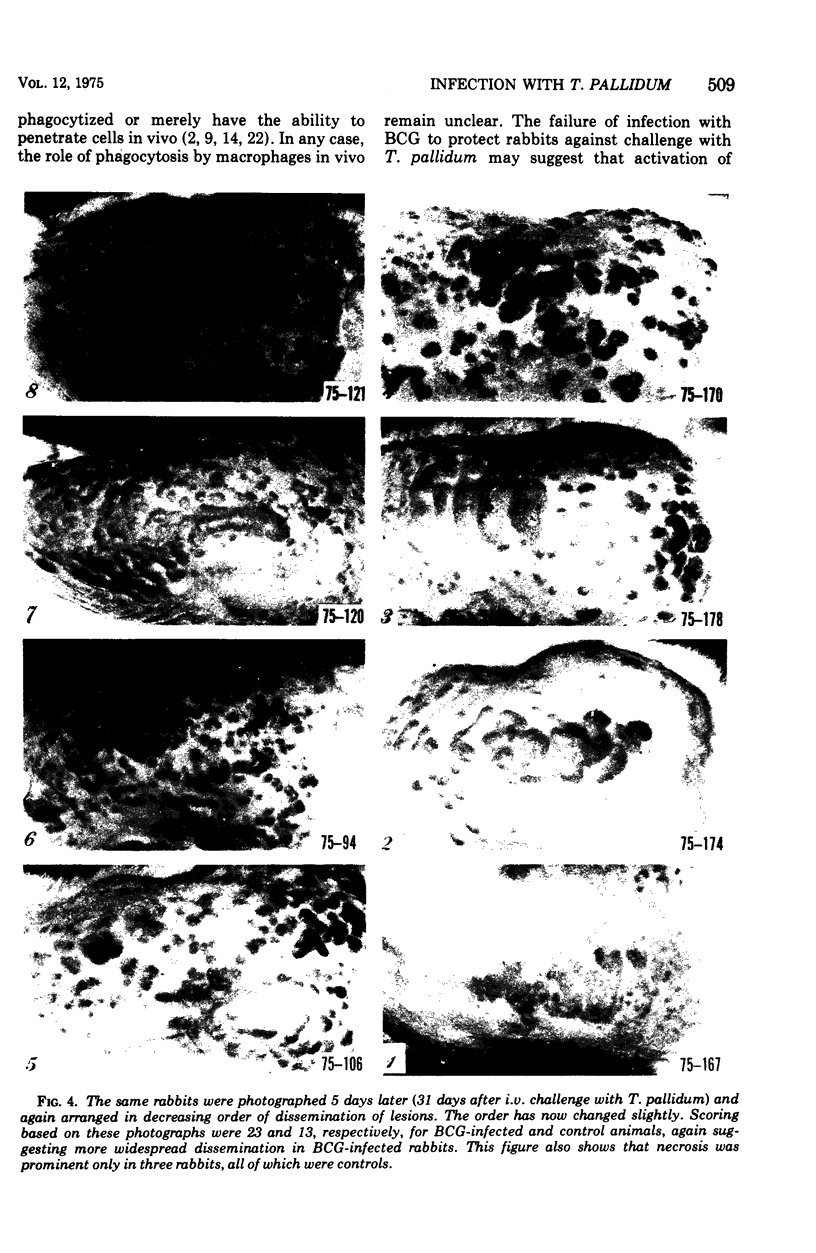
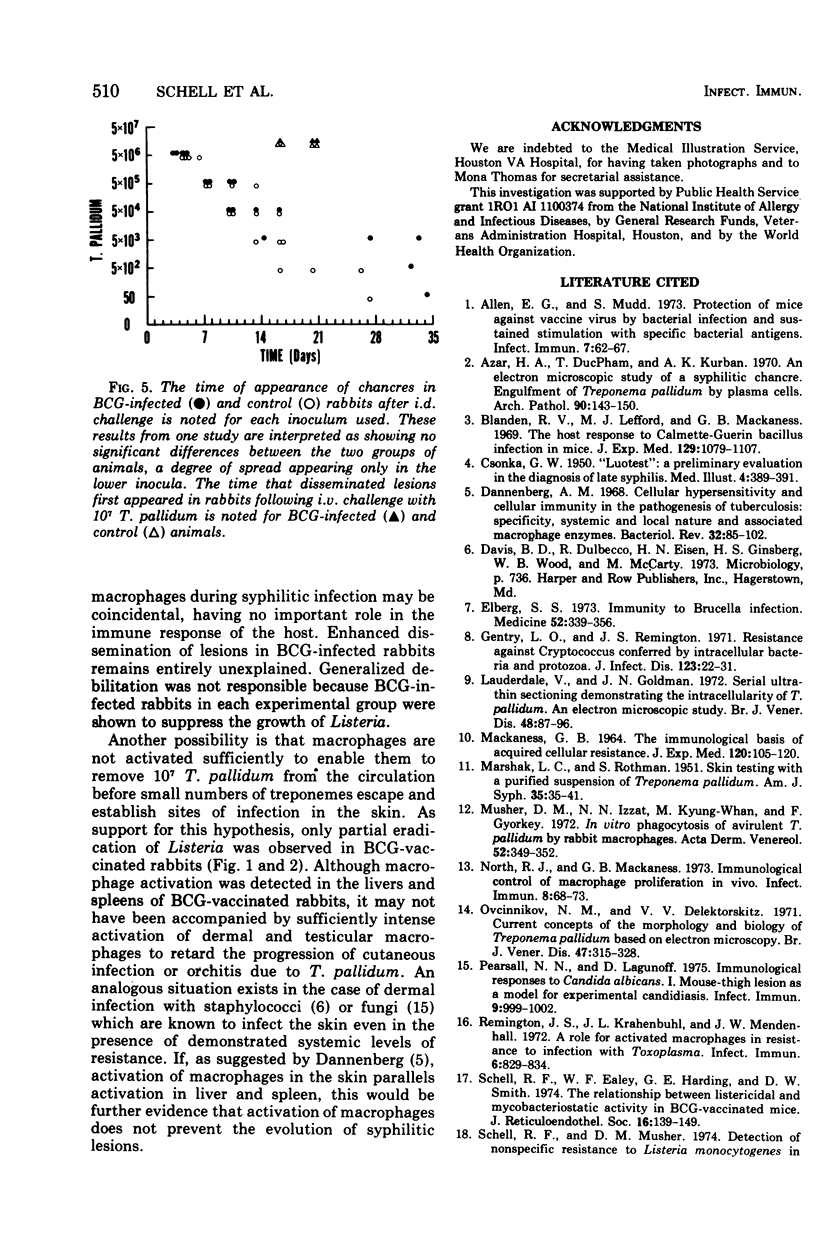
Abstract
Infection of rabbits with Treponema pallidum induces nonspecific acquired cellular resistance (ACR) to Listeria monocytogenes. This resistance can be adoptively transferred using thymus-dependent lymphocytes. Since infections that induce ACR are usually brought under control by cellular mechanisms, we sought to determine whether induction of ACR in rabbits stimulates resistance to challenge with T. pallidum. When BCG-infected rabbits which suppressed the growth of Listeria were challenged intravenously with T. pallidum, lesions appeared at the same time and progressed in a fashion similar to that in non-BCG-infected controls. There was a tendency for syphilitic lesions to disseminate more widely in BCG-infected animals and for the lesions to necrose more rapidly in controls. T. pallidum may resist phagocytosis by macrophages, as has been suggested previously, or macrophages may fail to be activated locally in the dermis. Although syphilitic infeciton appears to stimulate ACR, activation of the macrophages may not contribute significantly to the ability of the host to suppress T. pallidum.
Full text
PDF
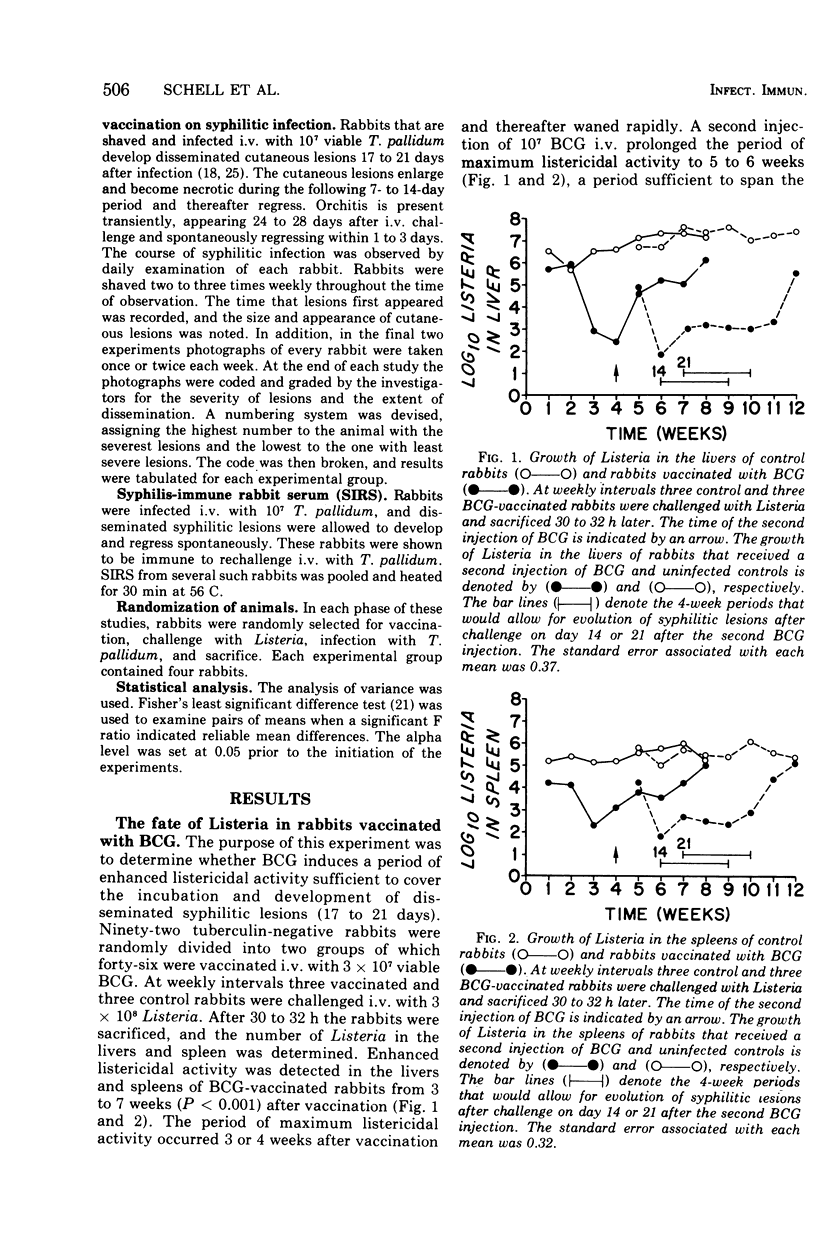

