Abstract
The anorexigenic gastrointestinal hormone Peptide YY plays an important role in the communication between the gastrointestinal tract and the central nervous system. PYY has been shown to modulate brain activity in regions implicated in reward and food related behavior. Its effects on brain structure however, remain unknown. Voxel-based morphometry was used to investigate the relationship between fasting and postprandial plasma PYY concentrations and regional gray matter volume (GMV). For this analysis twenty adult, non diabetic Caucasians were included (18F/2M, age 31±9 y, percentage of body fat [PFAT] 32±8%) who had volumetric brain magnetic resonance images and underwent H215O positron emission tomographic (PET) measurements of regional cerebral blood flow (rCBF), a marker of local neuronal activity, and measurements of plasma total PYY, prior to (fasting) and following a satiating liquid meal. Voxel-wise analysis revealed a regional positive association between postprandial PYY and gray matter volume bilaterally in the caudate nuclei. These associations remained significant (p<0.05) after small volume correction for multiple comparisons. Based on these findings we investigated whether postprandial PYY is associated with PET measured rCBF of the caudate nucleus. We found a significant negative association between average postprandial caudate rCBF and postprandial plasma PYY concentrations (r=−0.60, p<0.02, age, sex and PFAT adjusted). Average postprandial caudate rCBF was also negatively associated with rCBF in the right medial orbitofrontal cortex and the right hippocampal formation (p<0.05, corrected for multiple comparison). Total PYY is positively associated with gray matter but negatively with postprandial activity in the caudate nuclei while caudate activity is negatively associated with rCBF in prefrontal and paralimbic regions implicated in reward behavior. Thus, PYY may act centrally to modulate eating behavior via striatal networks.
Keywords: PYY, caudate nucleus, striatum, gray matter, VBM, MRI, PET, rCBF
1. Introduction
In the past decades, obesity has become a medical and socioeconomic problem of pandemic proportions in industrialized countries. A vast number of illnesses have been associated with excessive overweight including the major causes of death in western countries, cardiovascular disease, certain types of cancer, and stroke (Guh et al., 2009). In this respect, understanding the physiological events underlying feeding behavior and the development of obesity has become a research question of major importance. Gastrointestinal hormones play a crucial role in the communication between the gastrointestinal tract and the brain, mediating signals of both hunger and satiety. The gastrointestinal hormone Peptide YY (PYY) is a member of the pancreatic peptide fold family along with neuropeptide Y (NPY) and pancreatic polypeptide (PP) and is known to decrease appetite and food intake in lean and healthy humans (Batterham et al., 2003, 2007; Degen et al., 2005; Sloth et al., 2007), inhibit stomach emptying (Witte et al., 2009) and increase gastrointestinal water and electrolyte absorption (Cox, 2007). Synthesized by L-type Endocrine cells in the distal gastrointestinal tract, PYY is secreted into the circulation in response to a meal and postprandial plasma concentrations remain elevated for approximately 6 hours (Adrian et al., 1985). Two endogenous and physiologically active forms have been identified, PYY1–36 and PYY3–36. PYY3–36 is formed by the ubiquitously expressed enzyme dipeptidyl peptidase IV (DP-IV) via cleavage of the first three n-terminal amino acids of PYY1–36 (Mentlein et al., 1993). Effects of PYY are mediated by Y-receptors, a G-protein coupled receptor family (Cabrele and Beck-Sickinger, 2000), widely distributed throughout the gastrointestinal tract and the central nervous system (Widdowson, 1993; Parker and Herzog, 1999).
So far four subtypes have been identified in humans (i.e. Y1, Y2, Y4, Y5) (Gehlert, 1998; Michel et al., 1998; Berglund et al., 2003). The anorectic effects of PYY have been mainly attributed to PYY3–36 and the Y2-receptor. Animal and human studies have shown a reduction in appetite and food intake after systemic administration of PYY 3–36 (Batterham et al., 2003, 2007; Degen et al., 2005; Sloth et al., 2007). In rodents, this effect is absent in Y2R-knockouts (Batterham et al., 2002) or after administration of a Y2R antagonists (Abbott et al., 2005). While PYY3–36 exhibits a highly selective binding profile with a strong affinity for Y2-receptors, PYY1–36 has affinity for Y1, Y2 and Y5-receptors. (Dumont et al., 1995; Batterham and Bloom, 2003). Although the primary effect of PYY on appetite and eating behavior is believed to be mediated via the Y2 receptor of the arcuate nucleus within the hypothalamus, a significant decrease in high-fat food seeking in response to systemic PYY that was independent of arcuate nucleus Y2R signaling was reported in rodents (Ghitza et al., 2007). There is evidence indicating that postprandial rises of PYY are lower in obese compared to lean individuals leading to reduced satiety and relatively higher food intake, yet ratios of PYY1–36 and PYY3–36 do not seem to change with adiposity (le Roux et al., 2006). Importantly, obese individuals do not appear to develop a resistance to the anorectic effects of PYY as occurs with the adipokine leptin (Batterham et al., 2003). PYY may also play an important role in weight regain following gastric bypass surgery as attenuated postprandial PYY profiles have been found in individuals with poor weight loss (Meguid et al., 2008). Intravenous administration of PYY3–36 modulates brain regions implicated in both homeostatic (e.g. hypothalamus) and reward related feeding behavior (e.g. prefrontal cortical regions, ventral tegmental area, putamen, globus pallidus) (Batterham et al., 2007).
Peripheral PYY clearly has important effects on brain function and has sustained postprandial elevations. Furthermore, other peripherally circulating hormones have been shown to influence brain morphology (Starkman et al., 1999; Bourdeau et al., 2002; Matochik et al., 2005; Pannacciulli et al., 2007). Thus, we sought to investigate the effects of total PYY concentrations in the fasting state and in response to a satiating liquid meal on gray and white matter volume using voxel-based morphometry (VBM). After demonstrating associations with bilateral caudate gray matter, we investigated associations with Oxygen-15 water positron emission tomographic (PET) measurements of regional cerebral blood flow in a caudate nucleus region-of-interest (ROI). Associations between caudate nucleus rCBF and other brain regions were furthermore investigated in a voxel-based analysis, hypothesizing a modulation of prefrontal and limbic/paralimbic neuronal activity.
2. Subject and Methods
2.1. Subjects
All subjects in this study had previously participated in brain imaging studies of hunger, satiation, and the predisposition to obesity. Twenty adult, non-diabetic, right-handed Caucasians with a wide range of adiposity (18F/2M; age 31±9 y; percentage of body fat [PFAT] 32.2±8.4%; BMI 31.2±9.6 ) were included who had available MRI and PET scans and measurements of PYY. All subjects were recruited from the Phoenix, AZ metropolitan area by newspaper advertisements. All subjects were free of medical disorders, not taking any medications, as determined by medical history, physical examination, and screening laboratory tests. Female subjects were studied while in the follicular phase of the menstrual cycle. Subjects with a history of substance or alcohol abuse or addiction; endocrine disorders (including abnormal thyroid function and type 2 diabetes); hypertension, pulmonary, cardiovascular, gastrointestinal, hepatic, renal or central nervous system disorders were excluded from the study at screening. The Structured Clinical Interview for DSM-III-R (Spitzer et al., 1990) was used to screen for behavioral and psychiatric conditions (claustrophobia, major depression, the presence of psychotic symptoms, anorexia nervosa, or bulimia nervosa) incompatible with safe and successful participation in the study. All subjects were admitted for one week to the metabolic unit of the Obesity and Diabetes Clinical Research Section of the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) in Phoenix, AZ. Subjects were restricted to the research ward and were limited to sedentary activity for the duration of the study. The protocol was approved by the Institutional Review Boards of the NIDDK and the Banner Good Samaritan Regional Medical Center. All subjects provided a written informed consent prior to participation. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
2.2 Experimental protocol
The experimental procedures have been described previously (Tataranni et al., 1999). In brief, upon admission, subjects received a weight maintaining diet (50% of calories from carbohydrate, 30% fat and 20% protein). Body composition was assessed by dual energy x-ray absorptiomery (DPX-1; Lunar Corp, Madison, WI); resting energy expenditure (REE) was measured for 45 minutes using a ventilated-hood system (DeltaTrac, SensorMedics, Yorba Linda, CA). Prior to the brain imaging session, subjects fasted for 36 h. Study subjects had free access to water and non-caloric, non-caffeinated beverages during the fast.
