattoir (Mercazaragoza, Zaragoza, Spain), cleaned of fat and connective tissue, and cut into 3- to 4-mm rings. Rings were mounted on hooks connected to an isometric force transducer (Pioden UF1, Graham Bell House, Canterbury, UK) and prestretched to an initial tension of 1 g. Composition Krebs’ buffer (in mM) was as follows: NaCl 120, NaHCO3 24.5, CaCl2 2.4, KCl 4.7, MgSO4 1.2, KH2PO4 1, and glucose 5.6, pH 7.4, at 37°C and equilibrated with 95% O2/5% CO2. Changes in tension were registered using a Mac Laboratory System/8e program (AD Instruments Inc., Milford, MA). The buffer contained the NO-synthase blocker, Nω-nitro-l-arginine (l-NNA, 300 µM), and the cyclooxygenase blocker indomethacin (10 µM) to measure EDH-type relaxation. After three washes, rings were precontracted with the thromboxane analog U46619 (0.2 µM). Thereafter rings were exposed to bradykinin (BK, 1 µM) in combination with vehicle DMSO, SKA-111 (1 µM), SKA-111 plus TRAM-34 (1 µM), SKA-111 plus TRAM-34 plus UCL-1684 (1 µM), SKA-121 (1 µM), SKA-121 plus TRAM-34, or SKA-121 plus TRAM-34 plus UCL-1684. After washout, rings were contracted with a high KCl buffer (60 mM) to determine maximal contraction. TRAM-34 was synthesized as previously described (Wulff et al., 2000). UCL1684 and U46619 were purchased from Tocris (Wiesbaden-Nordenstadt, Germany). Data analysis was as follows: EDH-type relaxations were determined as percentage of change of U46619 contraction and are shown relative to the totally relaxed state (without U46619).
Telemetry
The experiments were in accordance with the Animal Research Reporting In Vivo Experiments guidelines and approved by the Institutional Animal Care and Use Committee of the IACS. Surgical implantation of TA11PA-C10 pressure transducers (Data Sciences International, St. Paul, MN) into the left carotid artery and telemetry were performed as described previously (Brahler et al., 2009). Four female wild-type (22 ± 1 g) and four female KCa3.1−/− (27 ± 2 g) mice were used in the present study. After surgery, mice were allowed to recover for 10 days before compounds or vehicle were injected and telemetry data were collected. After a washout phase of at least 48 hours after a first injection, animals were reused for injections of a higher dose of the SKA-111, SKA-121, or vehicle. Thereafter, mice were treated with 50 μg/ml Nω-nitro-l-arginine methyl ester (l-NAME, Sigma-Aldrich DK) in the drinking water. This l-NAME treatment over 2 days increased MAP by 10 ± 2 mm Hg in wild-type mice. Injection of compounds started on day 3 of l-NAME treatment. Preparation and injection of SKA-111 and SKA-121 were as follows: Appropriate amounts of SKA-111 and SKA-121 were dissolved in warmed peanut oil (SKA-111) or in a mixture of peanut oil/DMSO (9:1 v/v, both from Sigma-Aldrich DK) to give a dose of 30 or 100 mg/kg. Maximal injection volume was ±600 μl. SKA-111 solution, well-stirred suspension (SKA-121), or vehicle was injected i.p. during hour 3 of the dark phase. Mice were subjected to isoflurane anesthesia to minimize stress and pain during compound application. Telemetry data were collected and analyzed after the mice fully recovered from anesthesia (20 minutes after injection). Telemetry data were recorded over 1 minute every 10 minutes over 24 hours and averaged. Data were analyzed using Data Sciences International software.
Pharmacokinetics
Twelve-week-old male C57Bl/6J mice were purchased from Charles River Laboratories (Wilmington, MA) and housed in microisolator cages with rodent chow and autoclaved water ad libitum. All experiments were in accordance with National Institutes of Health guidelines and approved by the University of California, Davis Institutional Animal Care and Use Committee. For i.v. application, SKA-111 and SKA-121 were dissolved at 5 mg/ml in a mixture of 10% CremophorEL (Sigma-Aldrich, St. Louis, MO) and 90% phosphate-buffered saline and then injected at 10 mg/kg into the tail vein (n = 8 mice per compound). Another group of mice (n = 8) received SKA-121 orally. At various time points after the injection, blood was collected into EDTA blood sample collection tubes either from the saphenous vein or by cardiac puncture under deep isoflurane anesthesia. After the cardiac puncture, mice were sacrificed by cutting the heart, and then the brain was removed. Individual mice were typically used for three times points (two blood collections from the saphenous vein plus the terminal blood collection). Plasma was separated by centrifugation, and plasma and brain samples were stored at −80°C pending analysis. Brain samples were homogenized in 1 ml of H2O with a Brinkman Kinematica PT 1600E homogenizer, and the protein was precipitated with 1 ml of acetonitrile. The samples were then centrifuged at 3000 rpm, and supernatants concentrated to 1 ml. Plasma and homogenized brain samples were purified using C18 solid-phase extraction cartridges (ThermoFisher Scientific, Waltham, MA) preconditioned with acetonitrile, followed by 1 ml of water. The loaded column was washed with 2 ml of water. SKA-121 was eluted with 3 ml of acetonitrile. SKA-111 was eluted with 3 ml of methanol containing 1% NH4OH. Eluted fractions were dried under nitrogen and reconstituted in acetonitrile. LC/MS analysis was performed with a Waters Acquity UPLC (Waters, New York, NY) equipped with a Acquity UPLC BEH 1.7 µm RP-18 column (Waters, New York, NY) interfaced to a TSQ Quantum Access Max mass spectrometer (MS) (ThermoFisher Scientific, Waltham, MA). The isocratic mobile phase consisted of 80% acetonitrile and 20% water, both containing 0.1% formic acid with a flow rate of 0.25 ml per minute. Under these conditions SKA-111 had a retention time of 0.83 minute and SKA-121 a retention time of 0.96 minute. Using electrospray ionization, MS and selective reaction monitoring (capillary temperature 300°C, capillary voltage 4000 V, collision energy -34 eV, positive ion mode), SKA-121 was quantified by its base peak of 128.14 m/z and its concentration was calculated with a 5-point calibration curve from 100 nM to 10 μM. SKA-111 (capillary temperature 325°C, capillary voltage 4000 V, collision energy −28eV, positive ion mode) was quantified by its base peak of 200.045 m/z and its concentration was calculated with a six-point calibration curve from 100 nM to 20 μM.
The percentage of plasma protein binding for SKA-111 and SKA-121 was determined by ultrafiltration. Rat plasma (500 µl) was spiked with 10 µM of compound in 1% DMSO, and the sample was loaded onto a Microcon YM-30 Centrifugal Filter (Millipore Corp., Bedford, MA) and centrifuged at 13,500g for 30 minutes at RT. The retentate was collected by inverting the filter into an Eppendorf tube and spinning at 13,500g for 15 minutes. The retentate then underwent sample preparation as per the previously described procedure for SKA-111 or SKA-121. Plasma protein binding was found to be to be 59 ± 2% (n = 3) for SKA-111 and 81 ± 4% (n = 2) for SKA-121.
Results
SAR Study Aiming to Obtain Selectivity for KCa3.1 with SKA-31 as a Template.
