Abstract
Study Objectives:
To evaluate the efficacy of mindfulness meditation for the treatment of chronic insomnia.
Design:
Three-arm, single-site, randomized controlled trial.
Setting:
Academic medical center.
Participants:
Fifty-four adults with chronic insomnia.
Interventions:
Participants were randomized to either mindfulness-based stress reduction (MBSR), mindfulness-based therapy for insomnia (MBTI), or an eight-week self-monitoring (SM) condition.
Measurements and Results:
Patient-reported outcome measures were total wake time (TWT) from sleep diaries, the pre-sleep arousal scale (PSAS), measuring a prominent waking correlate of insomnia, and the Insomnia Severity Index (ISI) to determine remission and response as clinical endpoints. Objective sleep measures were derived from laboratory polysomnography and wrist actigraphy. Linear mixed models showed that those receiving a meditation-based intervention (MBSR or MBTI) had significantly greater reductions on TWT minutes (43.75 vs 1.09), PSAS (7.13 vs 0.16), and ISI (4.56 vs 0.06) from baseline-to-post compared to SM. Post hoc analyses revealed that each intervention was superior to SM on each of the patient-reported measures, but no significant differences were found when comparing MBSR to MBTI from baseline-to-post. From baseline to 6-month follow-up, MBTI had greater reductions in ISI scores than MBSR (P < 0.05), with the largest difference occurring at the 3-month follow-up. Remission and response rates in MBTI and MBSR were sustained from post-treatment through follow-up, with MBTI showing the highest rates of treatment remission (50%) and response (78.6%) at the 6-month follow-up.
Conclusions:
Mindfulness meditation appears to be a viable treatment option for adults with chronic insomnia and could provide an alternative to traditional treatments for insomnia.
Trial Registration:
Mindfulness-Based Approaches to Insomnia: clinicaltrials.gov, identifier: NCT00768781
Citation:
Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. SLEEP 2014;37(9):1553-1563.
Keywords: insomnia, mindfulness, meditation, behavior therapy, sleep, complementary and alternative medicine, clinical trial
INTRODUCTION
Sleep disturbance is a widespread public health concern with significant adverse consequences on quality of life for the individual and significant economic burden for society. Approximately 6% to 20% of adults suffer from an insomnia disorder, characterized as persistent difficulty falling or staying asleep with concomitant waking dysfunction, making it the most prevalent sleep disorder.1–3 Current guidelines for the treatment of insomnia include pharmacological and behavioral treatments,4 but each of these treatment modalities have limitations impeding its impact. Although hypnotic medications can reduce sleep latency and increase total sleep time at night,5–8 concerns regarding drug dependency, drug tolerance, and side effects remain (e.g., residual daytime sleepiness, acute memory impairments, impaired balance and gait), and many patients prefer non-pharmacological approaches.9,10 Multicomponent behavioral treatments, such as cognitive-behavior therapy for insomnia (CBTI), have strong empirical support with moderate to large effect sizes on sleep parameters,11–14 but only 26% to 43% of patients achieve full remission from insomnia when categorically defined criteria are applied.13–15 Therefore, the public health burden of insomnia remains substantial, and alternative approaches are needed to improve treatment outcomes.
Due to these shortcomings in traditional treatments, many individuals with insomnia are now turning to complementary and alternative medicine (CAM) to relieve their sleep problem. Common CAM treatments for sleep disturbances include acupuncture, herbal remedies, and mind-body therapies such as meditation and yoga. Data from the 2002 National Health Interview Survey (NHIS) revealed that 1.6 million were using CAM to treat insomnia,16 while data from the 2007 NHIS found that 45% of adults with insomnia symptoms use CAM annually.17 Mindfulness meditation is a mind-body CAM treatment using focused, non-judgmental awareness, and attention on the present moment experience as a means of self-regulation to promote mind-body calmness and relaxation.18 A program known as mindfulness-based stress reduction (MBSR)19 that teaches mindfulness meditation using a structured group intervention has gained popularity and has been shown to have several health benefits across stress-related conditions including sleep disturbance.20–25 MBSR has been adapted and integrated with cognitive-behavioral techniques tailored for specific disorders, including depression, binge eating, and substance abuse.26–28
We have been developing an adaptation of MBSR tailored specifically to insomnia that is called mindfulness-based therapy for insomnia (MBTI), which is a meditation-based program that integrates behavioral techniques for insomnia.29–31 The conceptual basis for MBTI is to improve sleep and daytime functioning by reducing hyperarousal,32 a prominent waking correlate that develops during the course of chronic insomnia.32–34 In contrast to CBTI that is primarily aimed at changing thoughts and behaviors to reduce unwanted wakefulness at night, MBTI is aimed at shifting metacognitions to reduce sleep-related arousal at night and during the day through mindfulness meditation practice. MBTI is similar in structure and format to MBSR but also includes behavioral strategies for insomnia that are delivered within the framework of mindfulness concepts during the meditations and group discussions. Empirical testing of MBTI began with a series of pilot studies that collectively supported the acceptability and feasibility of using mindfulness meditation to reduce sleep-related arousal and improve sleep parameters with evidence of clinically significant effect sizes.29–31 Our preliminary work utilized an uncontrolled design that did not include a standard MBSR treatment arm or control condition, and thus precluded testing of efficacy or determining the relative effects of mindfulness meditation without behavioral components.
The present study builds upon this work, using a small-scale randomized controlled trial to gather preliminary evidence for treatment efficacy, using current standards for the assessment of insomnia and validated clinical endpoints.35,36 The primary aim of this study was to evaluate the efficacy of meditation-based therapies on measures of sleep and arousal for people with chronic insomnia. The main research question was whether mindfulness meditation, delivered using MBSR—a standard meditation program—or delivered using MBTI—a tailored meditation program with behavior strategies for insomnia— would be superior to a self-monitoring (SM) control. It was hypothesized that the average effects of the two meditation arms would be superior to SM on acute treatment outcomes using a priori contrasts. The secondary research question was whether there were relative benefits for the tailored approach (MBTI) compared to the standard approach (MBSR) of delivering a meditation-based therapy. It was hypothesized that MBTI would show relative benefits on sleep measures compared to MBSR on acute and long-term outcomes. Together, these aims were designed to evaluate the viability of meditation-based approaches to the treatment of chronic insomnia.
METHODS
Participants
This was a single-site trial conducted at Rush University Medical Center. Participants were recruited between November 2008 and February 2012, primarily through advertisements posted in public transportation, local newspapers, internet bulletin boards, and fliers that described general non-pharmacological techniques (changing sleep behaviors, techniques to relax and calm the mind) for improving sleep. Participants were adults over the age of 21 who met research diagnostic criteria for an insomnia disorder, defined as difficulty initiating or maintaining sleep that occurs despite adequate opportunity to sleep with at least one symptom of an associated daytime impairment.37 Additional quantitative insomnia criteria following research recommendations38 were included for frequency, defined as sleep onset latency (SOL) or wake after sleep onset (WASO) > 30 minutes at least 3 nights per week and for chronicity, defined as symptoms lasting ≥ 6 months. In addition, at least one symptom of heightened cognitive or somatic arousal (e.g., anxiety about sleep, elevated muscle tension) was reported on the screening interview (see below) in order to meet criteria for the subtype of psychophysiological insomnia.37 Exclusion criteria were: (1) uncontrolled medical condition suspected to interfere with sleep or requiring immediate treatment outside of the study; (2) uncontrolled psychiatric condition requiring immediate treatment outside of the study, including current major depressive episode; (3) comorbid sleep disorders including obstructive sleep apnea (apnea/hypopnea index ≥ 5), periodic limb movement index with arousal ≥ 10, restless legs syndrome, or circadian rhythm sleep disorders; (4) use of hypnotic or sedating medications for the purpose of insomnia; or (5) inadequate proficiency in English to complete the protocol. All participants provided written informed consent, and the study was approved by the Institutional Review Board at Rush University Medical Center.