2.3 Metabolite analysis
Plasma concentrations of fasting and postprandial total PYY (including PYY1–36 and PYY3–36) were measured by a commercially available radioimmunoassay kit (Phoenix Pharmaceuticals, Belmont, California, USA) with a 100% crossreactivity between PYY1–36 and PYY3–36. Plasma glucose concentrations were measured by the glucose oxidase method (Beckman Instruments, Fullerton, CA). Plasma insulin concentrations were determined by an automated radioimmunoassay (Concept 4; ICN, Costa Mesa, CA).
2.4 Imaging procedures
Magnetic Resonance Imaging (MRI) and Positron Emission Tomography (PET) procedures were carried out at the Banner Good Samaritan Regional Medical Center (Phoenix, AZ). MRI scans were performed on a 1.5 Tesla Signa system (General Electric, Milwaukee, WI, USA). A set of high-resolution T1-weighted images was acquired with a fast spoiled gradient echo (FSPGR) 3d sequence (repetition time [TR]/ echo time [TE] = 12/5.2; inversion time (TI) = 300ms, number of excitations (NEX) = 1; field-of-view [FOV] = 24 × 24 cm; 256 × 256 matrix); the whole-brain data were acquired in an axial plane yielding 120 contiguous slices with slice thickness of 1 mm. Each subject’s MRI scans were evaluated by an experienced neuroradiologist in order to exclude individuals with anatomic abnormalities.
O-15 water positron emission tomographic measurement of regional cerebral blood flow (counts/voxel/min) was performed on an ECAT-951/31 scanner (Siemens, Knoxville, TN). To adjust for attenuation of γ-radiation by the brain and skull, a 10 min transmission scan was performed using a retractable external ring source of 68Ga/68Ge. For each 1-min PET scan, subjects remained motionless in the supine position and were requested to keep their eyes closed and pointing forward. Subjects received a 50-mCi intravenous bolus of 15O-water during each scan. Each individual underwent two scans at baseline (fasting, premeal condition) and two after oral administration of a satiating amount of a liquid formula meal (Ensure Plus, 1.5 kcal/ml, Ross-Abbott Laboratories, Columbus, OH) providing 50% of the subject’s measured REE, with intervals of 10 min between scans. Formula flavor (strawberry, vanilla or chocolate) was chosen by the subject and the meal was administered continuously over 25 min via a plastic tube placed into the subject’s mouth using a peristaltic pump. To avoid swallowing during the scan, subjects received 2 ml of water at room temperature via a plastic tube, administered by a syringe 30 s before each scan and were asked to retain and swallow. Immediately following each scan, blood samples were drawn for hormonal and metabolic measurements. Using 100-mm visual analogue scales (Raben et al., 1995) subjective ratings of hunger and fullness were obtained after each scan. All subjects had been fully acquainted with the experimental procedures, thus minimizing the risk of learning-related artifacts and anticipated receiving the satiating liquid meal.
2.5 Image data analysis
Voxel-based morphometry (Ashburner and Friston, 2000) was performed using the VBM8 toolkit in Statistical Parametric Mapping package (SPM8, Wellcome Department of Imaging Neuroscience, London, UK; www.fil.ion.ucl.ac.uk/spm). In brief, native MRI scans were normalized to the standard Montreal Neurological Institute (MNI) T1 MRI template (voxelsize:1.5mm×1.5mm×1.5mm) and segmented into grey matter (GM), white matter (WM), and cerebrospinal fluid (CSF) using automated algorithms implemented in VBM8 and the default settings. Finally, segmented images of GM and WM were smoothed with a 12mm full-width at half-maximum isotropic Gaussian kernel. Total intracranial volume (TIV) was calculated based on segmented GM, WM and CSF images over the individual participant’s native brain space and was included as a confounding covariate in an analysis of covariance (ANCOVA). In SPM8 voxel-wise multiple variable regression analysis of GM and WM images was performed, entering plasma PYY concentrations (fasting, postprandial, response), age, sex and percentage body fat (PFAT) as covariates with a p-threshold of <0.001 and an extent threshold of 100 continuous voxels (voxelsize:1.5mm×1.5mm×1.5mm). Small volume correction (SVC) for regional multiple comparisons was performed on regions (GM or adjacent WM) associated with PYY, eating and reward behavior respectively (i.e. caudate nucleus, globus pallidus, thalamus, prefrontal regions, anterior cingulate gyrus and the cerebellum (Batterham et al., 2007; Neary and Batterham, 2010)) by using anatomical masks or a sphere with a 10mm radius when masks were not applicable. Small volume corrected results are reported as significant at p<0.05 (family-wise error correction on the voxel-level). Masks for data extraction and SVC were created by using the WFU pickatlas tool (Maldjian et al., 2003) and the integrated automatic anatomic labeling (AAL) tool (Tzourio-Mazoyer et al., 2002).
PET scans were aligned, spatially normalized to the standard Montreal Neurological Institute (MNI) stereotactic space and smoothed using a 15- mm full-width-at-half-maximum Gaussian filter (Friston et al., 1995). SAS Software (SAS Institute Inc, version 9.2, Cary, NC) was used for statistical analyses of non-imaging data and extracted data. The hypothesis of brain regions modulated by caudate activity was tested by performing a multiple voxel-wise regression analysis of PET scans (fasting and postprandial) entering average bilateral caudate activity into a multiple regression model including age, sex and PFAT as covariates. Statistical parametric maps were thresholded at p<0.005 and an extent threshold of 50 continuous voxels (voxelsize: 2mm×2mm×2mm). Results are reported as significant at p<0.05 cluster-level corrected (FWE). ROI and whole brain analyses were adjusted for age, sex and PFAT. Pearson correlation coefficients were Fisher Z-transformed to test for differences between the pre- and postmeal condition. Anatomical regions of both VBM and PET analyses were defined by using the BioImage Suite MNI to Talaraich Coordinate Converter (www.bioimagesuite.org) and the Talaraich Client v2.4.2 (Lancaster et al., 2000) with a 2mm search range. For PET data a search range of 5mm was used to define closest Brodmann Areas.
3. Results
3.1 Subject characteristics
Table 1 summarizes subject characteristics, hormonal-metabolic measures and subjective appetite ratings in the fasting and postprandial state in our study group. As anticipated, plasma concentrations of glucose, insulin and total PYY significantly increased in response to the meal (all p<0.001), while hunger and fullness scores were respectively significantly decreased and increased (all p<0.001).
Table 1.