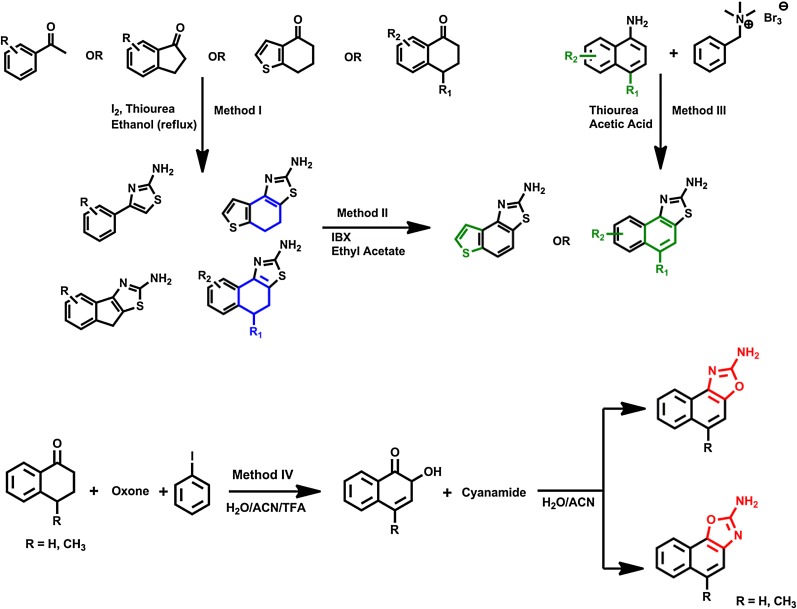
As described in the Introduction, the limited ability of existing benzimidazole/benzothiazole-type KCa2/3 activators such as SKA-31 to differentiate between KCa2 and KCa3.1 channels made it desirable to determine whether additional structural modification would increase selectivity for KCa3.1. Toward this goal, we synthesized a small focused library of 2-aminothiazoles, 2-aminobenzothiazoles or 2-aminonapthooxazoles (Fig. 1). Substituted 2-aminothiazoles were prepared by a one-step Hantzsch thiazole synthesis (Goblyos et al., 2005) from the appropriate substituted 1-tetralone, thiourea, and iodine (Method I in Fig. 1). This method allowed us to obtain both “open” 2-aminothiazoles as well as to replace the central aromatic ring of SKA-31 with aliphatic rings. Fully aromatic 2-aminobenzothiazoles could then be produced by aromatizing with 2-iodoxybenzoic acid (Method II in Fig. 1). An alternative route to 5-position substituted 2-aminobenzothiazole was the classic Hugerschoff benzothiazole synthesis (Jordan et al., 2003), in which appropriately substituted amines were transformed into the corresponding thioureas and then subsequently reacted with benzyltrimethyl ammonium tribromide to deliver bromine in stoichiometric amounts as an alternative to liquid bromine (Method III in Fig. 1). Lastly, naphthooxazoles were prepared by first oxidizing 4-methyl-1-tetralone with in situ formed bis(trifluoroacetoxy)iodo]benzene and then adding cyanamide (Schuart and Müller, 1973) to the intermediately produced 4-methylnaphthalene-1,2-dione (Method IV in Fig. 1).
Fig. 1.
General scheme for the synthesis of thiazoles, 2-aminonaphtho[1,2-d]thiazoles, and 2-aminonaphtho[1,2-d]oxazoles.
The compounds synthesized by these methods as well as five commercially available compounds were tested for their KCa2.3- and KCa3.1-activating activity using either manual or automated whole-cell patch-clamp. Our group previously described the establishment of a QPatch assay for KCa3.1 modulators. In this study, we benchmarked data obtained on the QPatch against manual patch-clamp electrophysiology by determining the potency of several commonly used KCa3.1 inhibitors (TRAM-34, NS6180, charybdotoxin) and activators (EBIO, riluzole, SKA-31) and found that the QPatch results were virtually identical to the IC50 and EC50 values obtained by manual patch clamp in our hands (Jenkins et al., 2013). We here made use of this assay and determined EC50 values for KCa3.1 activation using HEK-293 cells stably expressing human KCa3.1. Activities on human KCa2.3 were determined by manual electrophysiology since we currently only have KCa2.3 available in COS-7 cells, which are difficult to handle on the QPatch. For both channels we used 250 nM of free [Ca2+]i since positive gating-modulators like SKA-31 typically increase KCa currents at this Ca2+ concentration roughly 30-fold, creating a large assay window (Sankaranarayanan et al., 2009; Jenkins et al., 2013).
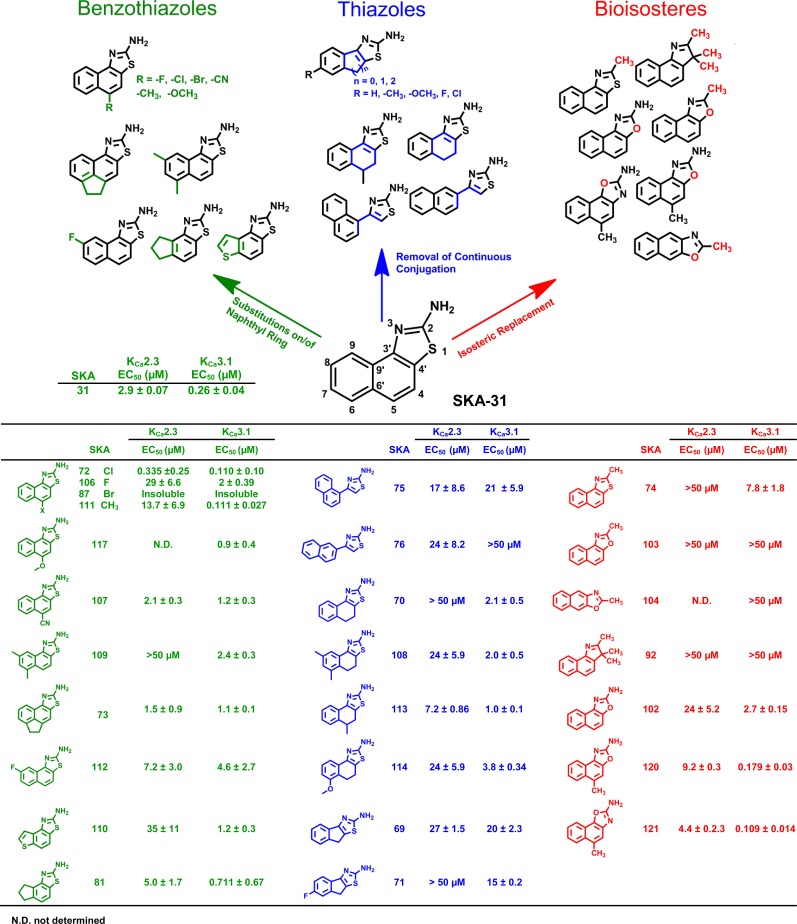
Removal of the continuous conjugation by opening of the napthothiazole system (SKA-75 and SKA-76) of SKA-31 or replacement of the internal aromatic ring with either a cyclohexyl (SKA-70, SKA-108, SKA-113, SKA-114) or a cyclopentyl ring (SKA-69, SKA-71) in general reduced both potency and selectivity irrespective of whether the compounds bore any substituents or not (Fig. 2, blue compounds). Since these results demonstrated that aromaticity of the internal ring was required for both KCa2.3 and KCa3.1 activation, we went back to benzothiazoles (Fig. 2 green compounds) and next explored substitutions on the napthothiazole system of SKA-31. Introduction of substituents in the 5-position had varying effects: chloride (SKA-72), which is both electron withdrawing and lipophilic, increased potency on both KCa2.3 (EC50 335 nM) and KCa3.1 (EC50 110 nM) but basically abolished any selectivity between the two channels. Fluoride in the 5-position (SKA-106) reduced potency roughly 10-fold compared with SKA-31 but preserved selectivity, whereas introduction of bromide (SKA-87) resulted in a compound that was too insoluble to be tested. Introduction of a methyl group in the 5-position, which is less lipophilic than chloride but has a positive inductive effect on the ring system, slightly increased potency for KCa3.1 (EC50 111 nM) in comparison with SKA-31 and dramatically increased selectivity for KCa3.1 over KCa2.3 to ∼100-fold (SKA-111). However, replacement of the CH3 group with other, larger carbon containing electron-donating groups such as −OCH3 (SKA-117) or electron withdrawing groups such as –CN (SKA-107) again reduced potency and selectivity. Attaching two of the obviously favorable CH3 groups in positions 6 and 8 of the ring system (SKA-109) instead of the 5-position preserved selectivity over KCa2.3 but reduced potency on KCa3.1 by 10-fold. Installation of an ethylene bridge connecting the 5- and 6- position reduced both potency and selectivity and resulted in a compound (SKA-73) that activated both KCa2.3 and KCa3.1 equipotently with an EC50 of 1 μM. We further tried replacing the terminal ring of SKA-31 with a thiophene (SKA-110) or an aliphatic cyclopentyl (SKA-81) but again only saw a reduction in potency.
Fig. 2.
Chemical structures and EC50 values for KCa2.3 and KCa3.1 activation. All compounds were tested at least three times at concentrations of 4 to 5, and EC50 values were determined by fitting the Hill equation to the increase in slope conductance between −80 and −65 mV.