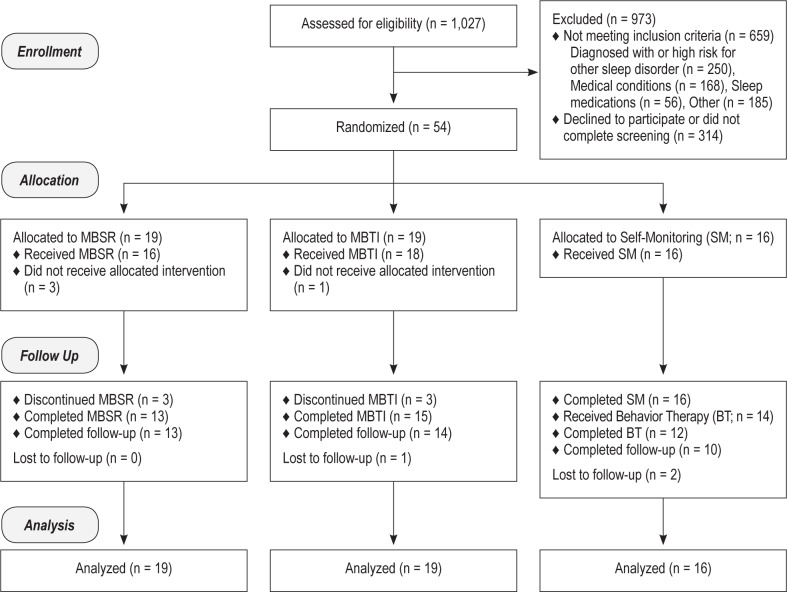
A 3-step screening process was employed (see Figure 1 for study flow diagram), which consisted of: (1) telephone screen for general eligibility; (2) in-person interview including review of medical history, the Structured Diagnostic Interview for DSM-IV39, and the Duke Structured Interview Schedule for Sleep Disorders40; and (3) overnight laboratory polysomnography (PSG; see below for PSG protocol).
Figure 1.
CONSORT study flow diagram. Participants in the self-monitoring (SM) arm were allocated to 8 weeks of SM, assessed at post SM, and then received 8 weeks of behavior therapy (BT), followed by assessments at post treatment and then at 3-month and 6-month follow-ups. Data for BT are not included in this report. MBSR, mindfulness-based stress reduction; MBTI, mindfulness-based therapy for insomnia.
Study Design and Procedures
After completing all screening procedures, participants were randomized to 1 of 3 study arms: (1) MBSR, (2) MBTI, and (3) a self-monitoring (SM) control consisting of an 8-week self-monitoring (SM) period using sleep diaries. Following the SM, participants received an 8-week multicomponent behavior therapy for insomnia (results not reported here). SM was selected as a comparator to control for the effects of sleep self-monitoring, which is used across all treatment conditions in this study. Self-monitoring using sleep diaries has been shown to have mild effects for reducing sleep latency41,42 and moderate effects on increasing sleep efficiency,42 suggesting clinical equipoise regarding the use of this condition as a control. Since treatments were intended to be delivered in groups, randomization was conducted in sequential cohorts, with most cohorts consisting of 4 to 6 participants. There were 2 cohorts that only had 2 participants due to a decreased tempo of enrollment. Therefore, “fillers” (participants not enrolled in this study but were similar on demographic characteristics) were used to maintain a minimum of 4 participants per treatment group for MBTI and MBSR to maintain integrity of the group intervention. Randomization with equal allocation was conducted by a study investigator with no participant contact who blindly selected from 3 sequentially numbered containers (for each study arm) without replacement until all study arms were assigned. Allocation was concealed from the study staff and participants during screening until randomization was completed.
Study Arms
Mindfulness-based stress reduction (MBSR)
The MBSR treatment consisted of 8 weekly group meetings lasting 2.5 h each plus one 6-h meditation retreat held between the 5th and 7th week.43 Each group meeting included meditation practice (breathing meditation, body scan meditations, walking meditations, Hatha Yoga), a period of general discussion about the at-home meditation practice, and education on the daily applications of meditation. MBSR was taught by 2 instructors with doctoral degrees (PhD or MD) who had > 2 years of experience teaching MBSR. Neither had formal training in sleep medicine or CBT for insomnia.
Mindfulness-based therapy for insomnia (MBTI)
MBTI was conducted as an 8-week group intervention that consisted of the same amount of contact and the same meditations as MBSR. Sessions typically began with formal mindfulness meditations that include one quiet (body scan, breathing, sitting meditation) and one movement meditation (yoga, walking, stretching meditation). A period of discussion led by the MBTI instructor followed, as participants discussed the application of mindfulness principles to the problem of insomnia and discussed challenges to maintaining a meditation practice. Instead of didactics on general health and education on meditation and stress that is part of MBSR, MBTI provided specific behavioral strategies for insomnia (sleep restriction therapy,44 stimulus control,45 and sleep hygiene46) delivered within the context of mindfulness principles. Further content of the 8-week MBTI and how the behavioral components are integrated has been described elsewhere.29 MBTI was delivered by the first author, who has specialized training in mindfulness meditation and behavioral treatments for insomnia. Participants in both MBSR and MBTI were instructed to practice meditation at home for 30-45 min ≥ 6 days/week and were asked to keep a meditation diary along with their sleep diary. In addition, they were provided with the book Full Catastrophe Living by Kabat-Zinn43 and a CD for guided meditation to aid in their home practice.
Self-monitoring (SM) condition
The SM condition consisted of self-monitoring using daily sleep/wake diaries and weekly pre-sleep arousal scale (PSAS) for 8 consecutive weeks. Upon randomization to SM, participants first attended an orientation session during which an overview of the SM period was explained and materials for monitoring were provided. To enhance motivation, participants were told that the diary data completed during the SM would be used to inform treatment planning during the behavior therapy that followed the SM period. SM was managed by the research staff and there was no structured contact with participants during this period. Following the SM period, participants were re-assessed with all outcome measures, which served as the post assessment time point.
Across all treatment conditions, treatment providers completed checklists and self-ratings of provider fidelity at each treatment session. A local data and safety monitoring committee reviewed progress with recruitment, retention, and adverse events.
Outcome Measures
Following standard guidelines for research assessment of insomnia,35 self-reported sleep patterns were completed each morning using sleep diaries. Sleep parameters derived from the sleep diaries include sleep onset latency (SOL), wake after sleep onset (WASO), number of awakenings (NWAK), total sleep time (TST), and time in bed (TIB). Sleep efficiency (SE) was a percentage calculated as TST / TIB × 100. Total wake time (TWT), consisting of SOL + WASO, was the primary outcome measure for sleep given the heterogeneous complaints of both sleep initiation and sleep maintenance difficulties in this sample. Diary data were averaged across one week with a minimum of 4 days required for inclusion. Sleep diaries were completed at baseline, each treatment/monitoring week, post, 3-month, and 6-month follow-up. The Pre-Sleep Arousal Scale (PSAS) is a 16-item self-report measure that assesses cognitive and somatic arousal in the period prior to sleep.47 In the current study, ratings across the week were used, and the internal consistency of the scale at baseline was α = 0.80.48 The PSAS was completed with sleep diaries at the same time points. The total PSAS score was the primary outcome measure for a waking correlate of insomnia, since all participants reported symptoms of psychophysiological arousal. The Insomnia Severity Index (ISI) is a 7-item scale that has been used as both a screening and outcome measure in insomnia treatment research.49 It assesses the severity of both nighttime and daytime symptoms of insomnia over the past week and has been validated as a clinical endpoint for a minimally important treatment response (ISI total score reduction > 7 points) and remission (ISI total score < 8).15,36,49 The ISI was given at baseline, post, 3-month, and 6-month follow-up to provide clinical endpoints for the treatment of chronic insomnia.
In addition to these patient-reported outcomes, sleep was measured objectively using laboratory PSG and wrist actigraphy as secondary outcome measures. Technician-monitored PSG was conducted for one night at baseline, post, and 6-month follow-up. For the screening PSG, a standard technician-monitored laboratory PSG was conducted following standard practices,50 using 19 channels for EEG, EOG, chin and bilateral anterior tibialis EMG, EKG, snoring, airflow, respiratory effort, and pulse oximetry. For the baseline, post, and 6-month follow-up, a reduced montage without respiratory and leg EMG measures were used. For all PSGs, scoring of sleep parameters followed AASM standards51 and were performed by research staff under the supervision of a registered polysomnography technologist (RPSGT). Bedtimes and wake times during the PSG were based upon habitual sleep/wake patterns derived from the screening diary with a target of 8 h as the TIB interval. At post and 6-month follow-up, participants who completed MBTI were allowed to reduce TIB based upon recommendations received during the intervention. The same sleep parameters used for sleep diaries were also derived for each night of PSG data. Wrist actigraphy (Actiwatch 2, Phillips Respironics, Bend, OR) was used to measure sleep/wake patterns at home for one week at baseline, post, and 6-month follow-up. Data were analyzed in 1-min epochs using medium sensitivity for determining wakefulness with Respironics Actiware version 5.70. Actigraphy is commonly used to assess sleep/wake patterns and has been well validated against PSG.52,53 In this study, TIB (or rest interval) was derived using data from the sleep diaries. If sleep diary data were unavailable or appeared invalid, then the event marker (pressed by the participant on the actigraph to indicate bedtime and wake time) was used to set the TIB interval. If neither of these was available, then no rest interval was set for that night and the data were considered incomplete. Data were averaged across the week with a minimum of 4 of 7 days required. TWT (SOL + WASO) from the PSG and actigraphy were the main secondary outcome measures of interest for assessing objective sleep outcomes.
