Abstract
Extracellular ATP is a signaling molecule which plays an important role in alerting the immune system in case of any tissue damage. Recent studies show that binding of ATP to the ionotropic P2X7 receptor of inflammatory cells (macrophages and monocytes) will induce caspase 1 activation. Stimulation of caspase 1 activity results in maturation and release of IL-1β in the inflammasome in Chronic Obstructive Pulmonary Disease (COPD) patients. COPD is an inflammatory disease characterized by emphysema and/or chronic bronchitis and is mostly associated with cigarette smoking. It is one of the leading causes of death in humans and there is currently no medication to stop the progression of disease. A deeper understanding of the mechanism by which the P2X7 receptor triggers IL-1β maturation and release, may open new opportunities for the treatment of inflammatory diseases such as COPD.
Keywords: Pulmonary inflammation, Interleukin-1β, P2X7 receptor
INTRODUCTION
P2X7 is a ligand-gated acid sensing ion channel (ASIC) receptor with 240 amino acid C-terminal domains (1, 2). The receptor is involved in interaction with other proteins and forms part of a multi-protein signal complex (2). The P2X7 receptor is proposed as an important therapeutic target in inflammation, pain and shock (2, 3). Activation of the P2X7 receptor by ATP through down-stream intracellular signaling pathways (4) can regulate inflammation by triggering changes in cell morphology after agonist exposure (5–7). Thus, extracellular ATP can act on inflammatory tissue as a danger signal which alerts the immune system (4, 8, 9).
P2X7 receptor activation by ATP stimulates caspase-1 activity which, in turn, leads to the regulated release of the cytokine interleukin-1 (IL-1β) from phagocytes and macrophages (7, 10, 11). Studies proved that P2X7 receptors have a supportive pro-inflammatory role in immune mediated reactions. For example, triggering of P2X7 receptors by ATP abolishes the maturation and exteriorization of IL-1β proteins in macrophages (2, 8). This suggests that P2X7 receptors are a potential therapeutic target in many inflammatory diseases.
IL-1β is one of the major cytokines involved in the activation and persistency of inflammation (12). Different studies have shown that IL-1β expression is enhanced in patients with chronic obstructive pulmonary disease (COPD) and asthma (13). Furthermore, elevated levels of IL-1β in COPD are associated with exacerbation of the disease (14). Human bronchial epithelial cells produce more IL-1β in smokers with COPD compared to nonsmokers (13, 15). In this review we examine the evidence for the P2X7 receptor as a potential therapeutic target for pulmonary diseases like COPD and asthma.
ATP (Extracellular communication)
In addition to its role as a source of chemical energy in the body, the purine nucleotide adenosine 5'triphosphate (ATP) is an active membrane transporter, a neurotransmitter and an important signaling molecule (16). Recent studies (17) demonstrate that there are two types of receptor (P2X and P2Y) for extracellular ATP. Extracellular ATP induces a large variety of responses in the body, including activation of receptors known as P2 (purinergic membrane receptors) (18). Purinergic receptors are activated by ATP in different scales of time and distance. As a consequence, purinergic receptors are classified as ‘slow’ P2Y and ‘fast’ P2X receptors (19, 20).
P2Y receptors are G protein-coupled receptors and at least 15 members of this family exist (P2Y1-P2Y15). Because of the wide metabolic changes by these receptors they are commonly called metabotropic receptors (16). ATP is secreted in the CNS (basal nuclei) and PNS (dorsal root ganglion neurons) and P2Y responses are activated when ATP is released from the ganglion neurons. P2Y activation leads to intracellular Ca2 + release in juxtaposed Schwann Cells (17).
The P2X ion tropic receptors, also called ligand gated ion channels (channel linked-receptors), comprise seven members (P2X1-P2X7) (2). When the receptors are ligand-bound they change their shape which allows ions to pass through the channel. P2X receptors are located close to the site of neurotransmitter release and open instantly when ligand binds (16). Activation of P2XR leads to an increase in the intracellular calcium (Ca2 +) concentration and activates several intracellular processes (21).