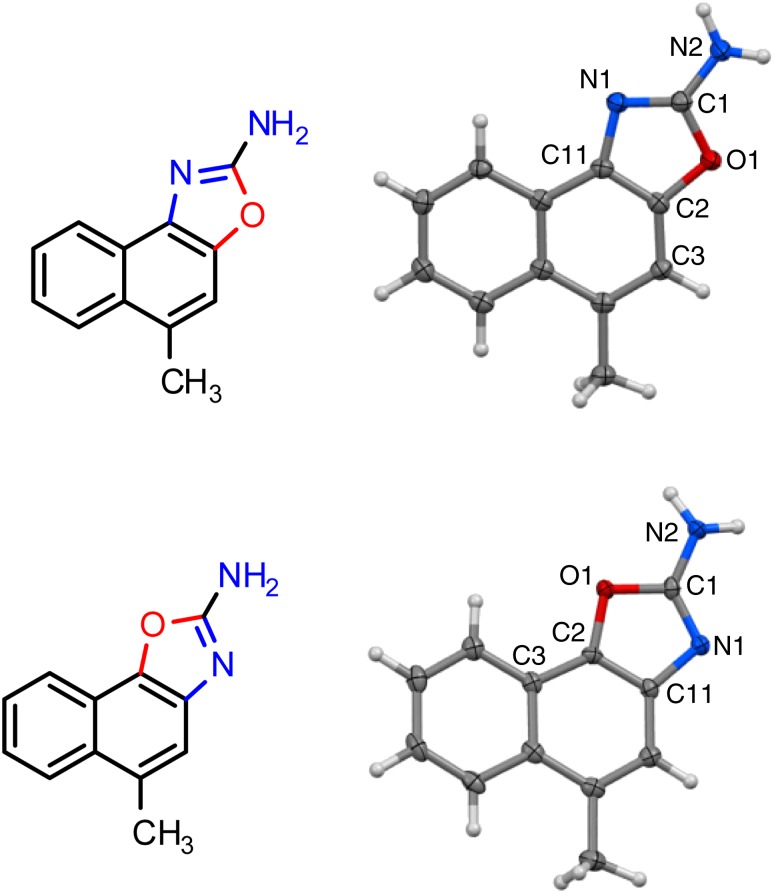
To better understand the full extent of the pharmacophore and potentially obtain patentable compounds, we explored alternative scaffolds (Fig. 2, red compounds). Moving away from the 2-aminothiazole system by replacing the 2-position NH2 group with a CH3 group (SKA-74), as well as isosterically replacing the S atom with an O (SKA-103 and SKA-104) or geminal CH3 groups (SKA-92), completely abolished activity. However, if the 2-position NH2 group was retained and only the S isosterically replaced with an O as in the SKA-102, which basically constitutes an oxazole analogous SKA-31, activity was regained, and the resulting compound activated KCa3.1 with an EC50 of 2.7 μM. Introduction of a CH3 group in 5-position, which previously was found to increase selectivity of the napthothiazole SKA-111 for KCa3.1 to 100-fold, had a similar effect on the 2-aminonaphthooxazole system. The two regioisomers, SKA-120 and SKA-121, which resulted from the synthesis and had to be separated by flash chromatography, exhibited EC50 values of 180 and 109 nM for KCa3.1 and EC50 values of 9.2 and 4.4 μM for KCa2.3, corresponding to a ∼50- or 40-fold selectivity. The correct structural assignment of the two regioisomers was confirmed by the different chemical shift of proton 9-H in the 800 MHz 1H-NMR since this proton is shielded differently depending on whether it is in proximity to either N or O in the adjacent oxazole ring. We further grew crystals of SKA-120 and SKA-121 and had them subjected to X-ray analysis, which allowed us to “see” the exact position of the N and O in the two compounds (Fig. 3). The crystal structures show two very different hydrogen bonding networks. Although SKA-120 exists as a dimer, SKA-121 has a hydrogen bonding motif that leads to a tetramer forming ribbon structures (Supplemental Fig. 1).
Fig. 3.
X-ray crystal structures of SKA-120 (top) and SKA-121 (bottom).
In summary, this SAR study demonstrated that it is possible to generate KCa3.1-selective activators using the naphthothiazole and the isosteric naphthooxazole scaffolds. In both cases, the presence of the 2-amino group was absolutely required for activity on both KCa2.3 and KCa3.1 channels. It was further necessary for the annulated three-ring system to be fully aromatic. Replacement of the terminal or the internal ring system with aliphatic rings reduced activity on both channels. The key position able to confer both high potency and selectivity for KCa3.1 seems to be the 5-position, which proved to have a very “tight” SAR. Whereas the large, lipophilic, and relatively “soft” chloride endowed the compounds with potency on both KCa2.3 and KCa3.1 (SKA-72), only CH3 in this position produced selectivity for KCa3.1 on both the naphthothiazole (SKA-111) and the isosteric naphthooxazole (SKA-121) system.
SKA-111 and SKA-121 Are Selective KCa3.1 Activators.
To fully evaluate the selectivity of the naphthothiazole SKA-111 and the naphthooxazole SKA-121, we determined seven-point concentration-response curves on KCa2.1, KCa2.2, KCa2.3 and KCa3.1 with 250 nM free Ca2+ in the internal solution (Fig. 4). SKA-111 and SKA-121 displayed nearly identical EC50 values on KCa3.1 (111 ± 27 nM and 109 ± 14 nM). Similar to the template SKA-31 (Sankaranarayanan et al., 2009), these effects plateaued at a roughly 30-fold maximal current increase with this intracellular Ca2+ concentration. Both compounds exhibited 40- to 120-fold selectivity over the three KCa2 channels (Fig. 4; Table 1). The Hill coefficient nH varied between 1.6 and 3.1 in most cases, which was again similar to what had been previously reported for the template SKA-31.
TABLE 1.
Selectivity of SKA-111 and SKA-121 over selected ion channels
The number in parentheses indicates the number of cells used to determining the EC50 values or the % of current inhibition.
| Channel | SKA-111 EC50 | SKA-121 EC50 |
|---|---|---|
| KCa1.1 | 120% of current at 25 µM (3) | 115% of current at 50 µM (3) |
| KCa2.1 | 8.1 ± 0.4 (10) | 8.7 ± 1.6 (8) |
| KCa2.2 | 7.7 ± 1.9 (10) | 6.8 ± 1.7 (12) |
| KCa2.3 | 13.7 ± 6.9 (15) | 4.4 ± 2.6 (18) |
| KCa3.1 | 0.111 ± 0.027 (24) | 0.019 ± 0.014 (21) |
| Channel | SKA-111% current inhibition at 25 µM | SKA-121% current inhibition at 50 µM |
| KV1.3 | 28.5 ± 2.3% (4) | 27.5 ± 7.2% (3) |
| KV2.1 | 37.3 ± 9.0% (4) | 43.2 ± 12.5% (3) |
| KV3.1 | 32.9 ± 1.3% (3) | 46.3 ± 16.5% (4) |
| KV11.1 (hERG) | 10.1 ± 7.7% (5) | 16.7 ± 9.7% (5) |
| NaV1.2 | 19.5 ± 6.9% (5) | 15.2 ± 12.7% (5) |
| NaV1.4 | 33.4 ± 10.5% (5) | 25.7 ± 1.3% (5) |
| NaV1.5 | 39.9 ± 11.1% (5) | 32.1 ± 11.1% (5) |
| NaV1.7 | 27.5 ± 0.9% (3) | 28.5 ± 1.8% (5) |
| CaV1.2 | 49.5 ± 17.0% (5) | 48.9 ± 2.7% (5) |
We next determined the selectivity of SKA-111 and SKA-121 over more distantly related channels. At the highest reasonable and well-dissolvable test concentrations, 25 μM for SKA-111 and 50 μM for SKA-121, both compounds blocked representative members of the major KV channel families (KV1.3, KV2.1, KV3.1, and KV11.1) by 10% to 46% (Table 1). Similarly, neuronal (NaV1.2, NaV1.7), skeletal muscle (NaV1.4), and cardiac (NaV1.5) sodium channels, as well as L-type Ca2+ channels (CaV1.2), were blocked by 20% to 50% by 25 μM of SKA-111 or 50 μM SKA-121. SKA-111 and SKA-121 thus displayed at least 200- to 400-fold selectivity for KCa3.1 over these physiologically relevant channels. Interestingly, while performing this selectivity screen, we found that N1E-115 neuroblastoma cells, a cell line established in the 1970s from a spontaneous mouse neuroblastoma tumor, are highly suitable for automated electrophysiology. The cell line produced large NaV1.2 currents comparable to NaV1.5 currents in a stable HEK-293 cell line (Supplemental Fig. 2).
SKA-121 is a Positive Gating Modulator of KCa3.1.