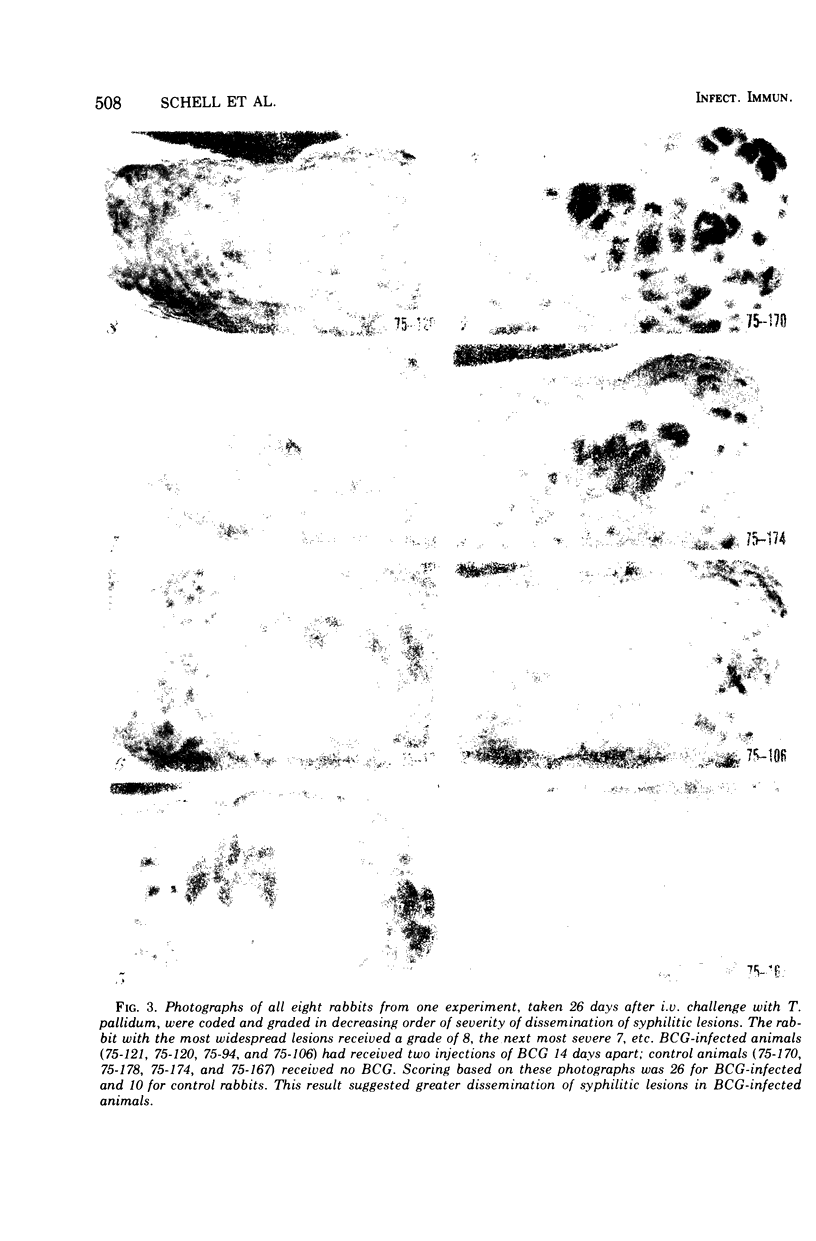

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen E. G., Mudd S. Protection of mice against vaccinia virus by bacterial infection and sustained stimulation with specific bacterial antigens. Infect Immun. 1973 Jan;7(1):62–67. doi: 10.1128/iai.7.1.62-67.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azar H. A., Pham T. D., Kurban A. K. An electron microscopic study of a syphilitic chancre. Engulfment of Treponema pallidum by plasma cells. Arch Pathol. 1970 Aug;90(2):143–150. [PubMed] [Google Scholar]
- Blanden R. V., Lefford M. J., Mackaness G. B. The host response to Calmette-Guérin bacillus infection in mice. J Exp Med. 1969 May 1;129(5):1079–1107. doi: 10.1084/jem.129.5.1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CSONKA G. W. 'Luotest' a preliminary evaluation in the diagnosis of late syphilis. Med Illus. 1950 Aug;4(8):389–391. [PubMed] [Google Scholar]
- Elberg S. S. Immunity to brucella infection. Medicine (Baltimore) 1973 Jul;52(4):339–356. doi: 10.1097/00005792-197307000-00013. [DOI] [PubMed] [Google Scholar]
- Gentry L. O., Remington J. S. Resistance against Cryptococcus conferred by intracellular bacteria and protozoa. J Infect Dis. 1971 Jan;123(1):22–31. doi: 10.1093/infdis/123.1.22. [DOI] [PubMed] [Google Scholar]