Data Analyses
All analyses were conducted on an intent-to-treat basis with no imputation for missing data, and all statistical tests were two-sided with P < 0.05 considered to be statistically significant (SAS version 9.3; SAS Institute, Inc., Cary, NC). Linear mixed models (LMM) were used to compare the rate of change in outcomes over the treatment phase (a total of 10 time points: baseline, 8 weekly treatment assessments, post) and separately over the entire study period including follow-up (a total of 4 time points: baseline, post, 3-month follow-up, 6-month follow-up). We refer to these as the post LMM and long-term LMM, respectively.
For the primary analyses, post LMMs were conducted on the following patient-reported outcomes: (1) TWT from sleep diaries, (2) PSAS, and (3) ISI total score. These models included study arm, time, and their interaction. For each outcome variable, a priori linear contrasts compared the average effects of the two meditation arms to the SM arm, followed by post hoc comparisons between each arm (MBSR vs. SM, MBTI vs. SM, and MBSR vs. MBTI). Given that the primary aim of this study was to test the efficacy of meditation-based approaches, it was hypothesized that the average effects of the 2 meditation arms would be superior to SM on TWT and PSAS. The long-term LMMs were conducted on the same outcome measures with only the 2 active treatment arms (MBSR vs. MBTI), time (baseline, post, 3-month follow-up, 6-month follow-up), and the interaction term. It was hypothesized that MBTI would show relative benefits compared to MBSR on long-term outcomes on TWT and ISI. To examine clinical endpoints, logistic regression analyses were conducted to test differences in treatment response and remission using cut-scores described above on the ISI across post, 3-month, and 6-month follow-up. Following the patient-reported outcomes, another set of LMMs were conducted on PSG and actigraphy variables to examine treatment effects on objective measures of sleep and wakefulness. These outcomes are considered secondary, given that effect sizes on objective measures are generally smaller than patient-reported outcomes and this study was not designed to be sufficiently powered to detect these treatment effects. The target sample size was 54, which was determined by a balance between power considerations informed by previous research,30 which found large within-group effect sizes for MBTI on both TWT (d = 1.17) and PSAS (d = 1.00) that would achieve adequate power on the primary analysis and the feasibility of resources available for this early-stage randomized controlled trial.54
RESULTS
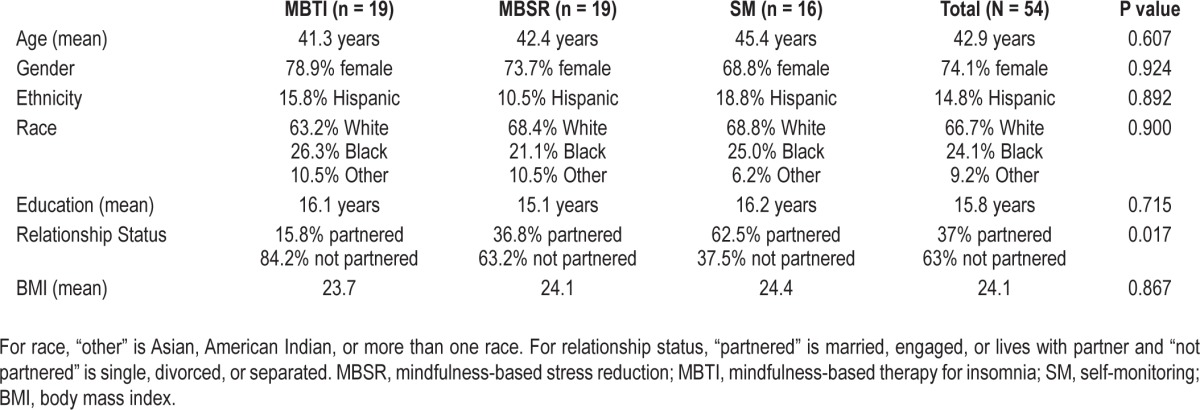
A total of 54 participants were enrolled in this study (see Figure 1 for participant flow). The most common reason for exclusion from the study was existing diagnosis or clinical features indicating high probability of other sleep disorders (n = 250), particularly obstructive sleep apnea (n = 179). The second most common reason for exclusion was a comorbid medical condition (e.g., pain condition, thyroid disease) that interferes with sleep. The average age at enrollment was 42.9 years (standard deviation [SD] = 12.2), and 74.1% of the sample was female. Demographic information for each study arm is presented in Table 1. There were no significant differences between groups on any of these demographic variables, except relationship status. Since this was a small sample with no preselected covariates of interest, all analyses were conducted without adjustments for covariates. Since the treatments were conducted in groups, the intra-class correlation (ICC) of each group within treatment condition was examined and found to be at zero or very small and did not have an impact on the analyses. Given the small sample size, and the ineligible ICCs, we did not model the nested data structure to include a group variable when comparing the treatment conditions. Preliminary inspection of the data revealed no outliers or significant differences on any of the primary outcome measures at baseline to indicate a need for entering covariates into the analytic model.
Table 1.
Participant characteristics
Patient-Reported Outcomes
Sleep Diary
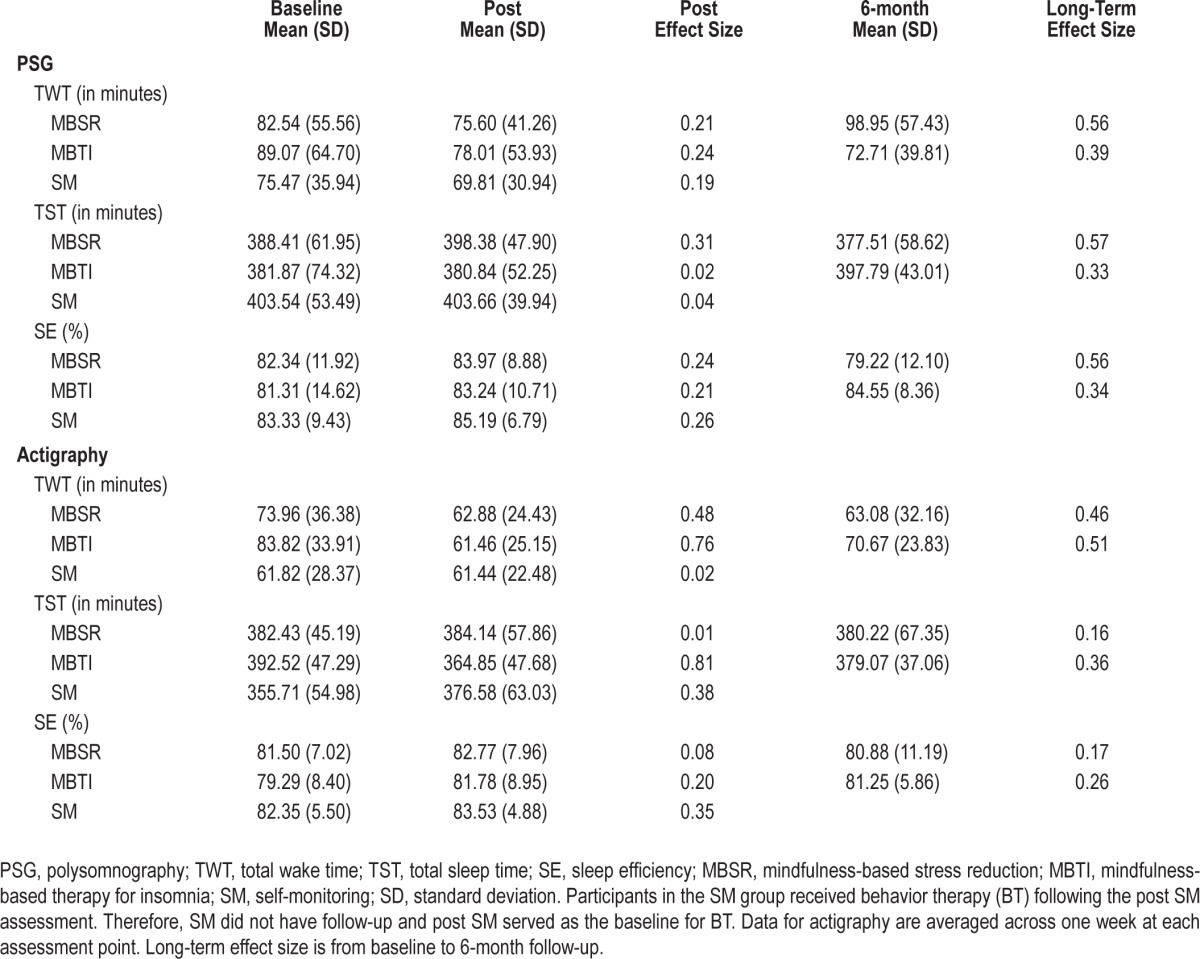
Means and standard deviations for TWT, PSAS, and ISI at baseline, post, and follow-up are presented in Table 2 with change scores from baseline shown in Figure 2. The post LMM on patient-reported TWT comparing the average effects of the meditation arms to SM revealed a significant interaction (P = 0.004), such that the meditation arms showed significantly greater rates of reduction in TWT (5.27 min per treatment week) relative to SM (between-group Cohen d = 0.67). Across all treatment weeks, the meditation arms had an average of 43.75 fewer min of TWT at post relative to baseline while the SM arm had only 1.09 fewer minutes of TWT at post relative to baseline (see Figure 2A). A significant main effect for time was also found (P = 0.01) on the post LMM. Post hoc comparisons were conducted for each intervention arm compared to SM on TWT. MBSR showed significantly greater reduction (4.30 min per treatment week) relative to SM (P < 0.05). MBTI also showed significantly greater reduction (6.00 min per treatment week) relative to SM (P < 0.01).