Classic KCa activators like EBIO and NS309 have been shown to increase the apparent Ca2+-sensitivity of KCa channels by stabilizing the interaction between CaM and KCa channels (Pedarzani et al., 2001; Li et al., 2009) Since this phenomenon manifests in a leftward shift of the Ca2+ concentration-response curve, we performed inside-out experiments in which we varied the intracellular [Ca2+]i concentration and investigated the ability of 1 μM of SKA-121 to further activate KCa3.1 currents at different [Ca2+]i concentrations. As shown in Fig. 4C, inside-out patches pulled from hKCa3.1-expressing HEK-293 cells exhibited Ca2+-dependent K+ currents, reversing at 0 mV in symmetrical K+, which could be increased further by SKA-121 at every Ca2+ concentration. The EC50 of the Ca2+-concentration response curve (Fig. 4E) shifted from 650 ± 50 nM to 380 ± 95 nM in the presence of 1 μM of SKA-121, whereas the Hill coefficient was not changed by SKA-121 (nH ∼3 in both cases). Interestingly, SKA-121 not only shifted the curve to the left, but it also substantially increased the maximal achievable current at 1 and 10 μM, suggesting that the compound might be able to further increase the open probability of KCa3.1 at these Ca2+ concentrations. As expected, KCa3.1 currents activated by 1 μM SKA-121 could be completely inhibited by 1 μM of the KCa3.1 pore blocker TRAM-34 (Wulff et al., 2000), whereas the KCa2 channel pore blocker UCL1684 (Rosa et al., 1998) had a similar effect on KCa2.3 currents activated by 20 μM SKA-121 (Fig. 4F).
SKA-111 and SKA-121 Increase Bradykinin-Induced Vasodilation.
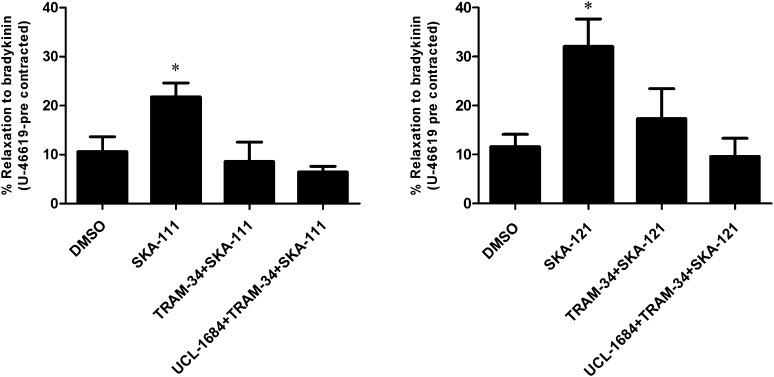
In addition to modulating the contractile state of the underlying vascular smooth muscle by releasing nitric oxide and prostacyclin, the vascular endothelium can induce an endothelium-derived hyperpolarization (EDH) in response to stimulation with acetylcholine or bradykinin (BK). These agonists increase [Ca2+]i in the endothelium, activate KCa3.1 and KCa2.3, and induce KCa, channel-mediated hyperpolarization and arterial relaxation (Ng et al., 2008; Grgic et al., 2009; Dalsgaard et al., 2010; Edwards et al., 2010; Köhler et al., 2010; Wulff and Köhler, 2013). To demonstrate that SKA-111 and SKA-121 efficiently augment native KCa3.1 in PCAs and thereby potentiate BK-induced relaxation, we performed isometric myography on PCA precontracted with 0.2 μM of the vasospasmic thromboxane mimetic, U46619. SKA-111, as well as SKA-121, both at 1 µM, potentiated BK (1 µM)-induced relaxation to ≈200% and ≈300%, respectively (Fig. 5). The KCa3.1 blocker TRAM-34 (1 µM) prevented this potentiation and the combination of TRAM-34 and the KCa2.3 blocker UCL-1684 (1 µM) inhibited this potentiation slightly more effectively than TRAM-34 alone (Fig. 5).
Fig. 5.
Isometric myography. SKA-111 and SKA-121 (both at 1 µM) increased BK-induced EDH-type relaxation of U46619-precontracted PCA rings in the presence of blockers of NO synthesis (l-NNA) and cyclooxygenases (indomethacin). TRAM-34 (1 µM) alone and the combination of TRAM-34 and UCL-1684 (1 µM) prevented the increase of relaxation. Data are mean ± S.E.M., n = 5–22 PCA. *P < 0.05, unpaired Student’s t test.
These data from ex vivo vessel experimentation demonstrate that the KCa3.1-selective activators SKA-111 and SKA-121 are capable of positively modulating a physiologic response, (e.g., EDH-type vasorelaxation, in which KCa3.1/KCa2.3 functions have been implicated before) (Edwards et al., 2010; Wulff and Köhler, 2013).
Systemic Cardiovascular Effects of SKA-111 and SKA-121.
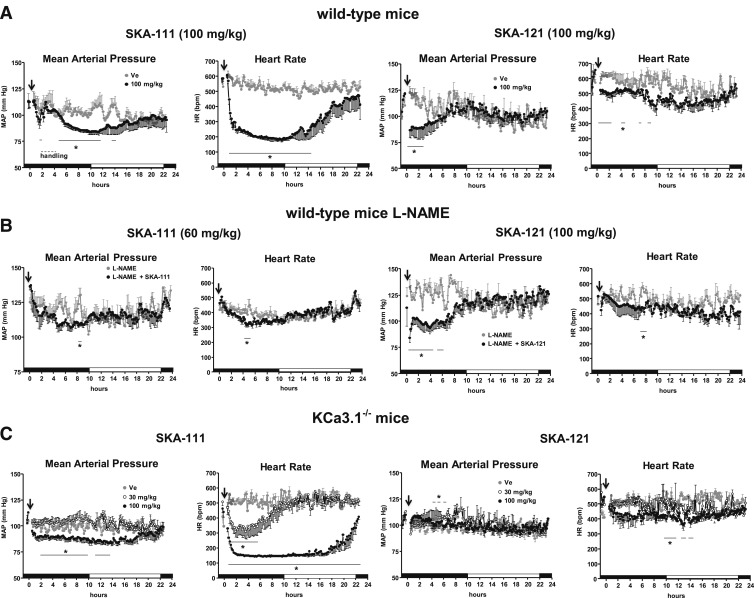
Since we had mice implanted with telemetry leads available, we performed telemetric blood pressure measurements on wild-type and KCa3.1−/− mice to evaluate the cardiovascular activity and selectivity of SKA-111 and SKA-121 before performing pharmacokinetic studies. However, since we did not know the half-life when these experiments were done, we chose to start with the relatively high dose of 100 mg/kg for both compounds, reasoning that we could lower the dose in subsequent experiments. In wild-type mice, i.p. injection of 100 mg/kg SKA-111 produced a substantial drop in mean arterial blood pressure (MAP) by ∼25 mmHg starting 20–30 minutes after injection (Fig. 6A, left). This decrease in MAP was significant compared with vehicle (peanut oil)-treated mice. The blood pressure drop was accompanied by a severe reduction in HR by ∼400 bpm (Fig. 6A, left). To avoid fatal hypothermia or circulatory collapse, we handled these severely bradycardic mice for ∼2 hours and increased RT to 34°C. As shown in Fig. 6A, these maneuvers increased MAP transiently, presumably because of a sympathetic input on total peripheral resistance, but not HR, and the low HR persisted over another 10 hours before the mice slowly recovered (Fig. 6A, left). SKA-121 at 100 mg/kg also lowered blood pressure by ∼20 mmHg (Fig. 6A, right). However, this drop was more transient and lasted ∼3 hours. HR was only moderately reduced (Fig. 6A, right). A lower dose of 30 mg/kg of both compounds did not produce significant alterations in MAP (Supplemental Fig. 3), whereas SKA-111 decreased HR to a minor extent (∼−50 bpm for ∼2 hours after injection (Supplemental Fig. 3). The vehicles, peanut oil (for SKA-111) or peanut oil/DMSO (9:1 v/v, for SKA-121), did not cause significant alterations in MAP or HR (Fig. 6A).
Fig. 6.
Blood pressure telemetry. (A) Injections (i.p.) of SKA-111 (panels on left) and SKA-121 (panels on right) reduced MAP in wild-type mice. SKA-111 (on left), but not SKA-121 (on right), severely reduced HR. As indicated by the dashed line (right panel), SKA-111–treated animals were initially handled and warmed to avoid fatal hypothermia during strong bradycardia. Ve, vehicle control. Black and white marked intervals of x-axis indicate dark and light periods. (B) SKA-121 (on right) but, to a lesser extent, SKA-111 (on left), reduced blood pressure in l-NAME–treated moderately hypertensive mice. HR remained virtually stable. (C) Cardiovascular actions of SKA-111 and SKA-121 in KCa3.1−/− mice. At 100 mg/kg, SKA-111 but not SKA-121 reduced MAP and HR. SKA-111 also reduced HR at 30 mg/kg. Data points are means ± S.E.M.; n = 3 or 4 experiments/strain and compound. Lines indicate time periods when pressures or HRs were significantly different from Ve. *P < 0.05, unpaired Student’s t test.
We next tested whether SKA-111 and SKA-121 are also efficient in lowering the higher MAP caused by systemic inhibition of nitric oxide production by l-NAME administered in the drinking water (Fig. 6B). However, for these experiments, we used a lower dose (60 mg/kg) of SKA-111 to avoid causing such a severe drop of HR as observed with 100 mg/kg. The 60 mg/kg dose produced only a minor drop in MAP and HR. In contrast, SKA-121 at 100 mg/kg produced a significant drop in MAP by ∼25 mmHg over 6 hours. This drop was accompanied by a minor decrease in HR (Fig. 6B, right).