Characteristics of the study population
| Age | 31.3±8.7 | ||
| Sex female/male* | 18/2 | ||
| PFAT (%) | 32.2±8.4 | ||
| BMI | 31.2±9.6 | ||
| Hormonal / metabolic measures | Fasting | Postprandial | |
| PYY (pg/ml) | 25.0±10.6 | 42.5±18.7 | |
| Glucose (mmol/l) | 4.5±0.5 | 5.4±0.5 | |
| Insulin (pmol/l) | 155.6±31.3 | 485.5±319.5 | |
| Appetite ratings | |||
| Hunger | 68.9±27.7 | 18.9±23.2 | |
| Desire | 69.9±25.5 | 21.5±25.5 | |
| Fullness | 16.6±16.3 | 75.1±24.7 | |
| Prospective foodintake | 64.3±24.7 | 20.0±18.3 | |
All results apart from *are presented as mean ± SD; PFAT percentage body fat;
3.2 VBM analysis
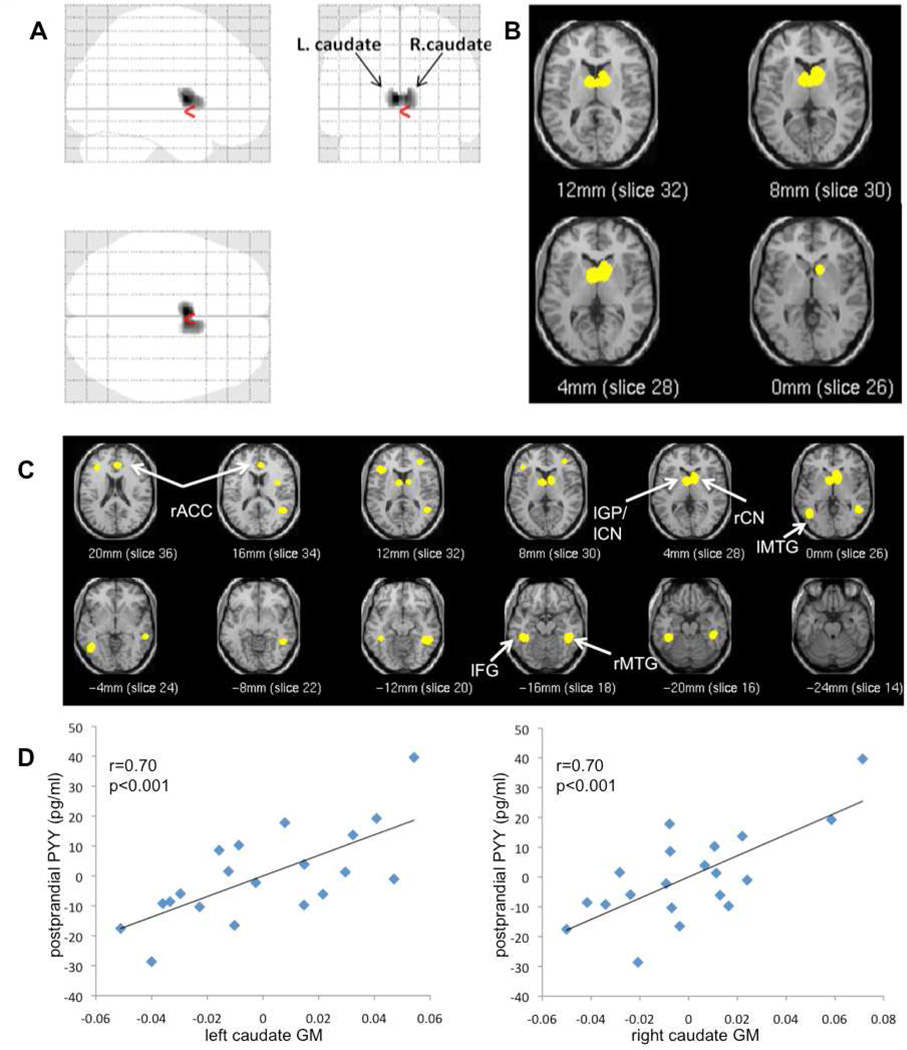
Fasting PYY concentrations were positively associated with regional GM volume in the bilateral cerebellum, reaching significance after SVC (pvoxel=0.047; kE=484; MNIxyz [−6 −64 −20]) (Table 2). Postprandial PYY levels were positively associated with GM volume bilaterally in caudate nuclei (i.e. caudate body) (Fig.1A, B, D). This association remained significant after SVC for the sublobar brain volume and comprised two significant peaks within the left and right caudate body (left: pvoxel=0.02; MNIxyz [−3 2 9]; right: pvoxel=0.049; MNIxyz [6 4 9]; kE =679).
Table 2.
Associations of gray and white matter regions with PYY*
| Region, Brodmann Areaa | MNIb coordinates |
T | Cluster size | ||
|---|---|---|---|---|---|
| x | y | z | |||
| GM regions positively associated with PYY | |||||
| Fasting PYY | |||||
| L. cerebellum, posterior lobe | −6 | −64 | −20 | 5.30 | 509 |
| R. cerebellum, posterior lobe | −10 | −58 | −21 | 3.19 | |
| Postprandial PYY | |||||
| L. caudate nucleus, body | −3 | 2 | 9 | 6.18 | 690 |
| R. caudate nucleus, body | 6 | 4 | 9 | 5.48 | |
| PYY response | |||||
| L. fusiform gyrus, BA37 | −39 | −40 | −15 | 6.71 | 168 |
| L. globus pallidus | −8 | 2 | 1 | 5.72 | 224 |
| R. temporal lobe, BA37 | 50 | −42 | −12 | 5.53 | 335 |
| R. caudate nucleus, head | 10 | 4 | 1 | 5.01 | 373 |
| L. middle temporal gyrus, BA37 | −45 | −58 | −2 | 4.75 | 170 |
| R. cingulate gyrus, BA32 | 15 | 33 | 28 | 4.37 | 139 |
| R. anterior cingulate gyrus, BA32 | 4 | 33 | 24 | 4.25 | |
| GM regions negatively associated with PYY | |||||
| none | |||||
| WM regions positively associated with PYY | |||||
| Fasting PYY | |||||
| R. inferior frontal gyrus, BA9 | 44 | 8 | 25 | 6.04 | 145 |
| Postprandial PYY | |||||
| none | |||||
| PYY response | |||||
| none | |||||
| WM regions negatively associated with PYY | |||||
| Fasting PYY | |||||
| none | |||||
| Postprandial PYY | |||||
| none | |||||
| PYY response | |||||
| Extranuclear WM, L. Thalamusa | −3 | −24 | 12 | 4.49 | 123 |
Results significant at a threshold of p<0.001 and a cluster size of 100 continuous voxels, uncorrected for multiple comparisons.
Indicates nearest gray matter.
MNI: Montreal Neurological Institute.
Figure 1.
Glass brain (A) and t-score map (B) of regional gray matter volume (GMV) positively associated with postprandial PYY concentrations. (C) t-score map of positive associations between regional GMV and PYY response.
Maps are thresholded at p<0.001 and a cluster size of 100 continuous voxels (glass brain only), uncorrected for multiple comparison; rACC right anterior cingulate, lGP left globus pallidus, lCN left caudate nucleus, rCN right caudate nucleus, lMTG left medial temporal gyrus, lFG left fusiform gyrus, rMTG right medial temporal gyrus;
(D) Correlation between gray matter (GM) values (adjusted for TIV) of the left and right caudate peak voxel with postprandial PYY values (adjusted for age, sex, PFAT).
PYY response (postprandial minus fasting PYY concentrations) was positively associated with GM volume in the left fusiform gyrus, left globus pallidus (extending to the left caudate), right caudate nucleus (i.e. head), left and right middle temporal gyrus and right anterior cingulate gyrus (ACC) (Fig. 1C), uncorrected for multiple comparisons.
After SVC for the sublobar brain volume, subcortical peaks partially reached significance (left globus pallidus pvoxel=0.038, kE=222, MNIxyz[−8 2 1]; right caudate, pvoxel=0.093, kE=362, MNIxyz[10 4 1]. However, SVC for the bilateral caudate volume revealed two significant peaks in the left (pvoxel= 0.019; kE =130; MNIxyz[−6 6 3]) and right caudate nucleus (pvoxel= 0.016; kE =18; MNIxyz[9 9 3]). SVC for the right ACC volume showed a significant peak (pvoxel= 0.023; kE =121; MNIxyz[15 23 27]) within the right ACC.