Table 2.
Patient-reported outcomes
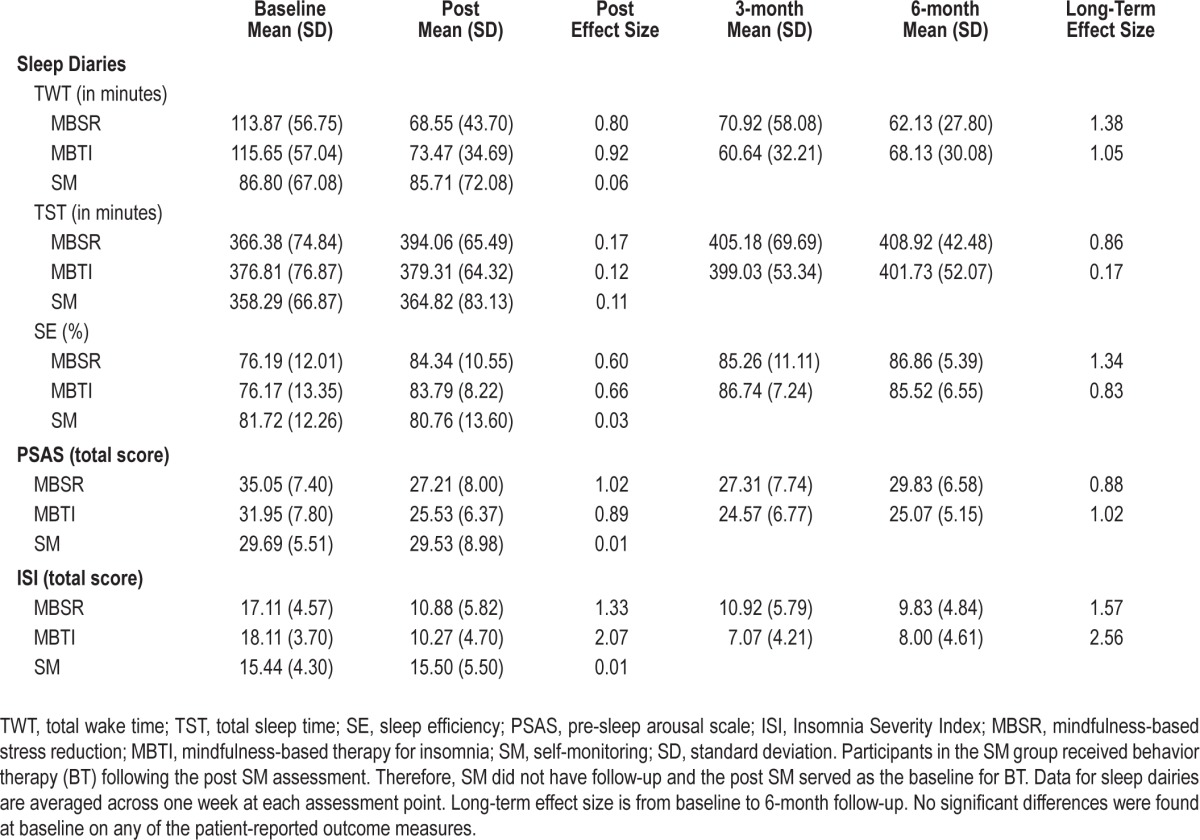
Figure 2.
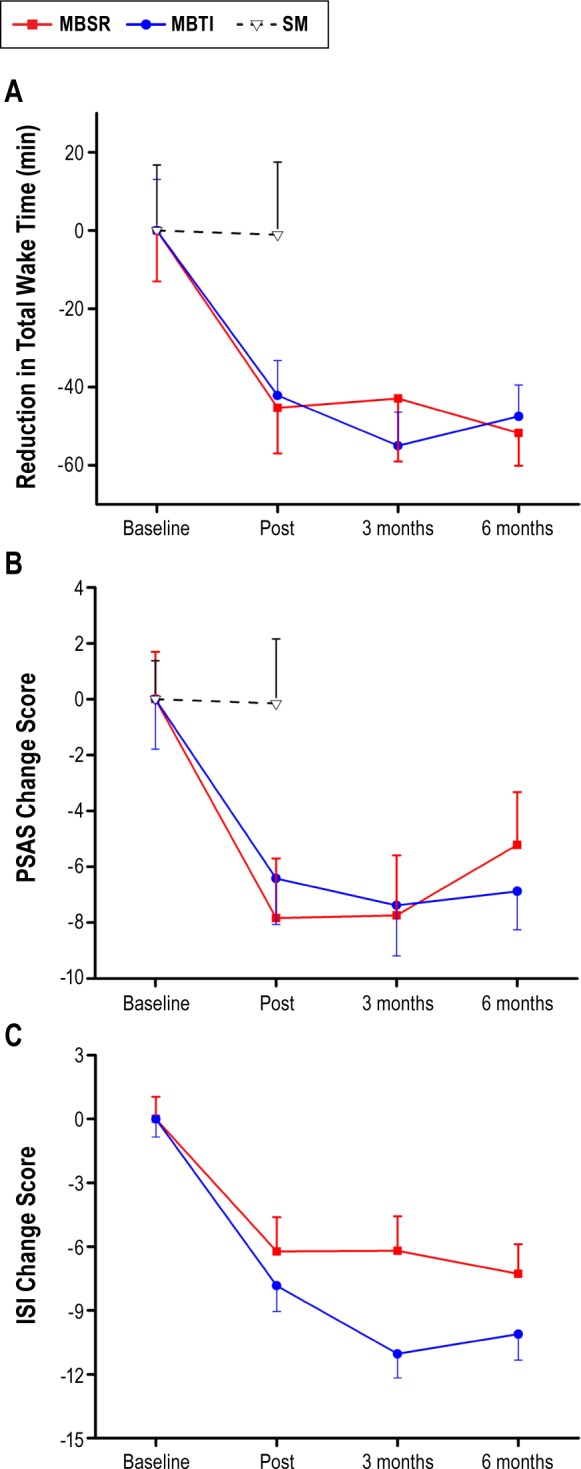
Patient-reported outcomes. (A) Total wake time (in minutes; with standard error of the mean) across study arms as reported on sleep diaries. Data presented are change scores from baseline to each assessment point. Raw data (mean, standard deviation, effect sizes) for each arm are reported in Table 2. (B) Pre-sleep arousal scale (PSAS) total scores (with standard error of the mean) across study arms. Data presented are change scores from baseline to each assessment point. Raw data (mean, standard deviation, effect sizes) for each arm are reported in Table 2. (C) Insomnia Severity Index (ISI) total scores (with standard error of the mean) across the MBSR and MBTI arms. Data presented are change scores from baseline to each assessment point. Raw data (mean, standard deviation, effect sizes) for each arm are reported in Table 2. MBSR, mindfulness-based stress reduction; MBTI, mindfulness-based therapy for insomnia; SM, self-monitoring.