We next evaluated the KCa3.1 selectivity by using KCa3.1−/− mice and found that SKA-111 at 100 mg/kg also produced a significant drop in MAP by ∼15 mmHg lasting ∼16 hours in these animals (Fig. 6C, left). Similar to wild-type mice, HR decreased substantially by ∼400 beats/minute over ∼22 hours (Fig. 6C, left). Similar to the results in wild-type mice, the lower dose of 30 mg/kg also significantly reduced HR, although less dramatically and not for as long as the higher dose. MAP did not change with the 30 mg/kg dose of SKA-111 (Fig. 6C, left). In contrast to SKA-111, SKA-121 at 100 mg/kg had no significant effects on MAP and HR in the KCa3.1−/− mice (Fig. 6C, right).
Taken together, these telemetry experiments showed that both compounds exhibited cardiovascular activity in vivo as they substantially reduce basal blood pressure and the higher blood pressure caused by NO deficiency. Moreover, SKA-121 produced this blood pressure–lowering action in a KCa3.1-dependent manner as suggested by lack of MAP-lowering effects in KCa3.1−/− mice. In contrast, SKA-111 lowered MAP and induced a strong HR reduction independently of KCa3.1.
Pharmacokinetics of SKA-111 and SKA-121.
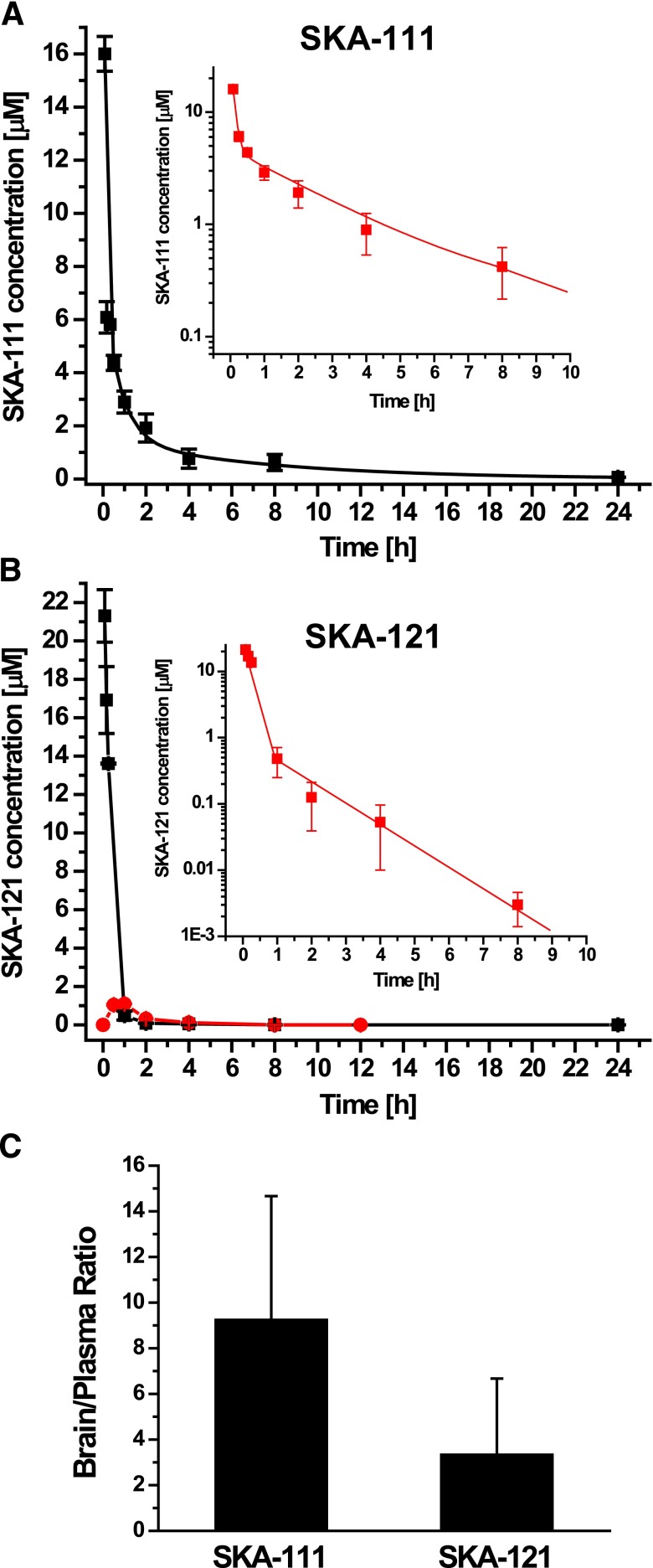
To help us better interpret the results of the telemetry experiments, we established ultraperformance liquid chromatography (UPLC)/mass spectrometry (MS) assays for SKA-111 and SKA-121 based on an high-performance LC/MS assay we had previously published for SKA-31 (Sankaranarayanan et al., 2009) and performed some basic pharmacokinetic studies with both compounds in mice. After i.v. injection at 10 mg/kg into the tail vein, total SKA-111 plasma concentrations fell biexponentially, reflecting a two-compartment model with very rapid distribution from blood into tissue (∼2 minutes), followed by elimination with a half-life of 4.7 ± 0.6 hours (Fig. 7A). SKA-121, in contrast, had a much shorter half-life (∼20 minutes), and plasma decay was extremely rapid (21.3 ± 2.4 μM at 5 minutes; 483 ± 231 nM at 1 hour and 53 ± 44 nM at 4 hours). Since SKA-121 is relatively well soluble (logP = 1.79) and could potentially be added to drinking water in animal experiments, we also administered it orally and found that it had an oral availability of roughly 25% (Fig. 7B). But again, plasma levels dropped rapidly from 1.1 ± 0.1 μM at 1 hour after oral administration to 27 ± 5 nM at 8 hours. Plasma protein binding was 59% ± 2% (n = 3) for SKA-111 and 81% ± 4% (n = 2) for SKA-121. Since we had also removed brains from the mice when obtaining blood samples by cardiac puncture (which had been done at every third blood collection), we further determined total brain concentrations at various time points and obtained averaged brain/plasma ratios for both compounds from times when the compounds were detectable in both plasma and brain. SKA-111 proved highly brain penetrant with a brain-to-plasma ratio of 9.3 ± 5.3. SKA-121 was less brain penetrant but still very effectively partitioned into the brain with a brain/plasma ratio of 3.3 ± 2.9 (Fig. 7C).
Fig. 7.
Pharmacokinetics of SKA-111 and SKA-121. (A) Total SKA-111 plasma concentration (mean ± S.D.) after i.v. administration of 10 mg/kg to mice (n = 2 to 3 per time point; t1/2 Distribution ∼2 minutes; t1/2 elimination = 4.7 ± 0.6 hours). Inset: The first 10 hours on a log scale. (B) Total SKA-121 plasma concentrations (mean ± S.D.) after i.v. (black) and oral (red) application of 10 mg/kg to mice (n = 3/time point; t1/2 ∼20 minutes; oral availability ∼25%). Inset: The first 10 hours after i.v. administration on a log scale. (C) Brain-to-plasma concentration ratios for SKA-111 and SKA-121 determined from multiple paired brain and plasma samples obtained during the experiments shown in (A) and (B) (n = 8 for SKA-111 and n = 5 for SKA-121).
Taken together, these results explain why SKA-111 had a much more prolonged blood pressure–lowering effect in the telemetry experiments in Fig. 6 than did SKA-121. The fact that SKA-111 is highly brain penetrant and probably achieved total brain concentrations in the range of 10–30 μM for hours after i.p. administration at 100 mg/kg also provides an explanation for why this KCa3.1 selective compound “lost” its selectivity in vivo and reduced blood pressure and heart rate, side effects that are presumably mediated by KCa2 channel activation in the central nervous system as well as KCa2 channel activation in resistance arteries and the heart (Radtke et al., 2013) and also occurred in KCa3.1−/− mice (Fig. 6C, left). In keeping with its shorter half-life of only 20 minutes and its lower brain penetration, SKA-121 induced a significant but less prolonged drop in MAP in the telemetry experiments when administered i.p. at 100 mg/kg (Fig. 6A right) and exhibited KCa3.1 selectivity in vivo as suggested by the lack of MAP-lowering effects in KCa3.1−/− mice and its insignificant effect on heart rate in wild-type mice.
