Abstract
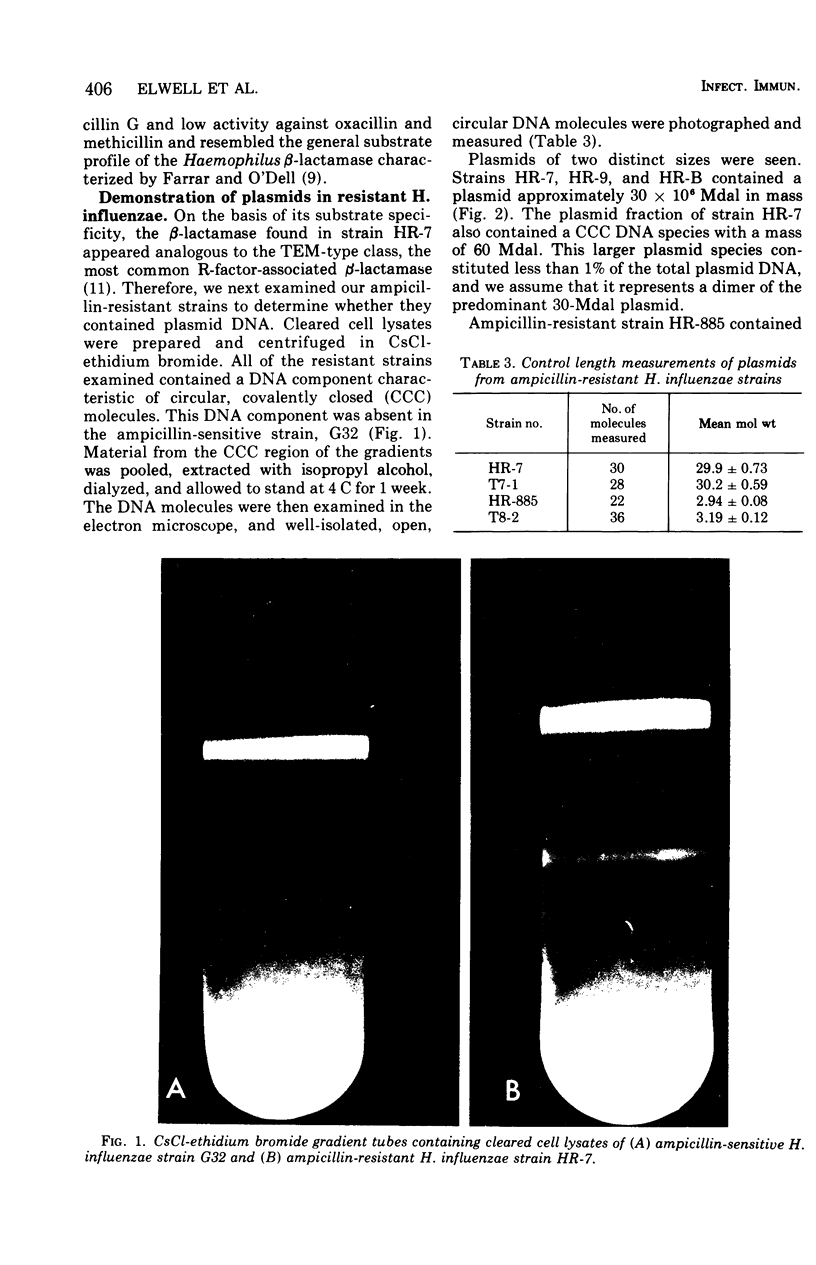
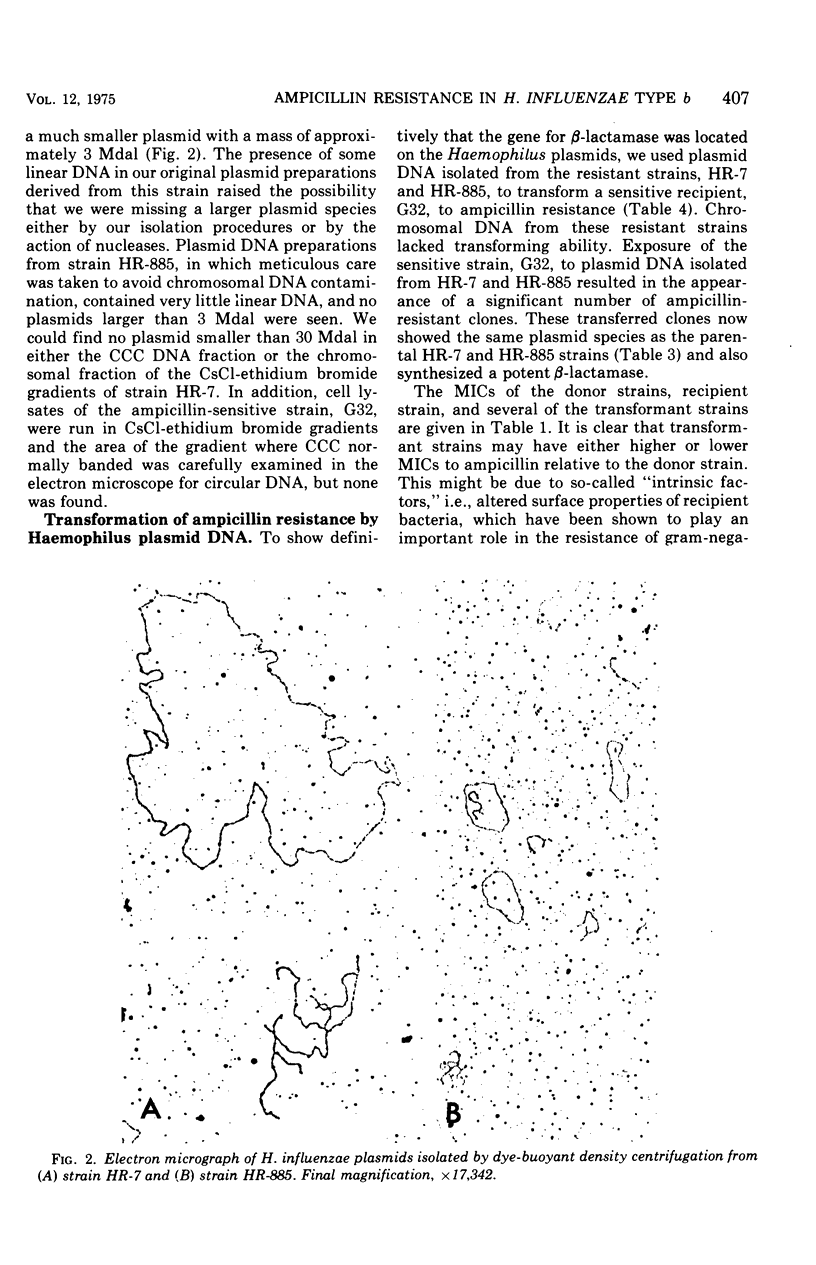
Four ampicillin-resistant, beta-lactamase-producing strains of Haempohilus influenzae type b were examined for the presence of plasmid deoxyribonucleic acid (DNA). Three resistant strains contained a 30 x 10-6-dalton (30Mdal) plasmid and one resitant strain contained a 3-Mdal plasmid. The ampicillin-sensitive Haemophilus strains examined did not contain plasmid DNA. Transformation of a sensitive H. influenzae strain to ampicillin resistance with isolated plasmid DNA preparations revealed that the structural gene for beta-lactamase resided on both plasmid species. DNA-DNA hybridization studies showed that the 30-Mdal Haemophilus plasmid contained the ampicillin translocation DNA segment (TnA) found on some R-factors of enteric origin of the H. influenzae plasmids.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Brenner D. J., Falkow S. Use of a single-strand specific nuclease for analysis of bacterial and plasmid deoxyribonucleic acid homo- and heteroduplexes. J Bacteriol. 1973 Sep;115(3):904–911. doi: 10.1128/jb.115.3.904-911.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosa J. H., Luttropp L. K., Falkow S. Nature of R-factor replication in the presence of chloramphenicol. Proc Natl Acad Sci U S A. 1975 Feb;72(2):654–658. doi: 10.1073/pnas.72.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W., Shaw E. J., Sykes R. B., Richmond M. H. Properties of an R factor from Pseudomonas aeruginosa. J Bacteriol. 