P2X receptors
The P2X receptors are comprised of 379 to 595 amino acids and have two transmembrane segments. A total of 50% to 70% of the receptor is composed of an extracellular loop. The C-terminal of P2X7 has the largest extracellular loop of all the family members consisting of 240 amino acids. Because of the large C-terminal loop the receptor can interact with other proteins to form a multi-protein signaling complex (2). Figure 1 depicts the P2X subunit structure and function. The ATP binding site is localized in the cysteine-rich extracellular loop of the P2X receptors (17).
Figure 1.
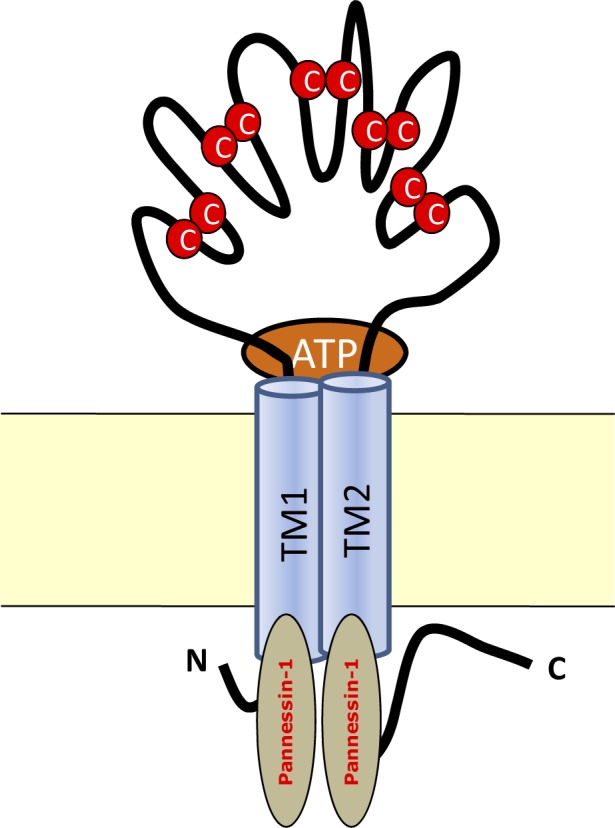
P2X7 receptor structure within the cell membrane  = Cysteine links; TM= Transmembrane
= Cysteine links; TM= Transmembrane
Activation of P2X receptor by ATP increases the receptor membrane pore permeability and leads to an influx of Na+, K+ and Ca2 + ions (23). This will depolarize the cell and generate an action potential that, in turn, modulates a wide range of cellular processes (24). Due to the juxtapositioning of the receptor with the sites of rapid transmitter release cellular activation can peak in less than 10 milliseconds (23, 24). Knockout of the P2X7 receptor results in an attenuated response of cells to ATP with respect to intracellular Ca 2 + response (25).
Binding of ATP to the P2X7 receptors of inflammatory cells (macrophages and monocytes) will open the rapid potassium-selective channels. Activated P2X7 receptors stimulate the hemi-channel pannesin-1, which leads to opening of a coupled larger receptor-associated pore (26, 22) which is critical for caspase-1 activation and hence in IL-1β maturation and release (27, 28).
P2X7 and IL-1β
Activation of P2X7 receptors on inflammatory cells induces caspase 1 activity. Caspase-1, also known as IL-1β converting enzyme (ICE), belongs to the nine cysteine proteases family and stimulates the intracellular processing of IL-1β (29). IL-1β is a key mediator of the host response to infections and inflammation (30). Cysteine proteases are mostly involved in mediating programmed cell death (apoptosis) through promoting the cleavage of critical intercellular proteins. Caspase-1 is the only member of the cysteine protease family that is also involved in the inflammatory response through cleavage of the IL-1β, IL-18 and IL-33 precursors (26). As stated above, caspase-1 activity is enhanced by P2X7 and hemichannel pannesin-1 stimulation. Inhibition of the hemichannel pannesin-1 blocks the activation of caspase-1 reaction even in presence of potassium efflux suggesting that K+ depletion alone does not induce caspase-1 activation (28, 31).
Activation of caspase-1 stimulates oligomerization of the cryopyrin/NALP3 inflammasome through its caspase-1 recruitment domain (CARD) (26). CARD is the amino-terminal sequence of nucleotide-binding and oligomerization domain (NOD)-like receptors (NLRs). This process is depicted in Figure 2.