Discussion
We here used our previously described mixed KCa2/3 channel activator SKA-31 (Sankaranarayanan et al., 2009) as a template for the design of two selective KCa3.1 activators. Both molecules, SKA-111 and SKA-121, activate KCa3.1 with EC50s of ∼110 nM and display 40- to 120-fold selectivity for KCa3.1 over the three KCa2 channels (KCa2.1, KCa2.2, and KCa2.3). These compounds constitute the first pharmacological or chemical biology tools that can be used to selectively activate KCa3.1 channels in tissue preparations or in vivo without performing additional manipulations such as genetically knocking out or pharmacologically blocking KCa2 channels.
According to the definition of positive-gating modulation, compounds like EBIO and NS309 act by shifting the Ca2+-activation curve of KCa2/3 channels to the left, meaning that the determined EC50 values for Ca2+-dependent channel activation calculated from Ca2+-concentration response curves decrease in the presence of the modulator molecule. For NS309, studies using CaM mutants making unstable association with the CaMB of KCa2.2 have shown that NS309 increases the apparent Ca2+-sensitivity of KCa channels by stabilizing the interaction between CaM and the CaMBD of the KCa channels (Pedarzani et al., 2001; Li et al., 2009). More recently, Zhang et al. (2012) crystallized CaM bound to the CaMBD of KCa2.2 and afterward soaked EBIO into the crystal. In a subsequent study, the same group obtained a cocrystal of the CaM/CaMBD with NS309 (Zhang et al., 2013). Both molecules reside in a pocket formed at the interface between CaM/CaMBD. Interestingly, on NS309 binding, an intrinsically disordered stretch of 16 amino acids that connects S6 to the CaMBD and was not visible in the EBIO/CaM/CaMBD crystal becomes visible, suggesting that it undergoes a transition to a well-defined structure. Other manipulations of this S6-CaMBD linker region, such as cross-linking a residue in the region to a residue in the CaMBD, also increase channel activity and apparent Ca2+ sensitivity, demonstrating that this linker region plays a crucial role in coupling Ca2+ binding to CaM to the mechanical opening of KCa channels (Zhang et al., 2013). By changing the confirmation of this linker region, NS309 is thus “truly” a gating modulator and we assume that SKA-111 and SKA-121 are exerting their effects in a similar manner. We have not yet mapped their binding sites, but we have also observed that mutations of the analogous residues in the CaMBD of KCa2.3, which had been reported to increase or decrease the potency of EBIO for activating KCa2.2 (Zhang et al., 2012), also significantly altered the potency of SKA-31 (Brown et al., 2014) suggesting that, similar to EBIO and NS309, benzothiazole-type KCa2/3 activators bind at the interface between CaM/CaMBD. This interface pocket is relatively “tight,” and the CaMBD shows a number of sequence differences between the four KCa channels, making it appear plausible that a “minor” structural change such as adding a -CH3 in 5-position of SKA-31 can increase selectivity for KCa3.1 from 10-fold to 100-fold in SKA-111. This hypothesis that SKA-31, SKA-111, and SKA-121 are binding to the same site in the CaM/CaMBD interface as NS309 agrees well with the relatively steep structure-activity-relationship we observed in our study. For the napthobenzothiazole and the isosteric naphthooxazole system, potency and selectivity for KCa3.1 over KCa2 channels were very sensitive to the exact position and electronic nature of substituents (e.g., SKA-106 and SKA-109 in Fig. 2). Another interesting observation in this context is that SKA-121 not only shifted the concentration-response curve for Ca2+-dependent KCa3.1 activation to the left but also increased the maximal achievable current at 1 and 10 μM in inside-out patches (Fig. 4). Since none of the benzothiazole-type KCa2/3 activators, including SKA-31, SKA-111, SKA-121, and their many derivatives, had ever increased KCa3.1 or KCa2 currents in our hands at Ca2+ concentrations lower than 100 nM or in the absence of Ca2+ with KF-based pipette solutions, we do not ascribe this effect to a direct channel opening component in their mechanism of action, like that reported for GW542573X and (-)-CM-TMPF for KCa2.1 (Hougaard et al., 2009, 2012). Unlike SKA-121, which we think is binding at the CaM/CaMBD interface, (-)CM-TMPF has been found to interact with positions deep within the inner pore vestibule (Hougaard et al., 2012) close to the selectivity filter, where the gate of KCa2/3 channels seems to be located (Bruening-Wright et al., 2002, 2007; Garneau et al., 2009; Klein et al., 2007). It therefore seems reasonable to attribute the Ca2+-independent KCa2.1 channel activation by (-)-CM-TMPF to a directly opening effect on the gate and use this explanation to account for the fact that (-)-CM-TMPF increases KCa2.1 currents to roughly 40% of their maximal activity at Ca2+ concentrations between 10 and 100 nM and then levels off in its opening/activating activity at higher Ca2+ concentrations (Hougaard et al., 2012). Since this is clearly not the case for SKA-121, which in contrast further increases maximal channel activity at 1 and 10 μM of Ca2+, we believe that SKA-121 is a “classic” positive gating modulator, which requires the presence of Ca2+ to enhance KCa channel activity but by stabilizing the interaction between CaM and the CaMBD of KCa3.1 is also able to further increase the Ca2+-dependent open channel probability Po(max) value of KCa3.1. Unlike KCa2 channels, which are assumed to be fully open at saturating [Ca2+]i concentrations, KCa3.1 channels have been reported to have a relatively low Ca2+-dependent Po(max), which can be increased significantly by the addition of 1.6 mM MgATP (Gerlach et al., 2001; Jones et al., 2007) or by mutations of residues in S5 (Garneau et al., 2014). We here left out ATP from the internal solutions for inside-out and whole-cell recordings on purpose to not confuse the analysis by having too many variables.
Since KCa3.1 is involved in EDH-mediated vasodilator responses and has been accordingly suggested as a potential new antihypertensive pharmacological target (Grgic et al., 2009; Dalsgaard et al., 2010; Edwards et al., 2010; Köhler et al., 2010), we tested the effect of both of our new KCa3.1 selective activators, SKA-111 and SKA-121, on BK-induced EDH responses in vitro on porcine coronary arteries and on blood pressure in mice. Both compounds potentiated BK effects in vitro and robustly lower blood pressure in mice in vivo. However, as these experiments and subsequently performed pharmacokinetic studies showed, both compounds do not have ideal properties for development into a potential antihypertensive drug candidate. SKA-111 is so highly brain penetrant that it achieves roughly ∼10-fold higher concentrations in the central nervous system and may thus cause complex neurologic side effects by activating neuronal KCa2 channels (Adelman et al., 2012). Moreover, SKA-111 induces the same severe bradycardia that had also been a problem when the unselective SKA-31 was dosed at 100 mg/kg in connexin 40–deficient mice (Radtke et al., 2013). This bradycardia is especially impressive in that it occurs also in KCa3.1−/− mice (Fig. 6) and is probably due to direct effects on KCa2 channels in cardiac pacemaker tissue (Radtke et al., 2013), as well as to a possible a central decrease in sympathetic drive through activation of neuronal KCa2 channels. SKA-121 is less brain penetrant and largely maintains its KCa3.1 selectivity in vivo. It lowers blood pressure in both normotensive and hypertensive mice without significantly reducing heart rate or affecting blood pressure in KCa3.1−/− mice. However, a problem with SKA-121 is the extremely short 20-minute half-life in mice, which would necessitate continuous infusion for blood pressure studies, a depot, or very frequently repeated drug applications. In this respect, it should, of course, be explored if SKA-121 possibly has a longer half-life in larger animals such as dogs, pigs, or primates.
In summary, with SKA-111 and SKA-121, we have identified two KCa3.1-selective positive gating modulators that constitute novel pharmacological tools for further dissecting the role of KCa3.1 in EDH and systemic blood pressure and that could help determine whether KCa3.1 activators could eventually be developed into a new class of endothelial targeted antihypertensive agents. Other potential indications for KCa3.1 activators could be intrasurgical hypertension, acute vasospasm, or preservation of endothelial function in large vascular organs such as the heart or kidneys or in vessel grafts during storage and transplantation. KCa3.1 activators have also long been suggested for enhancing fluid secretion in cystic fibrosis (Singh et al., 2001). Although SKA-111 and SKA-121 are not ideal candidate molecules, they could serve as templates for the design of derivatives with pharmacokinetic properties more suitable for further development and innovation.
Supplementary Material
Acknowledgments
The authors thank Dr. Eduardo Romanos-Alfonos and Susana Murillo-Pola of the Unit of Functional Evaluations of the IACS for excellent technical support (telemetry).