No negative associations were observed for fasting PYY, postprandial PYY or PYY response.
VBM analysis of the WM revealed a positive association between fasting PYY and the right inferior frontal gyrus (IFG) and a negative association between PYY response and extranuclear WM in the vicinity of the left thalamus, both significant after SVC correction using a 10mm sphere (IFG: pvoxel=0.001; kE =145; MNIxyz[44 8 25]; left Thalamus: pvoxel=0.01; kE =145; MNIxyz[−3 −24 12]). No negative associations were found for postprandial PYY concentrations.
3.3 ROI PET analysis
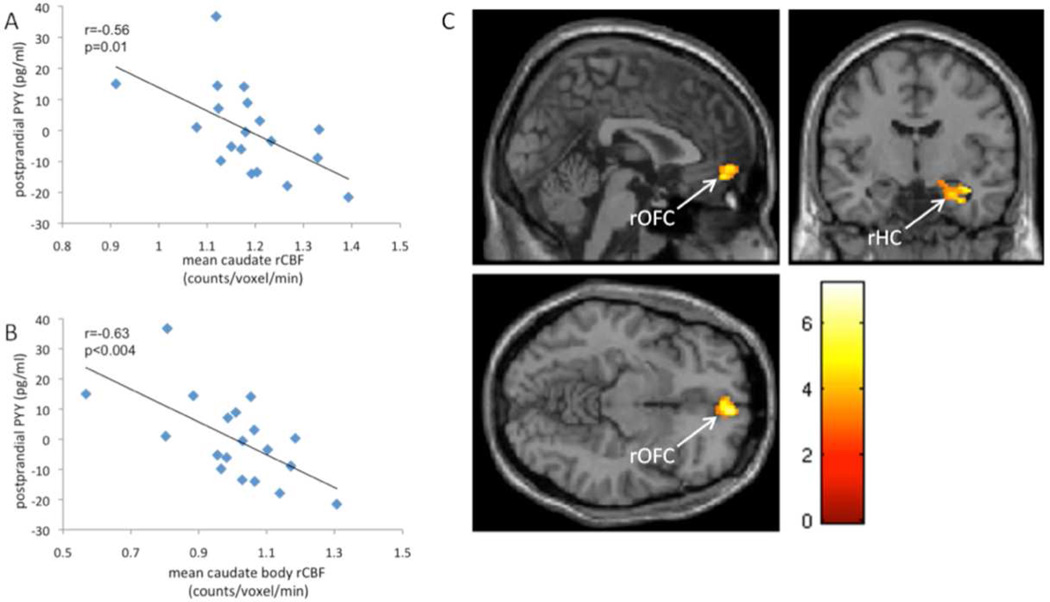
Caudate rCBF values (fasting and postprandial) were extracted from those with available PET measurements of rCBF in the fasting and postprandial state (n=19). Left and right caudate rCBF strongly correlated with each other(fasting: r=0.89, p<0.001; postprandial: r=0.94, p<0.001). Caudate rCBF significantly decreased in response to the liquid meal (p=0.03) and postprandial caudate rCBF was significantly negatively associated with postprandial PYY concentrations (bilateral mean: r=−0.60, p<0.02, left: r=−0.57, p=0.02, right: r=−0.61, p<0.01; Pearson partial correlation, adjusted for age, sex, PFAT). Further segmentation into the anatomical subdivision of the caudate nucleus revealed the strongest association in the caudate body and tail (Table 3, Fig.2A, B). Regarding possible correlations between ΔrCBF (fasting rCBF- postprandial rCBF) of the caudate and PYY response (postprandial - fasting) we only observed a positive correlation of ΔrCBF of the right caudate body with PYY response (r=0.56, p<0.03; Pearson partial correlation with age sex and PFAT as partial covariates). Total caudate ΔrCBF and all other anatomical subdivisions showed no significant correlations with PYY response (data not shown). All of the reported associations between caudate rCBF and postprandial PYY did not change after further adjustment for insulin concentrations (data not shown).
Table 3.
Correlations between caudate rCBF and PYY concentrations in the postprandial state*
| Region | R | P |
|---|---|---|
| Caudate nuclei, bilateral | −0.60 | <0.02 |
| Left | −0.57 | 0.02 |
| Right | −0.61 | 0.01 |
| Caudate body, bilateral | −0.65 | <0.01 |
| Left | −0.62 | <0.01 |
| Right | −0.64 | <0.01 |
| Caudate head, bilateral | −0.12 | 0.67 |
| Left | −0.25 | 0.36 |
| Right | 0.07 | 0.81 |
| Caudate tail, bilateral | −0.69 | <0.01 |
| Left | −0.81 | <0.001 |
| Right | −0.46 | 0.07 |
Pearson partial correlation adjusted for age, sex and PFAT; rCBF regional cerebral blood flow, PFAT percentage body fat;
Figure 2.
Pearson correlation of postprandial PYY (adjusted for age, sex, PFAT) and mean rCBF of the bilateral caudate nucleus (A) and the bilateral caudate body (B). T-score map (C) of prefrontal and limbic/paralimbic brain regions (rCBF) showing significant (p<0.05 cluster-level corrected) negative associations with mean caudate nuclei rCBF. Maps are thresholded at p<0.005 and a cluster size of 50 continuous voxels, uncorrected. T-score is indicated by color bar.
Pearson partial correlation (age, PFAT and sex as partial covariates) showed a significant negative association between TIV adjusted GM values of the left and right peak voxel and postprandial rCBF values of the left and right caudate (left: r=−0.64, p=0.008; right: r=−0.71p=0.002). These associations were no longer significant after adjusting for postprandial PYY concentrations (left: p=0.19, r=−0.36; right: p=0.09, r=−0.46).
3.4 Voxel-based PET analysis
Caudate activity appeared to modulate several other brain regions, as determined using our voxel-based analysis. Results are summarized in Table 4, S1–2. In the pre-meal condition, areas positively associated with mean caudate rCBF included the right thalamus (extending to the left thalamus), right anterior cingulate gyrus and the left cuneus (supplementary material, table S1). In the postmeal condition we observed positive associations in the right anterior cingulate gyrus, right putamen extending to the right thalamus, left inferior parietal lobule and the right superior and left occipital gyrus (supplementary material, table S2). Significant negative associations in the fasting state were only observed in the right cerebellum. Notably, in the postprandial state however negative associations were found in the right medial orbitofrontal cortex and the right hippocampal formation (Table 4, Fig. 2C).
Table 4.