Figure 2.
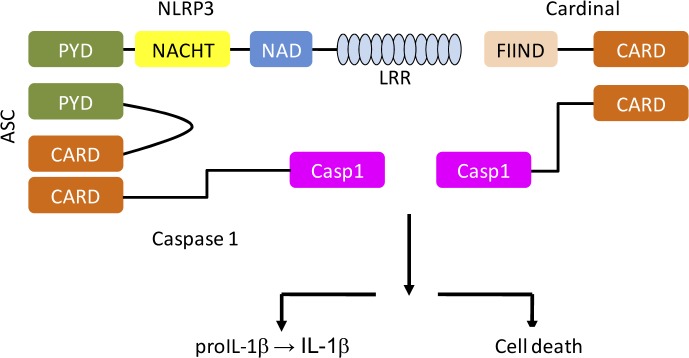
The multi-protein structure of the Inflammasome contains caspase-1 and NALP3
Interaction of pro-caspase-1 with NALP3 activates an autocatalytic reaction that stimulates the IL-1β maturation and release from inflammatory cells (17, 26). Studies suggest that P2X7R opening causes a drastic change in K+ homeostasis which has an important role in IL-1β maturation (8, 32). However, as mentioned above, this process is likely to be more complex since the release of IL-1β is triggered by other intracellular ions e.g., Ca2 +(32, 33).
Externalization of IL-1β has been investigated and proposed in a model by Surprenant et al. (34). In this model IL-1β is packaged into small plasma membrane blebs (microvesicles) which are released into the extracellular space. Microvesicles will be produced in cells stimulated via the P2X7 receptor (34, 35). The microvesicles express several markers including P2X7R itself. But the question how mature IL-1β gets through the microvesicle membrane remains to be elucidated. Studies indicate that enhanced release of IL-1β occurs at sites of high ATP expression (36). Exposure of microvesicles to ATP activates the P2X7 receptor leading to microvesicle lysis and IL-1β release into the extracellular space where it can activate IL-1βRs (8, 37). Figure 3 shows the pathways for the cleavage and release of IL-1β following stimulation by P2X7.
Figure 3.
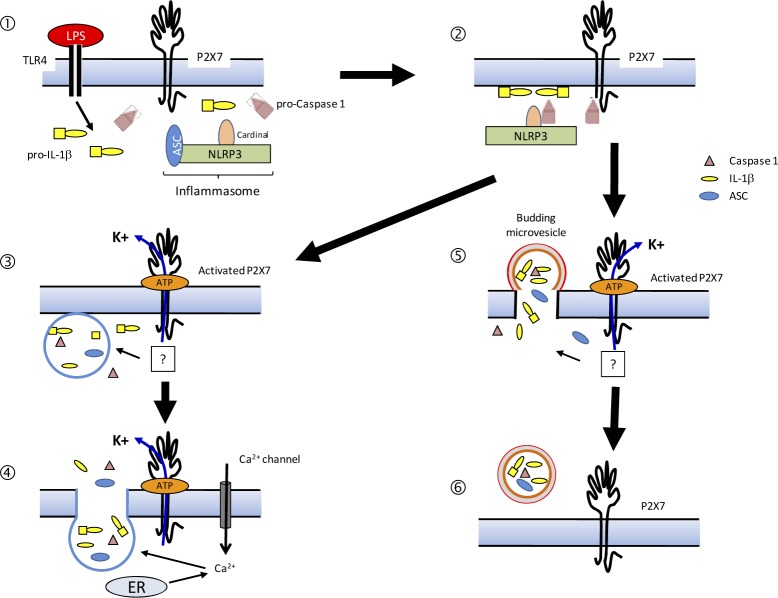
Pathways for cleavage and release of IL-1β following stimulation by P2X7. Aggregation of the inflammasome components ① Inflammasome localized ② P2X7 activation leads to K+ efflux and loading of inflammasome components (e.g. IL-1β) and IL-1β maturation .③ Secretion of lysosome content via a P2X7 K+ efflux and increase of Ca2+ ④ Budding of microvesicles that contain some of inflammasome components (e.g. IL-1β), IL-1β maturation ⑤ Microvesicles are released into the extracellular space ⑥
IL-1β and COPD
COPD is an inflammatory disease of the airways. It can be exemplified by chronic bronchitis and obstructive emphysema. High levels of IL-1β have been reported in induced sputum and BAL fluid of COPD patients (38) and a COPD-like phenotype consisting of lung inflammation and airway fibrosis has been displayed in mice over-expressing IL-1β in the lung epithelium (39). Furthermore, recent in vitro and in vivo models of COPD demonstrate increased ATP levels (40). Eltom et al. investigated whether modulation of P2X7 activation attenuated cigarette smoke (CS)-induced inflammation and caspase-1 activity. A correlation between markers of P2X7/inflammasome pathway activation and airway inflammation was shown in a 3-day CS model in vivo and this was blunted by both a P2X7 inhibitor and in a P2X7 knockout mouse (41).