Abbreviations
- BK
bradykinin
- CaM
calmodulin
- CamBD
calmodulin binding domain
- CAS
Chemical Abstracts Service
- CM-TMF
N-{7-[1-(4-chloro-2-methylphenoxy)ethyl]-[1,2,4]triazolo[1,5-a]pyrimidin-2-yl}-N'-methoxy-formamidine
- CyPPA
cyclohexyl-[2-(3,5-dimethyl-pyrazole-1-yl-)6-methyl-pyrimidin-4-yl]-amine
- DMSO
dimethylsulfoxide
- EBIO
1-ethylbenzimidazolin-2-one
- EDH
endothelium-derived hyperpolarization
- HEK
human embryonic kidney
- HR
heart rate
- HRMS
high-resolution mass spectrometry
- KCa
Ca2+-activated K+ channel
- KCa3.1
intermediate-conductance Ca2+-activated K+ channel
- KV
voltage-gated K+ channel
- LC
liquid chromatography
- MAP
mean arterial blood pressure
- m.p.
melting point
- MS
mass spectrometry
- NMR
nuclear magnetic resonance
- NS309
6,7-dichloro-1H-indole-2,3-dione 3 oxime
- PCA
porcine coronary arteries
- SK
small-conductance KCa channel
- SKA-31
naphtho[1,2-d]thiazol-2-ylamine
- SKA-111
5-methylnaphtho[1,2-d]thiazol-2-amine
- SKA-121
5-methylnaphtho[2,1-d]oxazol-2-amine
- TRAM-34
1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole
- UCL1684
6,10-diaza-3(1,3)8,(1,4)-dibenzena-1,5(1,4)-diquinolinacyclodecaphane
- U46619
(Z)-7-[(1S,4R,5R,6S)-5-[(E,3S)-3-hydroxyoct-1-enyl]-3-oxabicyclo[2.2.1]heptan-6-yl]hept-5-enoic acid
- UPLC
ultraperformance liquid chromatography
Authorship Contributions
Participated in research design: Coleman, Brown, Valero, Oliván-Viguera, Köhler, Wulff.
Conducted experiments: Coleman, Brown, Singh, Valero, Oliván-Viguera, Olmstead.
Performed data analysis: Coleman, Brown, Heike Wulff.
Wrote or contributed to the writing of the manuscript: Coleman, Brown, Singh, Oliván-Viguera, Köhler, Wulff.
Footnotes
This work was supported by the CounterACT Program, National Institutes of Health (NIH) Office of the Director [Grant U54NS079202] and National Institute of Neurological Disorders and Stroke [R21NS072585], the Deutsche Forschungsgemeinschaft [Grant KO1899/11-1], the Danish Hjerteforening, and the Fondo de Investigación Sanitaria [Red HERACLES RD12/0042/0014]. N.C. was supported by an NIH National Heart, Lung and Blood Institute T32 Training Program in Basic and Translational Cardiovascular Science [Grant T32HL086350]. B.M.B. was supported by an NIH National Institute of General Medical Sciences–funded Pharmacology Training Program [Grant T32GM099608].
The work forms part of the Ph.D. thesis of Nichole Coleman in fulfillment of the degree requirements of the University of California, Davis.
 This article has supplemental material available at molpharm.aspetjournals.org.
This article has supplemental material available at molpharm.aspetjournals.org.
References
- Adelman JP, Maylie J, Sah P. (2012) Small-conductance Ca2+-activated K+ channels: form and function. Annu Rev Physiol 74:245–269 [DOI] [PubMed] [Google Scholar]
- Balut CM, Hamilton KL, Devor DC. (2012) Trafficking of intermediate (KCa3.1) and small (KCa2.x) conductance, Ca(2+)-activated K(+) channels: a novel target for medicinal chemistry efforts? ChemMedChem 7:1741–1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blank T, Nijholt I, Kye MJ, Radulovic J, Spiess J. (2003) Small-conductance, Ca2+-activated K+ channel SK3 generates age-related memory and LTP deficits. Nat Neurosci 6:911–912 [DOI] [PubMed] [Google Scholar]
- Brähler S, Kaistha A, Schmidt VJ, Wölfle SE, Busch C, Kaistha BP, Kacik M, Hasenau AL, Grgic I, Si H, et al. (2009) Genetic deficit of SK3 and IK1 channels disrupts the endothelium-derived hyperpolarizing factor vasodilator pathway and causes hypertension. Circulation 119:2323–2332 [DOI] [PubMed] [Google Scholar]
- Brown BM, Coleman N, Oliván-Viguera A, Köhler R, Wulff H (2014) Positive KCa channel gating modulators with selectivity for KCa3.1. FASEB J 28:1057.6. [DOI] [PMC free article] [PubMed]
- Bruening-Wright A, Lee WS, Adelman JP, Maylie J. (2007) Evidence for a deep pore activation gate in small conductance Ca2+-activated K+ channels. J Gen Physiol 130:601–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruening-Wright A, Schumacher MA, Adelman JP, Maylie J. (2002) Localization of the activation gate for small conductance Ca2+-activated K+ channels. J Neurosci 22:6499–6506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Simonsen U. (2010) Calcium-activated potassium channels - a therapeutic target for modulating nitric oxide in cardiovascular disease? Expert Opin Ther Targets 14:825–837 [DOI] [PubMed] [Google Scholar]
- Damkjaer M, Nielsen G, Bodendiek S, Staehr M, Gramsbergen JB, de Wit C, Jensen BL, Simonsen U, Bie P, Wulff H, et al. (2012) Pharmacological activation of KCa3.1/KCa2.3 channels produces endothelial hyperpolarization and lowers blood pressure in conscious dogs. Br J Pharmacol 165:223–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debono MW, Le Guern J, Canton T, Doble A, Pradier L. (1993) Inhibition by riluzole of electrophysiological responses mediated by rat kainate and NMDA receptors expressed in Xenopus oocytes. Eur J Pharmacol 235:283–289 [DOI] [PubMed] [Google Scholar]
- Devor DC, Singh AK, Frizzell RA, Bridges RJ. (1996) Modulation of Cl- secretion by benzimidazolones. I. Direct activation of a Ca(2+)-dependent K+ channel. Am J Physiol 271:L775–L784 [DOI] [PubMed] [Google Scholar]
- Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M. (2000) The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol Pharmacol 57:906–912 [PubMed] [Google Scholar]
- Edwards G, Félétou M, Weston AH. (2010) Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch 459:863–879 [DOI] [PubMed] [Google Scholar]
- Fanger CM, Ghanshani S, Logsdon NJ, Rauer H, Kalman K, Zhou J, Beckingham K, Chandy KG, Cahalan MD, Aiyar J. (1999) Calmodulin mediates calcium-dependent activation of the intermediate conductance KCa channel, IKCa1. J Biol Chem 274:5746–5754 [DOI] [PubMed] [Google Scholar]
- Garneau L, Klein H, Banderali U, Longpré-Lauzon A, Parent L, Sauvé R. (2009) Hydrophobic interactions as key determinants to the KCa3.1 channel closed configuration: an analysis of KCa3.1 mutants constitutively active in zero Ca2+. J Biol Chem 284:389–403 [DOI] [PubMed] [Google Scholar]
- Garneau L, Klein H, Lavoie MF, Brochiero E, Parent L, Sauvé R. (2014) Aromatic-aromatic interactions between residues in KCa3.1 pore helix and S5 transmembrane segment control the channel gating process. J Gen Physiol 143:289–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach AC, Syme CA, Giltinan L, Adelman JP, Devor DC. (2001) ATP-dependent activation of the intermediate conductance, Ca2+-activated K+ channel, hIK1, is conferred by a C-terminal domain. J Biol Chem 276:10963–10970 [DOI] [PubMed] [Google Scholar]
- Göblyös A, Santiago SN, Pietra D, Mulder-Krieger T, von Frijtag Drabbe Künzel J, Brussee J, Ijzerman AP. (2005) Synthesis and biological evaluation of 2-aminothiazoles and their amide derivatives on human adenosine receptors. Lack of effect of 2-aminothiazoles as allosteric enhancers. Bioorg Med Chem 13:2079–2087 [DOI] [PubMed] [Google Scholar]
- Grgic I, Kaistha BP, Hoyer J, Köhler R. (2009) Endothelial Ca+-activated K+ channels in normal and impaired EDHF-dilator responses: relevance to cardiovascular pathologies and drug discovery. Br J Pharmacol 157:509–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grissmer S, Nguyen AN, Aiyar J, Hanson DC, Mather RJ, Gutman GA, Karmilowicz MJ, Auperin DD, Chandy KG. (1994) Pharmacological characterization of five cloned voltage-gated K+ channels, types Kv1.1, 1.2, 1.3, 1.5, and 3.1, stably expressed in mammalian cell lines. Mol Pharmacol 45:1227–1234 [PubMed] [Google Scholar]
- Grunnet M, Jespersen T, Angelo K, Frøkjaer-Jensen C, Klaerke DA, Olesen SP, Jensen BS. (2001) Pharmacological modulation of SK3 channels. Neuropharmacology 40:879–887 [DOI] [PubMed] [Google Scholar]