Brain regions negatively associated with caudate nucleus rCBF*
| Region, Brodmann Area (BA)a | MNIb coordinates |
T | Cluster size | ||
|---|---|---|---|---|---|
| x | y | z | |||
| Fasting state | |||||
| R. middle frontal gyrus, BA8 | 26 | 22 | 42 | 6.56 | 70 |
| R. rectal gyrus, BA11 | 2 | 28 | −24 | 6.52 | 85 |
| R. rectal gyrus, BA11 | 6 | 38 | −24 | 4.25 | |
| R. cerebllum, posterior lobe | 4 | −60 | −20 | 6.02 | 76 |
| R. cerebellum, anterior lobec | 32 | −40 | −28 | 5.12 | 216 |
| R. fusiform gyrus, BA37 | 56 | −46 | −28 | 4.48 | |
| R. fusiform gyrus, BA36 | 48 | −36 | −28 | 4.40 | |
| R. inferior occipital gyrus, BA18 | 30 | −88 | −14 | 4.82 | 52 |
| R. inferior occipital gyrus, BA18 | 42 | −86 | −14 | 4.11 | |
| R. lingual gyrus, BA19 | 32 | −76 | −16 | 3.14 | |
| R. medial frontal gyrus, BA10 | 8 | 50 | −8 | 4.17 | 50 |
| R. medial frontal gyrus, BA11 | 2 | 60 | −12 | 3.32 | |
| L. cerebellum, posterior lobe | −38 | −60 | −18 | 4.00 | 51 |
| L. cerebellum, posterior lobe | −30 | −62 | −16 | 3.39 | |
| L. fusiform gyrus, BA37 | −42 | −54 | −24 | 3.16 | |
| Postprandial state | |||||
| R. medial frontal gyrus, BA10c | 6 | 56 | −8 | 7.18 | 172 |
| R. medial frontal gyrus, BA11 | 6 | 48 | −14 | 3.40 | |
| L. middle temporal gyrus, BA21 | −44 | 10 | −36 | 6.89 | 55 |
| R. hippocampus c | 34 | −10 | −18 | 6.72 | 205 |
| R. hippocampus | 26 | −10 | −22 | 4.87 | |
| R. hippocampus | 32 | −6 | −28 | 4.61 | |
| R. cerebellum, anterior lobe | 18 | −34 | −30 | 6.49 | 69 |
| R. cerebellum, anterior lobe | 32 | −44 | −28 | 4.98 | |
| R. cerebellum, anterior lobe | 40 | −40 | −30 | 4.79 | |
| R. cerebellum, posterior lobec | 16 | −66 | −20 | 5.43 | 403 |
| R. middle occipital gyrus, BA19 | 36 | −80 | −14 | 5.19 | |
| R. lingual gyrus, BA18 | 20 | −84 | −12 | 5.16 | |
| L. cerebellum, anterior lobe | −24 | −42 | −26 | 5.25 | 133 |
| L. cerebellum, posterior lobe | −36 | −54 | −20 | 5.09 | |
| L. fusiform gyrus, BA20 | −42 | −40 | −24 | 4.67 | |
| R. cerebellum, posterior lobe | 56 | −58 | −24 | 4.91 | 82 |
| R. cerebellum, posterior lobe | 38 | −62 | −22 | 4.20 | |
| R. Occipital lobe, subgyral WM, BA37 | 44 | −52 | −18 | 3.95 | |
Results significant at a threshold of p<0.005 and a cluster size of 50 continuos voxels, uncorrected for multiple comparisons.
indicates nearest gray matter region
MNI: Montreal Neurological Institute.
p<0.05 cluster-level corrected
Fisher Z transformed Pearson moment-activity correlation coefficient of the right mOFC and right hippocampal formation did not show statistically significant differences between the pre-and postmeal condition (data not shown).
4. Discussion
We explored the associations of fasting and postprandial concentrations of the gastrointestinal hormone PYY using a voxel-based analysis of brain morphology and a subsequent ROI analysis of rCBF in a structurally affected brain region. We demonstrated that postprandial plasma concentrations of total PYY are positively associated with GM volume of the bilateral caudate nucleus. PYY response was positively associated with GM of the right anterior cingulate gyrus, temporal regions, the left globus pallidus (inclusive of the left caudate) as well as the right caudate nucleus. In addition, using a region of interest approach to examine rCBF, we found a significant decrease in caudate rCBF between fasting and post prandial states and a negative association between postpandrial PYY and caudate rCBF. Caudate activity was furthermore negatively associated with rCBF of the right orbitofrontal cortex and the right hippocampus. In humans, systemic application of PYY3–36 has been shown to modulate activity in several brain regions associated with feeding-behavior including prefrontal cortical regions, ventral tegmental area, putamen, globus pallidus and the hypothalamus (Batterham et al., 2007). As postprandial PYY concentrations remain elevated for approximately 6 hours after a meal (Adrian et al., 1985), variability in postprandial PYY profiles could affect human brain morphology. Other peripheral circulating hormones have been associated with effects on human brain tissue composition. Replacement therapy of the adipokine leptin in genetically leptin deficient humans increased cerebellar, inferior parietal lobule and anterior cingulate GM volume (Matochik et al., 2005). In addition, a VBM analysis of healthy humans demonstrated associations of fasting leptin concentrations and GM volume of several brain regions, independent of percent body fat (Pannacciulli et al., 2007). Furthermore changes in brain morphology, specifically in the hippocampus have been found following resolution of hypercortisolemia in individuals with Cushing's syndrome (Starkman et al., 1999; Bourdeau et al., 2002).
We further investigated rCBF changes in the caudate using an ROI approach and demonstrated that caudate activity decreases between fasting and postprandial states and is negatively associated with postprandial PYY. Batterham et al. showed a modulation of striatal BOLD levels in response to PYY3–36 infusion. However, this effect was restricted to the right putamen and the left globus pallidus and did not include the caudate. Furthermore, PYY3–36 infusion showed a positive association with striatal activity (Batterham et al., 2007). Several reasons might explain our divergent findings. First, the paradigms used in the study by Batterham et al. and ours differ substantially and therefore do not allow direct comparisons. Batterham et al. analyzed fMRI measured BOLD levels in response to systemic application of PYY3–36, while we analyzed metabolic/hormonal responses and PET measured rCBF in response to an actual satiating meal. Also, we measured total PYY, which includes both endogenous forms of PYY. These two subtypes, PYY1–36 and PYY3–36 have different receptor selectivity profiles (Y1,Y2,Y5 vs. Y2>>Y1) (Dumont et al., 1995; Batterham and Bloom, 2003) possibly leading to different neuronal responses as the central distribution of Y-receptor subtypes varies substantially between different brain regions (Widdowson, 1993; Parker and Herzog, 1999). The caudate has the highest Y1 receptor density based on a recent study using a specific PET radiotracer (Hostetler et al., 2011). Considering the strong affinity of PYY1–36 and the comparatively low affinity of PYY3–36 for the Y1 receptor (Keire et al., 2000) allows speculation that PYY1–36 is more responsible for the reported findings.
The reason for the positive association between postprandial PYY and caudate GMV is not clear. One possible explanation is that PYY mediates changes in the caudate neurochemical milieu, specifically via its effect on dopamine. Dopamine is known to have trophic effects on neuronal and synaptic plasticity (Nieoullon, 2002). Genetic variants of the dopamine D2 receptor (DRD2) and the dopamine transporter (DAT) that are associated with reduced dopaminergic neurotransmission (Bertolino et al., 2009) have been shown to modulate caudate GMV in healthy individuals. Rodent studies showed that orexigenic effects of centrally administered PYY are dependent on DA signaling in the caudate nucleus (Hnasko et al., 2004). In vitro experiments have furthermore revealed a modulation of striatal DA synthesis via Y-receptor activation. Selective activation of striatal Y2 receptors with the synthetic Y2 agonist PYY13–36 increased DA synthesis. However, the administration of PYY1–36 and PYY3–36 provoked a significant attenuation of striatal DA synthesis (PYY1–36>PYY3–36) (Adewale et al., 2005). In contrast to these in vitro studies, central administration of PYY in rodents, led to a significant increase of arcuate nucleus tyrosine hydroxylase (TH) mRNA expression, the rate-limiting enzyme for DA synthesis (Hong et al., 1995). Differentiating central versus peripheral effects of PYY is not straightforward as central administration of PYY stimulates appetite and feeding, while peripherally administered PYY has anorectic effects. In addition, central Y-receptor subtypes distribution is different between humans and rats so effects in rat brains may not apply to humans (Widdowson, 1993).