1971 Dec;108(3):1244–1249. doi: 10.1128/jb.108.3.1244-1249.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar W. E., Jr, O'Dell N. M. Beta-lactamase activity in ampicillin-resistant Haemophilus influenzae. Antimicrob Agents Chemother. 1974 Nov;6(5):625–629. doi: 10.1128/aac.6.5.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerry P., van Embden J., Falkow S. Molecular nature of two nonconjugative plasmids carrying drug resistance genes. J Bacteriol. 1974 Feb;117(2):619–630. doi: 10.1128/jb.117.2.619-630.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedges R. W., Jacob A. E. Transposition of ampicillin resistance from RP4 to other replicons. Mol Gen Genet. 1974;132(1):31–40. doi: 10.1007/BF00268228. [DOI] [PubMed] [Google Scholar]
- Heffron F., Sublett R., Hedges R. W., Jacob A., Falkow S. Origin of the TEM-beta-lactamase gene found on plasmids. J Bacteriol. 1975 Apr;122(1):250–256. doi: 10.1128/jb.122.1.250-256.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyobe S., Hasuda K., Fuse A., Mitsuhashi S. Demonstration of R factors from Pseudomonas aeruginosa. Antimicrob Agents Chemother. 1974 Jun;5(6):547–552. doi: 10.1128/aac.5.6.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan W., Ross S., Rodriguez W., Controni G., Saz A. K. Haemophilus influenzae type B resistant to ampicillin. A report of two cases. JAMA. 1974 Jul 15;229(3):298–301. [PubMed] [Google Scholar]