- Hougaard C, Eriksen BL, Jørgensen S, Johansen TH, Dyhring T, Madsen LS, Strøbaek D, Christophersen P. (2007) Selective positive modulation of the SK3 and SK2 subtypes of small conductance Ca2+-activated K+ channels. Br J Pharmacol 151:655–665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hougaard C, Hammami S, Eriksen BL, Sørensen US, Jensen ML, Strøbæk D, Christophersen P. (2012) Evidence for a common pharmacological interaction site on K(Ca)2 channels providing both selective activation and selective inhibition of the human K(Ca)2.1 subtype. Mol Pharmacol 81:210–219 [DOI] [PubMed] [Google Scholar]
- Hougaard C, Jensen ML, Dale TJ, Miller DD, Davies DJ, Eriksen BL, Strøbaek D, Trezise DJ, Christophersen P. (2009) Selective activation of the SK1 subtype of human small-conductance Ca2+-activated K+ channels by 4-(2-methoxyphenylcarbamoyloxymethyl)-piperidine-1-carboxylic acid tert-butyl ester (GW542573X) is dependent on serine 293 in the S5 segment. Mol Pharmacol 76:569–578 [DOI] [PubMed] [Google Scholar]
- Jenkins DP, Yu W, Brown BM, Løjkner LD, Wulff H. (2013) Development of a QPatch automated electrophysiology assay for identifying KCa3.1 inhibitors and activators. Assay Drug Dev Technol 11:551–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joiner WJ, Wang LY, Tang MD, Kaczmarek LK. (1997) hSK4, a member of a novel subfamily of calcium-activated potassium channels. Proc Natl Acad Sci USA 94:11013–11018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones HM, Bailey MA, Baty CJ, Macgregor GG, Syme CA, Hamilton KL, Devor DC. (2007) An NH2-terminal multi-basic RKR motif is required for the ATP-dependent regulation of hIK1. Channels (Austin) 1:80–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan AD, Luo C, Reitz AB. (2003) Efficient conversion of substituted aryl thioureas to 2-aminobenzothiazoles using benzyltrimethylammonium tribromide. J Org Chem 68:8693–8696 [DOI] [PubMed] [Google Scholar]
- Kasumu AW, Hougaard C, Rode F, Jacobsen TA, Sabatier JM, Eriksen BL, Strøbæk D, Liang X, Egorova P, Vorontsova D, et al. (2012) Selective positive modulator of calcium-activated potassium channels exerts beneficial effects in a mouse model of spinocerebellar ataxia type 2. Chem Biol 19:1340–1353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein H, Garneau L, Banderali U, Simoes M, Parent L, Sauvé R. (2007) Structural determinants of the closed KCa3.1 channel pore in relation to channel gating: results from a substituted cysteine accessibility analysis. J Gen Physiol 129:299–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler M, Hirschberg B, Bond CT, Kinzie JM, Marrion NV, Maylie J, Adelman JP. (1996) Small-conductance, calcium-activated potassium channels from mammalian brain. Science 273:1709–1714 [DOI] [PubMed] [Google Scholar]
- Köhler R (2012) Cardiovascular alterations in KCa3.1/KCa2.3-deficient mice and after acute treatment with KCa3.1/KCa2.3 activators. EDHF 2012: 10th Anniversary Meeting June 27th - 30th, 2012; Feletou M, Vanhoutte PM (eds) vol. 49, pp 1–54 Vaux-de-Cernay, France. [Google Scholar]
- Köhler R, Kaistha BP, Wulff H. (2010) Vascular KCa-channels as therapeutic targets in hypertension and restenosis disease. Expert Opin Ther Targets 14:143–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Halling DB, Hall AW, Aldrich RW. (2009) EF hands at the N-lobe of calmodulin are required for both SK channel gating and stable SK-calmodulin interaction. J Gen Physiol 134:281–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng KF, Leung SW, Man RY, Vanhoutte PM. (2008) Endothelium-derived hyperpolarizing factor mediated relaxations in pig coronary arteries do not involve Gi/o proteins. Acta Pharmacol Sin 29:1419–1424 [DOI] [PubMed] [Google Scholar]
- Pedarzani P, Mosbacher J, Rivard A, Cingolani LA, Oliver D, Stocker M, Adelman JP, Fakler B. (2001) Control of electrical activity in central neurons by modulating the gating of small conductance Ca2+-activated K+ channels. J Biol Chem 276:9762–9769 [DOI] [PubMed] [Google Scholar]
- Radtke J, Schmidt K, Wulff H, Köhler R, de Wit C. (2013) Activation of KCa3.1 by SKA-31 induces arteriolar dilatation and lowers blood pressure in normo- and hypertensive connexin40-deficient mice. Br J Pharmacol 170:293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosa JC, Galanakis D, Ganellin CR, Dunn PM, Jenkinson DH. (1998) Bis-quinolinium cyclophanes: 6,10-diaza-3(1,3),8(1,4)-dibenzena-1,5(1,4)- diquinolinacyclodecaphane (UCL 1684), the first nanomolar, non-peptidic blocker of the apamin-sensitive Ca(2+)-activated K+ channel. J Med Chem 41:2–5 [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan A, Raman G, Busch C, Schultz T, Zimin PI, Hoyer J, Köhler R, Wulff H. (2009) Naphtho[1,2-d]thiazol-2-ylamine (SKA-31), a new activator of KCa2 and KCa3.1 potassium channels, potentiates the endothelium-derived hyperpolarizing factor response and lowers blood pressure. Mol Pharmacol 75:281–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hänsel W, Wulff H. (2005) Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol Pharmacol 68:1254–1270 [DOI] [PubMed] [Google Scholar]
- Schuart J, Müller HK. (1973) [2-Aminooxazoles and 2-iminooxazolines. 1: reaction of racemic alpha-methylaminopropiophenone using cyanobromide]. Pharmazie 28:438–439 [PubMed] [Google Scholar]
- Sheldrick GM. (2008) A short history of SHELX. Acta Crystallogr A 64:112–122 [DOI] [PubMed] [Google Scholar]
- Singh S, Syme CA, Singh AK, Devor DC, Bridges RJ. (2001) Benzimidazolone activators of chloride secretion: potential therapeutics for cystic fibrosis and chronic obstructive pulmonary disease. J Pharmacol Exp Ther 296:600–611 [PubMed] [Google Scholar]
- Strøbaek D, Teuber L, Jørgensen TD, Ahring PK, Kjaer K, Hansen RS, Olesen SP, Christophersen P, Skaaning-Jensen B. (2004) Activation of human IK and SK Ca2+ -activated K+ channels by NS309 (6,7-dichloro-1H-indole-2,3-dione 3-oxime). Biochim Biophys Acta 1665:1–5 [DOI] [PubMed] [Google Scholar]
- Wei AD, Gutman GA, Aldrich R, Chandy KG, Grissmer S, Wulff H. (2005) International Union of Pharmacology. LII. Nomenclature and molecular relationships of calcium-activated potassium channels. Pharmacol Rev 57:463–472 [DOI] [PubMed] [Google Scholar]
- Wulff H, Köhler R. (2013) Endothelial small-conductance and intermediate-conductance KCa channels: an update on their pharmacology and usefulness as cardiovascular targets. J Cardiovasc Pharmacol 61:102–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Miller MJ, Hansel W, Grissmer S, Cahalan MD, Chandy KG. (2000) Design of a potent and selective inhibitor of the intermediate-conductance Ca2+-activated K+ channel, IKCa1: a potential immunosuppressant. Proc Natl Acad Sci USA 97:8151–8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulff H, Zhorov BS. (2008) K+ channel modulators for the treatment of neurological disorders and autoimmune diseases. Chem Rev 108:1744–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia XM, Fakler B, Rivard A, Wayman G, Johnson-Pais T, Keen JE, Ishii T, Hirschberg B, Bond CT, Lutsenko S, et al. (1998) Mechanism of calcium gating in small-conductance calcium-activated potassium channels. Nature 395:503–507 [DOI] [PubMed] [Google Scholar]
- Zhang M, Pascal JM, Schumann M, Armen RS, Zhang JF. (2012) Identification of the functional binding pocket for compounds targeting small-conductance Ca²⁺-activated potassium channels. Nat Commun 3:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Pascal JM, Zhang JF. (2013) Unstructured to structured transition of an intrinsically disordered protein peptide in coupling Ca²⁺-sensing and SK channel activation. Proc Natl Acad Sci USA 110:4828–4833 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.