Regarding the observed negative association between postprandial PYY and postprandial caudate rCBF, it has to be noted that the relationship between striatal rCBF and dopaminergic activity remains controversial as animal and human studies using pharmacological dopaminergic challenges have shown differing results. D-amphetamine, a dopamine-releasing agent, administered in four different doses followed by measurements of local DA (via microdialysis) and regional cerebral blood volume (rCBV) by MRI demonstrated increased rCBV in response to higher doses of D-amphetamine which also produced dose related higher concentrations of extracellular DA but an actual decrease in rCBV with lower doses. This finding was interpreted as a switch in dopamine receptor subtype stimulation (D2/D3 vs. D1/D5) associated with dose escalation (Ren et al., 2009) and indicates that different effects on brain structure and function may be seen in pharmacologic versus physiologic situations. L-DOPA, the direct precursor of dopamine, increased striatal rCBF in awake and but decreased rCBF in anaesthesized healthy, nonhuman primates (Hershey et al., 2000). In awake humans, L-DOPA increased striatal rCBF (i.e. bilateral putamen) (Hershey et al., 1998). However, pharmacologic challenge studies using dopamine receptor blocking agents have shown increases in striatal rCBF after systemic administration of haloperidol in both healthy humans and schizophrenic patients(Bartlett et al., 1994; Lahti et al., 2003, 2005).
Historically, the striatum has been most commonly associated with motor control. However, the caudate nucleus has complex interconnections within the striato-thalamo-cortical networks (Alexander et al., 1990) and recent studies indicate a more diverse role for the caudate in human behavior and cognition, including working memory, decision-making, reward and reward based learning (Haruno et al., 2004; Balleine et al., 2007; Ding and Gold, 2010) and food-related behavior (Stice et al., 2008; Neary and Batterham, 2010; Green et al., 2011). We showed significant positive associations between relative mean caudate rCBF and rCBF in the right cingulate gyrus and subcortical nuclei (i.e. thalamic nuclei, putamen, globus pallidus) in both fasting and postprandial state and significant positive associations with parietal and occipital region rCBF in the postprandial state only. Most interestingly, in the postprandial but not the fasting state we observed significant negative associations with right mOFC and right hippocampus rCBF, both significant on the cluster-level. The OFC is a polyfunctional region that has been linked to reward processing, decision-making and inhibitory control (i.e. the capacity to control a pre-potent reaction in response to a stimulus) (Kringelbach and Rolls, 2004; Perry et al., 2011). Alterations of these cognitive processes and their underlying neuronal systems (i.e. frontostriatal networks) have been associated with several disorders, including obesity, eating disorders (ED) and addictive behavior in general (Volkow, Wang, Telang, Fowler, Goldstein, et al., 2008; Volkow, Wang, Telang, Fowler, Thanos, et al., 2008; Brogan et al., 2010, 2011; Koob and Volkow, 2010; Oberndorfer et al., 2011). Interestingly, the caudate nucleus also seems to play an important role in inhibitory control, as disturbed caudate function and low inhibitory control are characteristics of obsessive-compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD) (Tripp and Wickens, 2009; Fineberg et al., 2010). The hippocampus is primarily implicated in memory but also appears to be part of the regulation of reward related behavior as demonstrated in human neuroimaging studies (Liu et al., 2011). The dorsal striatum together with the hippocampus, prefrontal cortical regions and the amygdala may form a network relevant in reward processing and reward based learning in particular (Haber et al., 2006).
Several other limitations must be acknowledged. This is a post-hoc analysis of a study designed to assess neuronal correlates of hunger and satiety. Although we adjusted for sex, results of this study might not entirely apply to the male gender due to the unbalanced sex distribution of our study population, however the results were consistent when women only were analyzed. Imaging procedures and hormonal/metabolic measurements were done after a prolonged fast of 36h. Furthermore, we measured only total PYY concentrations even though the anorectic effects of PYY are majorly attributed to PYY3–36. However, ratios of PYY1–36 and PYY3–36 appear to be stable in both healthy and obese individuals (le Roux et al., 2006) and both endogenous forms (PYY1–36 and PYY3–36) are physiologically active (Pfluger et al., 2007).
As this study is of correlational nature only, we cannot rule out the possibility of other physiological events that occur in response to a meal contributing to the reported associations, as for example changes in plasma concentrations of other hormones (e.g. GLP1, CCK, Pancreatic Polypeptide) or changes in vagal tone. However, adjusting for insulin concentrations did not show an effect on our results. MRI scans were performed on a 1.5T scanner and the VBM analysis presented here is of an exploratory nature in a limited number of subjects. Although the segmentation, especially of subcortical structures, has been improved in VBM8 by including the Maximum A Posterior (MAP) technique and Partial Volume Estimation (PVE) (Gaser, 2009) these results need to be repeated in a larger cohort using different volumetric methodologies. We did not observe differences in functional connectivity between the fasting and the postprandial state of the caudate nucleus and the hippocampus and right mOFC respectively. Nevertheless this may be due to our relatively small sample size. We also acknowledge that no attempt was made in this current study to correct the effects of combined partial volume effects and existence of variation of regional gray matter volume. Thus, additional studies are needed to address this issue and to confirm the significance of our findings independently of such effects.
5. Conclusions
We demonstrate that postprandial PYY concentrations are positively associated with gray matter volume of the bilateral caudate nucleus and negatively correlated with caudate rCBF. Furthermore, caudate activity is negatively associated with prefrontal and limbic regions implicated in reward and food related behavior, indicating that peripheral PYY may modulate eating behavior via central effects on striatal networks.
Supplementary Material
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott CR, Small CJ, Kennedy AR, Neary NM, Sajedi A, Ghatei MA, Bloom SR. Blockade of the neuropeptide Y Y2 receptor with the specific antagonist BIIE0246 attenuates the effect of endogenous and exogenous peptide YY(3-36) on food intake. Brain Res. 2005;1043(1–2):139–144. doi: 10.1016/j.brainres.2005.02.065. [DOI] [PubMed] [Google Scholar]
- Adewale AS, Macarthur H, Westfall TC. Neuropeptide Y induced modulation of dopamine synthesis in the striatum. Regul. Pept. 2005;129(1–3):73–78. doi: 10.1016/j.regpep.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Adrian TE, Ferri GL, Bacarese-Hamilton AJ, Fuessl HS, Polak JM, Bloom SR. Human distribution and release of a putative new gut hormone, peptide YY. Gastroenterology. 1985;89(5):1070–1077. doi: 10.1016/0016-5085(85)90211-2. [DOI] [PubMed] [Google Scholar]