On the long-term LMM for TWT, a significant main effect for time was found (P < 0.001), with participants in the meditation arms reporting an average reduction of 49.63 min in TWT from baseline to the 6-month follow-up. No significant interactions were found on the long-term LMM.
PSAS
For treatment effects on sleep-related arousal (PSAS), the post LMM comparing the average effects of the 2 meditation arms relative to SM revealed a significant interaction (P = 0.002), such that the meditation arms showed significantly greater rates of reduction on PSAS than SM (between-group Cohen d = 0.58). The meditation arms had an average reduction in PSAS total score of 7.13 points from baseline to post compared to a 0.16 point decrease in SM (see Figure 2B). There was also a significant main effect of time (P < 0.001). Post hoc comparisons were conducted for each intervention arm compared to SM. MBSR showed significantly greater reduction in PSAS relative to SM (P < 0.01), and MBTI also showed significantly greater reduction in PSAS relative to SM (P < 0.01). No significant differences were found on the long-term LMM for PSAS, but the long-term pattern indicates that MBTI maintains a relatively stable reduction in PSAS throughout follow-up (between-group d = 0.75).
ISI
For treatment effects on the ISI, the post LMM comparing the average effects of the 2 meditation arms to SM revealed a significant interaction (P < 0.0001), such that the meditation arms showed significantly greater rates of reduction on the ISI compared to SM. The meditation arms had an average reduction in ISI score of 5.03 from baseline to post, and the SM arm had an average increase in ISI score of 0.06 (see Figure 2C). Post hoc comparisons were conducted for each intervention arm compared to SM. MBSR had an average reduction in ISI score of 4.56 points, which was significantly greater than SM (P < 0.05). MBTI had an average reduction in ISI score of 5.41 points, which was significantly greater than SM (P < 0.01). The long-term LMM comparing ISI scores between the 2 meditation arms (MBSR vs MBTI) revealed a significant interaction (P < 0.05). MBTI had significantly greater rates of reduction on ISI scores compared to MBSR from baseline to 6-month follow-up. Specifically, the MBTI arm had significantly lower scores compared to the MBSR arm at the 3-month follow-up (P < 0.05), but the difference was not significant at the 6-month follow-up (P = 0.16).
Remission and Response
Logistic regression analyses on remission and response status were conducted to compare the 2 meditation arms (MBSR vs. MBTI) at post, 3-month follow-up, and 6-month follow-up. No significant differences in remission or response were found between MBSR and MBTI (Figures 3A and 3B). Remission rates in the MBSR group were largely stable over time, (46.2% at post, 38.5% at 3 months, and 41.7% at 6 months.) Remission rates for MBTI increased steadily from 33.3% at post to 42.9% at 3-month follow-up and 50% at 6-month follow-up. Similarly, treatment response remained relatively steady between post and follow-up in MBSR (38.5% and 41.7%) but showed a steady increase from post (60%), 3-month (71.4%), and 6-month follow-up (78.6%) in MBTI.
Figure 3.
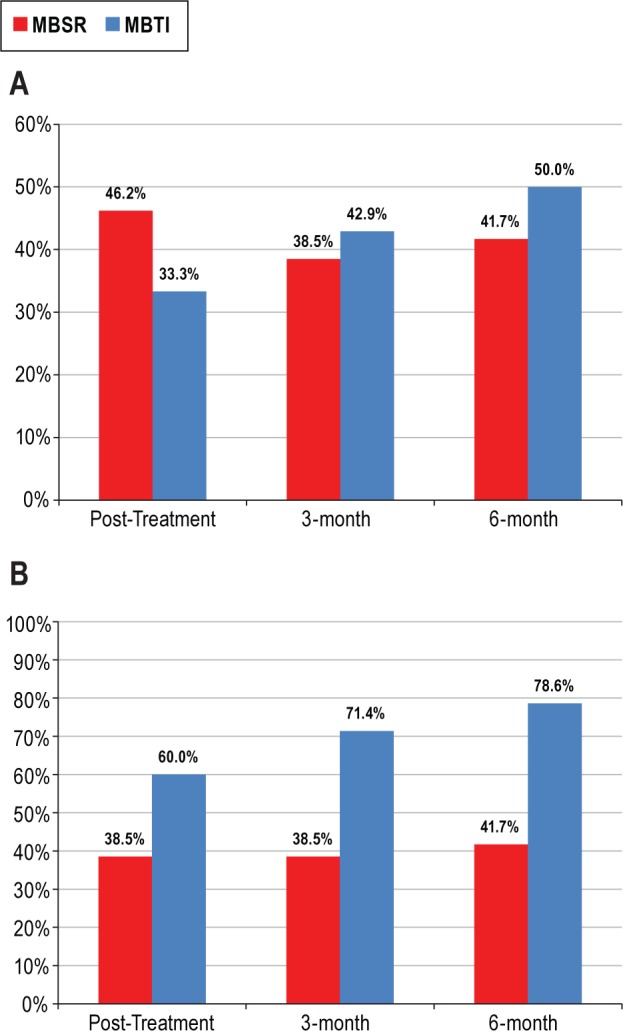
Treatment remission and treatment response. (A) Treatment remission. Percentage of patients who met criteria for treatment remission defined as Insomnia Severity Index (ISI) total score < 8 at each assessment point. Treatment remission for self-monitoring (SM) at post was 6.3% (not pictured). (B) Treatment response. Percentage of patients who met criteria for a minimally important treatment response defined as ISI total score reduction from baseline > 7 points at each assessment point. Treatment response at post for SM was 0.0% (not pictured). MBSR, mindfulness-based stress reduction; MBTI, mindfulness-based therapy for insomnia.
Objective Sleep Outcomes
Post treatment LMM analyses conducted on objective sleep parameters using PSG and actigraphy revealed significant findings only for 2 actigraphy-measured variables (TWT and TST) and no significant findings for PSG-based sleep parameters. First, the post LMM on actigraphy-measured TWT comparing the average effects of the 2 meditation arms to SM revealed a significant interaction (P < 0.05), such that the meditation arms showed significantly greater rates of reduction in TWT (17.97 min) relative to SM. However, no significant differences between the groups were found at post (P = 0.92). Second, the post LMM on actigraphy-measured TST comparing the meditation arms to SM revealed a significant interaction (P < 0.01). Again, no significant differences between the groups were found at post (P = 0.76). See Table 3 for means and standard deviations on objective sleep outcomes.
Table 3.
Objective measures of sleep
Treatment Integrity and Safety Monitoring
The average attendance for all randomized participants was not significantly different between MBSR (mean = 4.95, SD = 2.93) and MBTI (mean = 5.74, SD = 2.45). Three participants dropped out prior to receiving MBSR, and one participant dropped out prior to receiving MBTI. Twelve of the 16 participants who received MBSR attended ≥ 6 sessions (mean = 5.88, SD = 2.13), and 11 of the 18 participants who received MBTI attended ≥ 6 sessions (mean = 6.06, SD = 2.07). The majority of absences were due to scheduling conflicts or unexpected emergencies; whenever possible, instructors would review the content of the missed session and homework assignments over the phone at an alternate time. If the participant failed to attend without prior notice, the instructor would attempt to contact the participant and review the session materials and homework assignments to keep the participant engaged and maintain program continuity.