- Nelson J. D. Editorial: Should ampicillin be abandoned for treatment of Haemophilus influenzae disease? JAMA. 1974 Jul 15;229(3):322–324. [PubMed] [Google Scholar]
- Olsen R. H., Shipley P. Host range and properties of the Pseudomonas aeruginosa R factor R1822. J Bacteriol. 1973 Feb;113(2):772–780. doi: 10.1128/jb.113.2.772-780.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond M. H., Curtis N. A. The interplay of beta-lactamases and intrinsic factors in the resistance of gram-negative bacteria to penicillins and cephalosporins. Ann N Y Acad Sci. 1974 May 10;235(0):553–568. doi: 10.1111/j.1749-6632.1974.tb43290.x. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The chromosomal integration of a -lactamase gene derived from the P-type R-factor RP1 in Escherichia coli. Genet Res. 1972 Oct;20(2):231–237. doi: 10.1017/s0016672300013732. [DOI] [PubMed] [Google Scholar]
- Rubin F. A., Smith D. H. Characterization of R factor beta-lactamases by the acidimetric method. Antimicrob Agents Chemother. 1973 Jan;3(1):68–73. doi: 10.1128/aac.3.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So M., Crosa J. H., Falkow S. Polynucleotide sequence relationships among Ent plasmids and the relationship between Ent and other plasmids. J Bacteriol. 1975 Jan;121(1):234–238. doi: 10.1128/jb.121.1.234-238.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomeh M. O., Starr S. E., McGowan J. E., Jr, Terry P. M., Nahmias A. J. Ampicillin-resistant Haemophilus influenzae type B infection. JAMA. 1974 Jul 15;229(3):295–297. [PubMed] [Google Scholar]