- Alexander GE, Crutcher MD, DeLong MR. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11(6 Pt 1):805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- Balleine BW, Delgado MR, Hikosaka O. The role of the dorsal striatum in reward and decision-making. J. Neurosci. 2007;27(31):8161–8165. doi: 10.1523/JNEUROSCI.1554-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett EJ, Brodie JD, Simkowitz P, Dewey SL, Rusinek H, Wolf AP, Fowler JS, Volkow ND, Smith G, Wolkin A. Effects of haloperidol challenge on regional cerebral glucose utilization in normal human subjects. Am J Psychiatry. 1994;151(5):681–686. doi: 10.1176/ajp.151.5.681. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Bloom SR. The gut hormone peptide YY regulates appetite. Ann. N. Y. Acad. Sci. 2003;994:162–168. doi: 10.1111/j.1749-6632.2003.tb03176.x. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cohen MA, Ellis SM, Le Roux CW, Withers DJ, Frost GS, Ghatei MA, Bloom SR. Inhibition of food intake in obese subjects by peptide YY3-36. N. Engl. J. Med. 2003;349(10):941–948. doi: 10.1056/NEJMoa030204. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, Wren AM, Brynes AE, Low MJ, Ghatei MA, Cone RD, Bloom SR. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–654. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- Batterham RL, ffytche DH, Rosenthal JM, Zelaya FO, Barker GJ, Withers DJ, Williams SCR. PYY modulation of cortical and hypothalamic brain areas predicts feeding behaviour in humans. Nature. 2007;450(7166):106–109. doi: 10.1038/nature06212. [DOI] [PubMed] [Google Scholar]
- Berglund MM, Hipskind PA, Gehlert DR. Recent developments in our understanding of the physiological role of PP-fold peptide receptor subtypes. Exp. Biol. Med. (Maywood) 2003;228(3):217–244. doi: 10.1177/153537020322800301. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, Caforio G, Sinibaldi L, Ursini G, Popolizio T, Tirotta E, Papp A, Dallapiccola B, Borrelli E, Sadee W. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J. Neurosci. 2009;29(4):1224–1234. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourdeau I, Bard C, Noël B, Leclerc I, Cordeau M-P, Bélair M, Lesage J, Lafontaine L, Lacroix A. Loss of brain volume in endogenous Cushing’s syndrome and its reversibility after correction of hypercortisolism. J. Clin. Endocrinol. Metab. 2002;87(5):1949–1954. doi: 10.1210/jcem.87.5.8493. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, O’Callaghan G, Yoder R, O’Shea D. Impaired decision making among morbidly obese adults. J Psychosom Res. 2011;70(2):189–196. doi: 10.1016/j.jpsychores.2010.07.012. [DOI] [PubMed] [Google Scholar]
- Brogan A, Hevey D, Pignatti R. Anorexia, bulimia, and obesity: shared decision making deficits on the Iowa Gambling Task (IGT) J Int Neuropsychol Soc. 2010;16(4):711–715. doi: 10.1017/S1355617710000354. [DOI] [PubMed] [Google Scholar]
- Cabrele C, Beck-Sickinger AG. Molecular characterization of the ligand-receptor interaction of the neuropeptide Y family. J. Pept. Sci. 2000;6(3):97–122. doi: 10.1002/(SICI)1099-1387(200003)6:3<97::AID-PSC236>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Cox HM. Peptide YY: a neuroendocrine neighbor of note. Peptides. 2007;28(2):345–351. doi: 10.1016/j.peptides.2006.07.023. [DOI] [PubMed] [Google Scholar]
- Degen L, Oesch S, Casanova M, Graf S, Ketterer S, Drewe J, Beglinger C. Effect of peptide YY3-36 on food intake in humans. Gastroenterology. 2005;129(5):1430–1436. doi: 10.1053/j.gastro.2005.09.001. [DOI] [PubMed] [Google Scholar]
- Ding L, Gold JI. Caudate encodes multiple computations for perceptual decisions. J. Neurosci. 2010;30(47):15747–15759. doi: 10.1523/JNEUROSCI.2894-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont Y, Fournier A, St-Pierre S, Quirion R. Characterization of neuropeptide Y binding sites in rat brain membrane preparations using [125I][Leu31,Pro34]peptide YY and [125I]peptide YY3-36 as selective Y1 and Y2 radioligands. J. Pharmacol. Exp. Ther. 1995;272(2):673–680. [PubMed] [Google Scholar]
- Fineberg NA, Potenza MN, Chamberlain SR, Berlin HA, Menzies L, Bechara A, Sahakian BJ, Robbins TW, Bullmore ET, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes: a narrative review. Neuropsychopharmacology. 2010;35(3):591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Frith CD, Frackowiak RS, Turner R. Characterizing dynamic brain responses with fMRI: a multivariate approach. Neuroimage. 1995;2(2):166–172. doi: 10.1006/nimg.1995.1019. [DOI] [PubMed] [Google Scholar]
- Gaser C. Partial Volume Segmentation with Adaptive Maximum A Posteriori (MAP) Approach. NeuroImage. 2009;47:S121. [Google Scholar]
- Gehlert DR. Multiple receptors for the pancreatic polypeptide (PP-fold) family: physiological implications. Proc. Soc. Exp. Biol. Med. 1998;218(1):7–22. doi: 10.3181/00379727-218-44263. [DOI] [PubMed] [Google Scholar]
- Ghitza UE, Nair SG, Golden SA, Gray SM, Uejima JL, Bossert JM, Shaham Y. Peptide YY3-36 decreases reinstatement of high-fat food seeking during dieting in a rat relapse model. J. Neurosci. 2007;27(43):11522–11532. doi: 10.1523/JNEUROSCI.5405-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green E, Jacobson A, Haase L, Murphy C. Reduced nucleus accumbens and caudate nucleus activation to a pleasant taste is associated with obesity in older adults. Brain Res. 2011;1386:109–117. doi: 10.1016/j.brainres.2011.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guh DP, Zhang W, Bansback N, Amarsi Z, Birmingham CL, Anis AH. The incidence of co-morbidities related to obesity and overweight: a systematic review and meta-analysis. BMC Public Health. 2009;9:88. doi: 10.1186/1471-2458-9-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Kim K-S, Mailly P, Calzavara R. Reward-Related Cortical Inputs Define a Large Striatal Region in Primates That Interface with Associative Cortical Connections, Providing a Substrate for Incentive-Based Learning. The Journal of Neuroscience. 2006;26(32):8368–8376. doi: 10.1523/JNEUROSCI.0271-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M, Kuroda T, Doya K, Toyama K, Kimura M, Samejima K, Imamizu H, Kawato M. A neural correlate of reward-based behavioral learning in caudate nucleus: a functional magnetic resonance imaging study of a stochastic decision task. J. Neurosci. 2004;24(7):1660–1665. doi: 10.1523/JNEUROSCI.3417-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Carl JL, Perlmutter JS. Dopa-induced blood flow responses in nonhuman primates. Exp. Neurol. 2000;166(2):342–349. doi: 10.1006/exnr.2000.7522. [DOI] [PubMed] [Google Scholar]
- Hershey T, Black KJ, Stambuk MK, Carl JL, McGee-Minnich LA, Perlmutter JS. Altered thalamic response to levodopa in Parkinson’s patients with dopa-induced dyskinesias. Proc. Natl. Acad. Sci. U.S.A. 1998;95(20):12016–12021. doi: 10.1073/pnas.95.20.12016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hnasko TS, Szczypka MS, Alaynick WA, During MJ, Palmiter RD. A role for dopamine in feeding responses produced by orexigenic agents. Brain Res. 2004;1023(2):309–318. doi: 10.1016/j.brainres.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Hong M, Li S, Pelletier G. Role of neuropeptide Y in the regulation of tyrosine hydroxylase messenger ribonucleic acid levels in the male rat arcuate nucleus as evaluated by in situ hybridization. J. Neuroendocrinol. 1995;7(1):25–28. doi: 10.1111/j.1365-2826.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Hostetler ED, Sanabria-Bohórquez S, Fan H, Zeng Z, Gantert L, Williams M, Miller P, O’Malley S, Kameda M, Ando M, Sato N, Ozaki S, Tokita S, Ohta H, Williams D, Sur C, Cook JJ, Burns HD, Hargreaves R. Synthesis, characterization, and monkey positron emission tomography (PET) studies of [18F]Y1-973, a PET tracer for the neuropeptide Y Y1 receptor. Neuroimage. 2011;54(4):2635–2642. doi: 10.1016/j.neuroimage.2010.11.014. [DOI] [PubMed] [Google Scholar]