- Lauderdale V., Goldman J. N. Serial ultrathin sectioning demonstrating the intracellularity of T. Pallidum. An electron microscopic study. Br J Vener Dis. 1972 Apr;48(2):87–96. doi: 10.1136/sti.48.2.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musher D. M., Izzat N. N., Min K. W., Györkey F. In vitro phagocytosis of avirulent T. pallidum by rabbit macrophages. Acta Derm Venereol. 1972;52(5):349–352. [PubMed] [Google Scholar]
- North R. J., Mackaness G. B. Immunological control of macrophage proliferation in vivo. Infect Immun. 1973 Jul;8(1):68–73. doi: 10.1128/iai.8.1.68-73.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovcinnikov N. M., Delektorskij V. V. Current concepts of the morphology and biology of Treponema pallidum based on electron microscopy. Br J Vener Dis. 1971 Oct;47(5):315–328. doi: 10.1136/sti.47.5.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearsall N. N., Lagunoff D. Immunological responses to Candida albicans. I. Mouse-thigh lesion as a model for experimental candidiasis. Infect Immun. 1974 Jun;9(6):999–1002. doi: 10.1128/iai.9.6.999-1002.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remington J. S., Krahenbuhl J. L., Mendenhall J. W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972 Nov;6(5):829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schell R. F., Ealey W. F., Harding G. E., Smith D. W. The relationship between listericidal and mycobacteriostatic activity in BCG-vaccinated mice. J Reticuloendothel Soc. 1974 Sep;16(3):139–149. [PubMed] [Google Scholar]
- Schell R. F., Musher D. M. Transfer of nonspecific resistance to Listeria monocytogenes using spleen cells from syphilitic rabbits (38574). Proc Soc Exp Biol Med. 1975 Feb;148(2):516–518. doi: 10.3181/00379727-148-38574. [DOI] [PubMed] [Google Scholar]
- Schell R., Musher D., Jacobson K., Schwethelm P. Induction of acquired cellular resistance following transfer of thymus-dependent lymphocytes from syphilitic rabbits. J Immunol. 1975 Feb;114(2 Pt 1):550–553. [PubMed] [Google Scholar]
- Sykes J. A., Miller J. N. Intracellular location of Treponema pallidum (Nichols strain) in the rabbit testis. Infect Immun. 1971 Sep;4(3):307–314. doi: 10.1128/iai.4.3.307-314.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THIVOLET J., SIMERAY A., ROLLAND M., CHALLUT F. Etude de l'intradermoréaction aux suspensions de Tréponèmes formolées souche Nichols pathogène chez les syphilitiques et les sujets normaux. Ann Inst Pasteur (Paris) 1953 Jul;85(1):23–33. [PubMed] [Google Scholar]