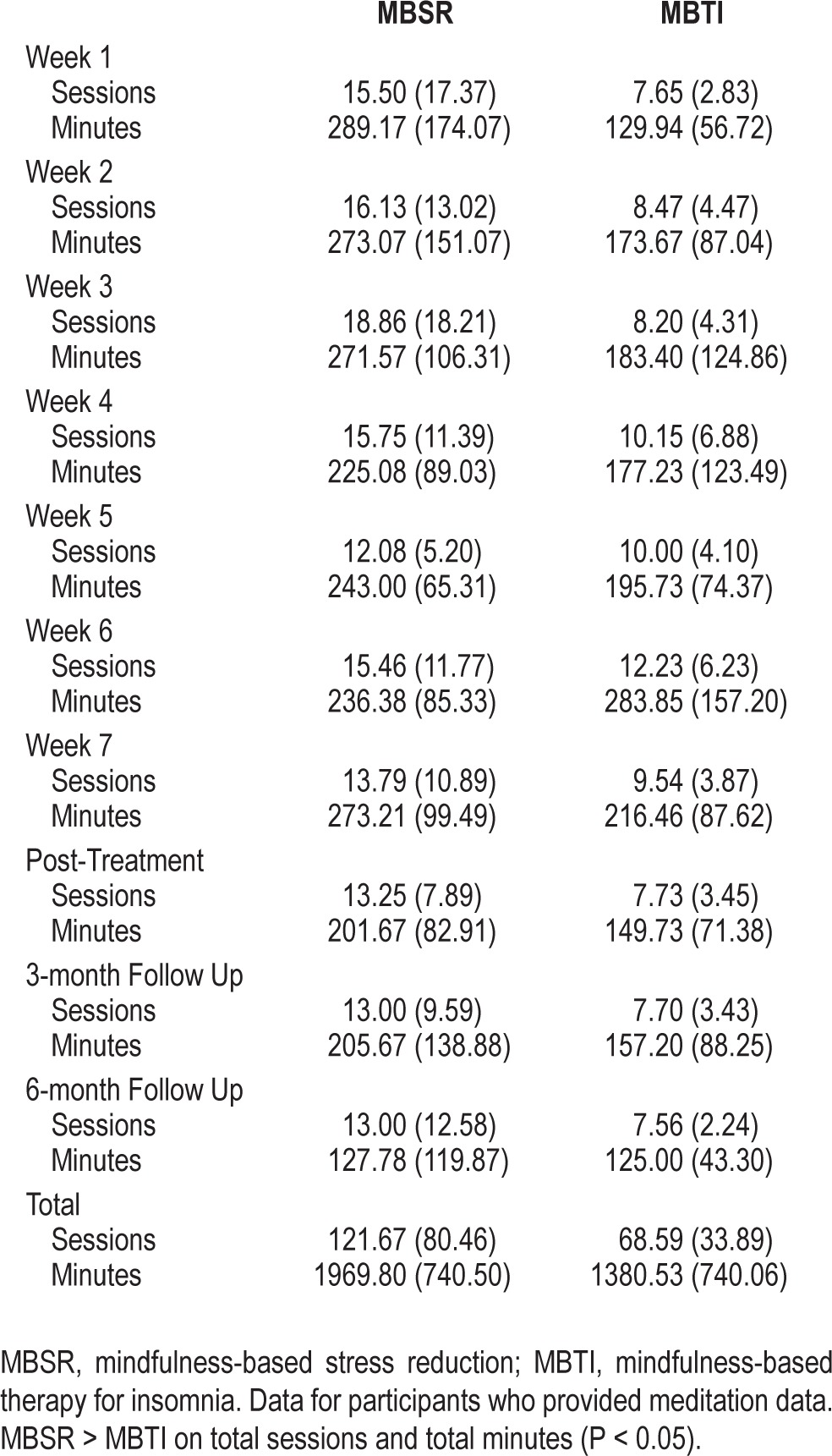
A treatment credibility and expectancy questionnaire55 was completed by participants before session 3 commenced. No significant differences were found between treatment conditions on credibility (logicalness, success in reducing symptoms, or confidence in recommending to friend) or expectancy (degree of expected improvement from treatment). Data from meditation diaries were used to monitor the extent of daily practice of mindfulness meditations during MBSR and MBTI (Table 4). Overall, participants in MBSR averaged 1,969.80 min of meditation practice during the study period, and participants in MBTI averaged 1,380.53 min of meditation practice. MBSR participants reported significantly more home meditation sessions (P < 0.05) and more total minutes of home meditation practice (P < 0.05) than participants in MBTI. No treatment-related adverse events were reported.
Table 4.
Weekly meditation diaries
DISCUSSION
The aim of this study was to evaluate the efficacy of mindfulness meditation as a treatment for chronic insomnia in a randomized controlled trial. Overall, these findings provide important new evidence for the efficacy, credibility, and safety of meditation-based therapies. First, the findings revealed evidence of treatment efficacy for meditation-based treatments to reduce patient-reported TWT in bed and sleep-related arousal along with clinically significant changes in treatment response and remission. Participants who received either MBSR or MBTI reported a mean reduction in TWT from baseline to post-treatment of 43.75 minutes and from baseline to the 6-month follow-up of 49.63 minutes, corresponding to large within-group effect sizes (Cohen's d > 0.8). These improvements in sleep are significantly larger than the SM control, indicating the results are not due to the effects of completing sleep diaries. Moreover, the effect sizes are generally similar to effect sizes reported in behavioral clinical trials on chronic insomnia,11,14,56 providing preliminary support that meditation-based treatments are viable non-pharmacological treatments for adults with insomnia. The observed magnitude of change in TWT is similar to two previous randomized studies using mindfulness-based cognitive therapy57,58 and greater than one study using MBSR.24
The findings on patient-reported pre-sleep arousal provide support for the treatment effects of mindfulness meditation to reduce psychophysiological arousal, a common waking correlate of chronic insomnia disorders.34,35,59 The large within-group effects for both MBSR and MBTI compared to the small effects for SM are consistent with the hypothesized conceptual mechanisms,32 indicating that mindfulness meditation decreases sleep-related arousal at a rate superior to self-monitoring. Given that insomnia disorders are defined by nocturnal sleep disturbance and waking dysfunction, the present findings demonstrate the efficacy of mindfulness meditation to improve both nocturnal symptoms and waking distress in those patients with elevated sleep-related arousal. No significant differences were found between MBSR and MBTI on TWT or PSAS, but the long-term pattern appears to favor the stability of MBTI for reducing sleep-related arousal.
MBTI showed significantly greater reduction in insomnia severity, as measured by the ISI, compared to MBSR across the entire study period. The difference between MBTI and MBSR was largest at the 3-month follow-up, such that participants who received MBTI reported significantly less insomnia symptom severity compared to MBSR three months after treatment ended. This finding is consistent with the hypothesis that a tailored mindfulness meditation program that integrates behavioral strategies for sleep has favorable long-term benefits relative to the standard mindfulness meditation program without behavioral components. The rates for remission and response over the follow-up study period were generally positive for both groups, with no significant differences between MBTI and MBSR on these clinical endpoints. Sixty percent of participants who completed MBTI had a minimally important treatment response at post-treatment, with the rates rising to 78.6% at the 6-month follow-up. In contrast, the rate of response for MBSR was relatively stable between post-treatment to 6-month follow-up (38.5% and 41.7%). The rates of treatment remission were not significantly different between the groups, with MBSR and MBTI showing similar rates of remission between 38% and 50% at the 3-month and 6-month follow-ups. Although the remission and response rates should be interpreted with caution given the small sample size, it appears that meditation-based treatments show positive long-term benefits on key clinical endpoints. Finally, patients considered both MBTI and MBSR as credible treatments for insomnia and were willing to practice meditation, with those in MBSR reporting almost 2,000 minutes of mindfulness meditation practice and those in MBTI reporting almost 1,400 minutes of practice during the study period. No treatment-related adverse events were reported.
These encouraging findings should be interpreted with consideration to the limitations of this study. First, the study included a small sample size consisting primarily of Caucasian females. Furthermore, participants had to report elevated sleep-related arousal, a criterion that is not commonly used in studies on the efficacy of treatments for insomnia. As a result, these findings might not generalize to all patients with chronic insomnia. Notably, the prevalence of insomnia is higher among women,60 and highly educated, Caucasian women are known to be the primary users of CAM in the United States.16 Thus, it might be the case that meditation-based treatments match the ideology and lifestyle of this demographic group and could be particularly suitable for women with insomnia who have elevated arousal levels. Further research with larger and more diverse samples is needed to examine these characteristics as potential moderators of treatment outcome to identify who is likely to seek out and benefit from meditation-based interventions. The study also had a limited set of providers, including a study investigator who only delivered treatment in one arm. Although the intention was to select the best available therapist for each treatment arm, we cannot rule out investigator bias, treatment allegiance, or therapist skill as plausible explanations for the observed differences between treatment arms. The SM condition also did not control for treatment expectations, therapist contact, and other nonspecific factors that might have been present and could have accounted for the differences observed between the active treatments and the SM. In addition, this study was not sufficiently powered to detect treatment effects on objective measures of sleep and no formal inter-rater reliability was conducted for the PSG scoring. The meditation groups showed a reduction on actigraphy-measured TWT and TST from baseline to post, but the difference between the meditation groups and SM was not significant at post. Two previous studies on meditation-based treatments found only small effect sizes on objective measures of sleep.24,57 It appears that mindfulness meditation has larger effects on patient-reported sleep outcomes relative to objectively measured sleep outcomes. Finally, blinding was not employed in this protocol so differential expectations between SM and the meditation treatments could inflate the observed differences between SM and the active treatments.