- Keire DA, Mannon P, Kobayashi M, Walsh JH, Solomon TE, Reeve JR., Jr Primary structures of PYY, [Pro(34)]PYY, and PYY-(3-36) confer different conformations and receptor selectivity. Am. J. Physiol. Gastrointest. Liver Physiol. 2000;279(1):G126–G131. doi: 10.1152/ajpgi.2000.279.1.G126. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35(1):217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: evidence from neuroimaging and neuropsychology. Prog. Neurobiol. 2004;72(5):341–372. doi: 10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Holcomb HH, Weiler MA, Medoff DR, Tamminga CA. Functional effects of antipsychotic drugs: comparing clozapine with haloperidol. Biol. Psychiatry. 2003;53(7):601–608. doi: 10.1016/s0006-3223(02)01602-5. [DOI] [PubMed] [Google Scholar]
- Lahti AC, Weiler MA, Medoff DR, Tamminga CA, Holcomb HH. Functional effects of single dose first- and second-generation antipsychotic administration in subjects with schizophrenia. Psychiatry Res. 2005;139(1):19–30. doi: 10.1016/j.pscychresns.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Woldorff MG, Parsons LM, Liotti M, Freitas CS, Rainey L, Kochunov PV, Nickerson D, Mikiten SA, Fox PT. Automated Talairach atlas labels for functional brain mapping. Hum Brain Mapp. 2000;10(3):120–131. doi: 10.1002/1097-0193(200007)10:3<120::AID-HBM30>3.0.CO;2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hairston J, Schrier M, Fan J. Common and distinct networks underlying reward valence and processing stages: a meta-analysis of functional neuroimaging studies. Neurosci Biobehav Rev. 2011;35(5):1219–1236. doi: 10.1016/j.neubiorev.2010.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003;19(3):1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Matochik JA, London ED, Yildiz BO, Ozata M, Caglayan S, DePaoli AM, Wong M-L, Licinio J. Effect of leptin replacement on brain structure in genetically leptin-deficient adults. J. Clin. Endocrinol. Metab. 2005;90(5):2851–2854. doi: 10.1210/jc.2004-1979. [DOI] [PubMed] [Google Scholar]
- Meguid MM, Glade MJ, Middleton FA. Weight regain after Roux-en-Y: A significant 20% complication related to PYY. Nutrition. 2008;24(9):832–842. doi: 10.1016/j.nut.2008.06.027. [DOI] [PubMed] [Google Scholar]
- Mentlein R, Dahms P, Grandt D, Krüger R. Proteolytic processing of neuropeptide Y and peptide YY by dipeptidyl peptidase IV. Regul. Pept. 1993;49(2):133–144. doi: 10.1016/0167-0115(93)90435-b. [DOI] [PubMed] [Google Scholar]
- Michel MC, Beck-Sickinger A, Cox H, Doods HN, Herzog H, Larhammar D, Quirion R, Schwartz T, Westfall T. XVI. International Union of Pharmacology recommendations for the nomenclature of neuropeptide Y, peptide YY, pancreatic polypeptide receptors. Pharmacol. Rev. 1998;50(1):143–150. [PubMed] [Google Scholar]
- Neary MT, Batterham RL. Gaining new insights into food reward with functional neuroimaging. Forum Nutr. 2010;63:152–163. doi: 10.1159/000264403. [DOI] [PubMed] [Google Scholar]
- Nieoullon A. Dopamine and the regulation of cognition and attention. Prog. Neurobiol. 2002;67(1):53–83. doi: 10.1016/s0301-0082(02)00011-4. [DOI] [PubMed] [Google Scholar]
- Oberndorfer TA, Kaye WH, Simmons AN, Strigo IA, Matthews SC. Demand-specific alteration of medial prefrontal cortex response during an inhibition task in recovered anorexic women. Int J Eat Disord. 2011;44(1):1–8. doi: 10.1002/eat.20750. [DOI] [PubMed] [Google Scholar]
- Pannacciulli N, Le DSNT, Chen K, Reiman EM, Krakoff J. Relationships between plasma leptin concentrations and human brain structure: a voxel-based morphometric study. Neurosci. Lett. 2007;412(3):248–253. doi: 10.1016/j.neulet.2006.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker RM, Herzog H. Regional distribution of Y-receptor subtype mRNAs in rat brain. Eur. J. Neurosci. 1999;11(4):1431–1448. doi: 10.1046/j.1460-9568.1999.00553.x. [DOI] [PubMed] [Google Scholar]
- Perry JL, Joseph JE, Jiang Y, Zimmerman RS, Kelly TH, Darna M, Huettl P, Dwoskin LP, Bardo MT. Prefrontal cortex and drug abuse vulnerability: translation to prevention and treatment interventions. Brain Res Rev. 2011;65(2):124–149. doi: 10.1016/j.brainresrev.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Kampe J, Castaneda TR, Vahl T, D’Alessio DA, Kruthaupt T, Benoit SC, Cuntz U, Rochlitz HJ, Moehlig M, Pfeiffer AFH, Koebnick C, Weickert MO, Otto B, Spranger J, Tschöp MH. Effect of human body weight changes on circulating levels of peptide YY and peptide YY3-36. J. Clin. Endocrinol. Metab. 2007;92(2):583–588. doi: 10.1210/jc.2006-1425. [DOI] [PubMed] [Google Scholar]
- Raben A, Tagliabue A, Astrup A. The reproducibility of subjective appetite scores. Br. J. Nutr. 1995;73(4):517–530. doi: 10.1079/bjn19950056. [DOI] [PubMed] [Google Scholar]
- Ren J, Xu H, Choi J-K, Jenkins BG, Chen YI. Dopaminergic response to graded dopamine concentration elicited by four amphetamine doses. Synapse. 2009;63(9):764–772. doi: 10.1002/syn.20659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- le Roux CW, Batterham RL, Aylwin SJB, Patterson M, Borg CM, Wynne KJ, Kent A, Vincent RP, Gardiner J, Ghatei MA, Bloom SR. Attenuated peptide YY release in obese subjects is associated with reduced satiety. Endocrinology. 2006;147(1):3–8. doi: 10.1210/en.2005-0972. [DOI] [PubMed] [Google Scholar]
- Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am. J. Physiol. Endocrinol. Metab. 2007;292(4):E1062–E1068. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Williams JB, Gibbon M, First MB. Structured Clinical Interview for DSM-III-R, Patient Edition/Non-patient Edition,(SCID-P/SCID-NP) Washington, D.C.: American Psychiatric Press, Inc.; 1990. [Google Scholar]
- Starkman MN, Giordani B, Gebarski SS, Berent S, Schork MA, Schteingart DE. Decrease in cortisol reverses human hippocampal atrophy following treatment of Cushing’s disease. Biol. Psychiatry. 1999;46(12):1595–1602. doi: 10.1016/s0006-3223(99)00203-6. [DOI] [PubMed] [Google Scholar]
- Stice E, Spoor S, Bohon C, Veldhuizen M, Small D. Relation of Reward from Food Intake and Anticipated Food Intake to Obesity: A Functional Magnetic Resonance Imaging Study. J Abnorm Psychol. 2008;117(4):924–935. doi: 10.1037/a0013600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tataranni PA, Gautier JF, Chen K, Uecker A, Bandy D, Salbe AD, Pratley RE, Lawson M, Reiman EM, Ravussin E. Neuroanatomical correlates of hunger and satiation in humans using positron emission tomography. Proc. Natl. Acad. Sci. U.S.A. 1999;96(8):4569–4574. doi: 10.1073/pnas.96.8.4569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripp G, Wickens JR. Neurobiology of ADHD. Neuropharmacology. 2009;57(7–8):579–589. doi: 10.1016/j.neuropharm.2009.07.026. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. Neuroimage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Goldstein RZ, Alia-Klein N, Logan J, Wong C, Thanos PK, Ma Y, et al. Inverse association between BMI and prefrontal metabolic activity in healthy adults. Obesity. 2008;17(1):60–65. doi: 10.1038/oby.2008.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding Y-S, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42(4):1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widdowson PS. Quantitative receptor autoradiography demonstrates a differential distribution of neuropeptide-Y Y1 and Y2 receptor subtypes in human and rat brain. Brain Res. 1993;631(1):27–38. doi: 10.1016/0006-8993(93)91182-r. [DOI] [PubMed] [Google Scholar]
- Witte A-B, Grybäck P, Holst JJ, Hilsted L, Hellström PM, Jacobsson H, Schmidt PT. Differential effect of PYY1-36 and PYY3-36 on gastric emptying in man. Regul. Pept. 2009;158(1–3):57–62. doi: 10.1016/j.regpep.2009.07.013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.