In summary, these findings indicate that interventions featuring mindfulness meditation have positive patient-reported benefits and could be a viable treatment option for chronic insomnia. Additional research is needed to determine the position of meditation-based therapies among treatment options. This study was designed as a small-scale, early-stage randomized controlled trial.54 As such, the mindfulness meditation arms were not compared against a standard treatment such as CBTI and it remains possible that a proportion of the variance observed for the benefits of MBTI were accounted for by the inclusion of sleep restriction and stimulus control instructions. Future research should consider large-scale studies using comparative effectiveness designs that directly compare MBSR or MBTI with CBTI. For example, Garland and colleagues61 found that MBSR was non-inferior to CBTI on the ISI at the 3-month follow-up for patients with insomnia comorbid with cancer. In addition, future research should examine variables that could inform patient decisions related to different nonpharmacological approaches to insomnia. For example, meditation-based therapies might appeal to those seeking to learn stress management techniques outside of the traditional health care system, while CBTI might appeal to those more comfortable with traditional psychotherapy. These future directions provide considerations for further testing and potential implementation of meditation-based therapies for insomnia.
DISCLOSURE STATEMENT
This was not an industry supported study. This research project was supported by a grant from the National Institutes of Health, National Center for Complementary and Alternative Medicine, awarded to Dr. Ong (K23AT003678). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The sponsor had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication. Portions of the findings in this paper were presented at the 2013 and 2014 Annual Meetings of the Associated Professional Sleep Societies and the 2013 meeting of the Association for Behavior and Cognitive Therapies. Dr. Manber serves on the advisory boards for General Sleep and Sleep Rate. This activity is unrelated to the current manuscript. The other authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Dr. Ong had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Ong, Manber, Segal, Shapiro, Wyatt; Acquisition of data: Ong, Wyatt; Analysis and interpretation of data: Ong, Manber, Xia, Wyatt; Drafting of the manuscript: Ong, Manber, Wyatt; Critical revision of the manuscript for important intellectual content: Ong, Manber, Segal, Xia, Shapiro, Wyatt; Statistical analysis: Xia; Obtained funding: Ong, Manber; Study supervision: Ong, Wyatt.
The authors thank M. Isabel Crisostomo, MD, for her role as medical advisor on the DSMB. We thank David Sholtes and Christina Khou for their contributions as study coordinators on this project. We thank Arthur Hoffman, MD, and Vered Hankin, PhD, for serving as MBSR teachers and Jamie Cvengros, PhD, Megan Hood, PhD, Jamie Jackson, PhD, Heather Gunn, PhD, and Lisa Hantsoo, PhD, for serving as study therapists. Finally, we thank all of the participants in our study for their time, energy, and effort in helping us conduct this study.
SUPPLEMENTAL MATERIAL
CONSORT 2010 checklist of information to include when reporting a randomized trial*
REFERENCES
- 1.Ohayon MM. Epidemiology of insomnia: What we know and what we still need to learn. Sleep Med Rev. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- 2.Morin CM, LeBlanc M, Belanger L, Ivers H, Merette C, Savard J. Prevalence of insomnia and its treatment in canada. Can J Psychiatry. 2011;56:540–8. doi: 10.1177/070674371105600905. [DOI] [PubMed] [Google Scholar]
- 3.Roth T, Coulouvrat C, Hajak G, et al. Prevalence and perceived health associated with insomnia based on DSM-IV-TR; international statistical classification of diseases and related health problems, tenth revision; and research diagnostic criteria/international classification of sleep disorders, second edition criteria: results from the America insomnia survey. Biol Psychiatry. 2011;69:592–600. doi: 10.1016/j.biopsych.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 4.Morgenthaler T, Kramer M, Alessi C, et al. Practice parameters for the psychological and behavioral treatment of insomnia: an update. an American Academy of Sleep Medicine report. Sleep. 2006;29:1415–9. [PubMed] [Google Scholar]
- 5.Buysse DJ. Insomnia. JAMA. 2013;309:706–16. doi: 10.1001/jama.2013.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holbrook AM, Crowther R, Lotter A, Cheng C, King D. Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ. 2000;162:225–33. [PMC free article] [PubMed] [Google Scholar]
- 7.Randall S, Roehrs TA, Roth T. Efficacy of eight months of nightly zolpidem: A prospective placebo-controlled study. Sleep. 2012;35:1551–7. doi: 10.5665/sleep.2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ancoli-Israel S, Krystal AD, McCall WV, et al. A 12-week, randomized, double-blind, placebo-controlled study evaluating the effect of eszopiclone 2 mg on sleep/wake function in older adults with primary and comorbid insomnia. Sleep. 2010;33:225–34. doi: 10.1093/sleep/33.2.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morin CM, Gaulier B, Barry T, Kowatch RA. Patients' acceptance of psychological and pharmacological therapies for insomnia. Sleep. 1992;15:302–5. doi: 10.1093/sleep/15.4.302. [DOI] [PubMed] [Google Scholar]
- 10.Vincent N, Lionberg C. Treatment preference and patient satisfaction in chronic insomnia. Sleep. 2001;24:411–17. doi: 10.1093/sleep/24.4.411. [DOI] [PubMed] [Google Scholar]
- 11.Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: A randomized controlled trial. JAMA. 2001;285:1856–64. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- 12.Morin CM, Colecchi C, Stone J, Sood R, Brink D. Behavioral and pharmacological therapies for late-life insomnia: a randomized controlled trial. JAMA. 1999;281:991–9. doi: 10.1001/jama.281.11.991. [DOI] [PubMed] [Google Scholar]
- 13.Epstein DR, Sidani S, Bootzin RR, Belyea MJ. Dismantling multicomponent behavioral treatment for insomnia in older adults: a randomized controlled trial. Sleep. 2012;35:797. doi: 10.5665/sleep.1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buysse DJ, Germain A, Moul DE, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171:887–95. doi: 10.1001/archinternmed.2010.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Morin CM, Vallieres A, Guay B, et al. Cognitive behavioral therapy, singly and combined with medication, for persistent insomnia: A randomized controlled trial. JAMA. 2009;301:2005–15. doi: 10.1001/jama.2009.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pearson NJ, Johnson LL, Nahin RL. Insomnia, trouble sleeping, and complementary and alternative medicine: analysis of the 2002 national health interview survey data. Arch Intern Med. 2006;166:1775–82. doi: 10.1001/archinte.166.16.1775. [DOI] [PubMed] [Google Scholar]
- 17.Bertisch SM, Wells RE, Smith MT, McCarthy EP. Use of relaxation techniques and complementary and alternative medicine by American adults with insomnia symptoms: results from a national survey. J Clin Sleep Med. 2012;8:681–91. doi: 10.5664/jcsm.2264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ludwig DS, Kabat-Zinn J. Mindfulness in medicine. JAMA. 2008;300:1350–2. doi: 10.1001/jama.300.11.1350. [DOI] [PubMed] [Google Scholar]
- 19.Kabat-Zinn J. An outpatient program in behavioral medicine for chronic pain patients based on the practice of mindfulness meditation: Theoretical considerations and preliminary results. Gen Hosp Psychiatry. 1982;4:33–47. doi: 10.1016/0163-8343(82)90026-3. [DOI] [PubMed] [Google Scholar]
- 20.Birnie K, Garland SN, Carlson LE. Psychological benefits for cancer patients and their partners participating in mindfulness-based stress reduction (MBSR) Psychooncology. 2010;19:1004–9. doi: 10.1002/pon.1651. [DOI] [PubMed] [Google Scholar]
- 21.Morone NE, Greco CM, Weiner DK. Mindfulness meditation for the treatment of chronic low back pain in older adults: A randomized controlled pilot study. Pain. 2008;134:310–19. doi: 10.1016/j.pain.2007.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoge EA, Bui E, Marques L, et al. Randomized controlled trial of mindfulness meditation for generalized anxiety disorder: Effects on anxiety and stress reactivity. J Clin Psychiatry. 2013;74:786–92. doi: 10.4088/JCP.12m08083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kabat-Zinn J, Wheeler E, Light T, et al. Influence of a mindfulness meditation-based stress reduction intervention on rates of skin clearing in patients with moderate to severe psoriasis undergoing phototherapy (UVB) and photochemotherapy (PUVA) Psychosom Med. 1998;60:625–32. doi: 10.1097/00006842-199809000-00020. [DOI] [PubMed] [Google Scholar]
- 24.Gross CR, Kreitzer MJ, Reilly-Spong M, et al. Mindfulness-based stress reduction versus pharmacotherapy for chronic primary insomnia: a randomized controlled clinical trial. Explore (NY) 2011;7:76–87. doi: 10.1016/j.explore.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shapiro SL, Bootzin RR, Figueredo AJ, Lopez AM, Schwartz GE. The efficacy of mindfulness-based stress reduction in the treatment of sleep disturbance in women with breast cancer: an exploratory study. J Psychosom Res. 2003:85–91. doi: 10.1016/s0022-3999(02)00546-9. [DOI] [PubMed] [Google Scholar]
- 26.Teasdale JD, Segal ZV, Williams JM, Ridgeway VA, Soulsby JM, Lau MA. Prevention of relapse/recurrence in major depression by mindfulness-based cognitive therapy. J Consult Clin Psychol. 2000;68:615–23. doi: 10.1037//0022-006x.68.4.615. [DOI] [PubMed] [Google Scholar]
- 27.Kristeller JL, Wolever RQ. Mindfulness-based eating awareness training for treating binge eating disorder: the conceptual foundation. Eat Disord. 2011;19:49–61. doi: 10.1080/10640266.2011.533605. [DOI] [PubMed] [Google Scholar]
- 28.Bowen S, Chawla N, Collins SE, et al. Mindfulness-based relapse prevention for substance use disorders: a pilot efficacy trial. Subst Abus. 2009;30:295–305. doi: 10.1080/08897070903250084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther. 2008;39:171–82. doi: 10.1016/j.beth.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore (NY) 2009;5:30–6. doi: 10.1016/j.explore.2008.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ong J, Sholtes D. A mindfulness-based approach to the treatment of insomnia. J Clin Psychol. 2010;66:1175–84. doi: 10.1002/jclp.20736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ong JC, Ulmer CS, Manber R. Improving sleep with mindfulness and acceptance: A metacognitive model of insomnia. Behav Res Ther. 2012;50:651–60. doi: 10.1016/j.brat.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spiegelhalder K, Regen W, Feige B, et al. Sleep-related arousal versus general cognitive arousal in primary insomnia. J Clin Sleep Med. 2012;8:431. doi: 10.5664/jcsm.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bonnet MH, Arand DL. Hyperarousal and insomnia: state of the science. Sleep Med Rev. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 35.Buysse DJ, Ancoli-Israel S, Edinger JD, Lichstein KL, Morin CM. Recommendations for a standard research assessment of insomnia. Sleep. 2006;29:1155–73. doi: 10.1093/sleep/29.9.1155. [DOI] [PubMed] [Google Scholar]
- 36.Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: Psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34:601–8. doi: 10.1093/sleep/34.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine work group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 38.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 39.First MB, Spitzer RL, Gibbon M, Williams JB. New York: Biometrics Research, New York State Psychiatric Institute; 2002. Structured clinical interview for DSM-IV-TR axis I disorders, research version. Non-patient ed. [Google Scholar]
- 40.Edinger JD, Wyatt JK, Stepanski EJ, et al. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses: results of a multitrait-multimethod analysis. Arch Gen Psychiatry. 2011;68:992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- 41.Espie CA, Inglis SJ, Tessier S, Harvey L. The clinical effectiveness of cognitive behaviour therapy for chronic insomnia: implementation and evaluation of a sleep clinic in general medical practice. Behav Res Ther. 2001;39:45–60. doi: 10.1016/s0005-7967(99)00157-6. [DOI] [PubMed] [Google Scholar]
- 42.Espie CA, Kyle SD, Williams C, et al. A randomized, placebo-controlled trial of online cognitive behavioral therapy for chronic insomnia disorder delivered via an automated media-rich web application. Sleep. 2012;35:769–81. doi: 10.5665/sleep.1872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kabat-Zinn J. New York: Delacorte Press; 1990. Full catastrophe living: using the wisdom of your body and mind to face stress, pain, and illness. [Google Scholar]
- 44.Spielman AJ, Saskin P, Thorpy MJ. Treatment of chronic insomnia by restriction of time in bed. Sleep. 1987;10:45–56. [PubMed] [Google Scholar]
- 45.Bootzin RR. Stimulus control treatment for insomnia. Proc Am Psychol Assoc. 1972:395–6. [Google Scholar]
- 46.Hauri PJ. Current Concepts: The Sleep Disorders. Kalamazoo, MI: The Upjohn Company; 1977. [Google Scholar]
- 47.Nicassio PM, Mendlowitz DR, Fussell JJ, Petras L. The phenomenology of the pre-sleep state: the development of the pre-sleep arousal scale. Behav Res Ther. 1985;23:263–71. doi: 10.1016/0005-7967(85)90004-x. [DOI] [PubMed] [Google Scholar]
- 48.Hantsoo L, Khou CS, White CN, Ong JC. Gender and cognitive-emotional factors as predictors of pre-sleep arousal and trait hyperarousal in insomnia. J Psychosom Res. 2013;74:283–9. doi: 10.1016/j.jpsychores.2013.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 50.Carskadon MA, Rechtschaffen A. Monitoring and staging human sleep. In: Kryger M, Roth T, Dement W, editors. Principles and practice of sleep medicine. 4th ed. Philadelphia, PA: Elsevier; 2005. pp. 1359–77. [Google Scholar]
- 51.Iber C, Ancoli-Israel S, Chesson A, Quan S. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. [Google Scholar]
- 52.Ancoli-Israel S, Cole R, Alessi C, Chambers M, Moorcroft W, Pollak CP. The role of actigraphy in the study of sleep and circadian rhythms. Sleep. 2003;26:342–92. doi: 10.1093/sleep/26.3.342. [DOI] [PubMed] [Google Scholar]
- 53.Hyde M, O'Driscoll DM, Binette S, et al. Validation of actigraphy for determining sleep and wake in children with sleep disordered breathing. J Sleep Res. 2007;16:213–6. doi: 10.1111/j.1365-2869.2007.00588.x. [DOI] [PubMed] [Google Scholar]
- 54.Rounsaville BJ, Carroll KM, Onken LS. A stage model of behavioral therapies research: Getting started and moving on from stage I. Clin Psychol. 2001;8:133–42. [Google Scholar]
- 55.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Behav Ther Exp Psychiatry. 2000;31:73–86. doi: 10.1016/s0005-7916(00)00012-4. [DOI] [PubMed] [Google Scholar]
- 56.Sivertsen B, Omvik S, Pallesen S, et al. Cognitive behavioral therapy vs zopiclone for treatment of chronic primary insomnia in older adults: a randomized controlled trial. JAMA. 2006;295:2851–8. doi: 10.1001/jama.295.24.2851. [DOI] [PubMed] [Google Scholar]
- 57.Britton WB, Haynes PL, Fridel KW, Bootzin RR. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med. 2010;72:539–48. doi: 10.1097/PSY.0b013e3181dc1bad. [DOI] [PubMed] [Google Scholar]
- 58.Britton WB, Haynes PL, Fridel KW, Bootzin RR. Mindfulness-based cognitive therapy improves polysomnographic and subjective sleep profiles in antidepressant users with sleep complaints. Psychother Psychosom. 2012;81:296–304. doi: 10.1159/000332755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Riemann D, Spiegelhalder K, Feige B, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14:19–31. doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 60.Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29:85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
- 61.Garland SN, Carlson LE, Stephens AJ, Antle MC, Samuels C, Campbell TS. Mindfulness-based stress reduction compared with cognitive behavioral therapy for the treatment of insomnia comorbid with cancer: a randomized, partially blinded, noninferiority trial. J Clin Oncol. 2014;32:449–57. doi: 10.1200/JCO.2012.47.7265. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT 2010 checklist of information to include when reporting a randomized trial*