Elthom and colleagues demonstrated that P2X7-/-mice failed to increase caspase -1 activity and IL-1β expression in the airway to the same extent as wild-type mice following CS exposure (41) providing strong evidence for an important role of the P2X7 receptor in CS-induced airway neutrophilia.
The same authors also examined whether P2X7 is important in modulation of an inflammatory response in a chronic CS-induced COPD model (41). These authors reported an increase in both caspase -1 activity and IL-1β levels in this chronic CS-exposed model. Furthermore, this increase in IL-1β expression and caspase-1 activity correlated with an increase in lung macrophages and neutrophils (41).
To investigate if the results observed in the pre-clinical murine CS-model could be translated into the human disease, caspase-1 activity in the lung tissue of non-smoking, smoking and emphysematous patients undergoing surgery for lung cancer was examined (41).
Caspase-1 activity was increased in the lungs of smokers and in patients with emphysema mimicking the responses seen in the pre-clinical models.
Perspectives
In the past few years, there has been an increase in the number of studies investigating the pathophysiology of airway inflammation in COPD. This has led to a greater understanding of the disease and the pathways behind this complex disorder. The results from more recent studies advocate a critical role for ATP, P2X7 and IL-1β in CS-induced lung inflammation.
Because of the significant role of P2X7 in the inflammasome pathway, it is important to understand the physiological role of these receptors. Finding a link between purinergic signaling and the inflammasome pathway in disease is challenging. However, this can help us to create opportunities for a whole new therapeutic class of drugs that will suppress lung inflammation. Hereby P2X7 receptors can be considered as an important therapeutic target in COPD patients.
REFERENCES
- 1.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, et al. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168(12):6436–45. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 2.Murrell-Lagnado RD, Qureshi OS. Assembly and trafficking of P2X purinergic receptors (Review) Mol Membr Biol. 2008;25(4):321–31. doi: 10.1080/09687680802050385. [DOI] [PubMed] [Google Scholar]
- 3.North RA. Families of ion channels with two hydrophobic segments. Curr Opin Cell Biol. 1996;8(4):474–83. doi: 10.1016/s0955-0674(96)80023-8. [DOI] [PubMed] [Google Scholar]
- 4.Mortaz E, Braber S, Nazary M, Givi ME, Nijkamp FP, Folkerts G. ATP in the pathogenesis of lung emphysema. Eur J Pharmacol. 2009;619(1-3):92–6. doi: 10.1016/j.ejphar.2009.07.022. [DOI] [PubMed] [Google Scholar]
- 5.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172(8):4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 6.Cohn ZA, Parks E. The regulation of pinocytosis in mouse macrophages. II. Factors inducing vesicle formation. J Exp Med. 1967;125(2):213–32. doi: 10.1084/jem.125.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Virgilio F, Borea PA, Illes P. P2 receptors meet the immune system. Trends Pharmacol Sci. 2001;22(1):5–7. doi: 10.1016/s0165-6147(00)01574-1. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari D, Pizzirani C, Adinolfi E, Lemoli RM, Curti A, Idzko M, et al. The P2X7 receptor: a key player in IL-1 processing and release. J Immunol. 2006;176(7):3877–83. doi: 10.4049/jimmunol.176.7.3877. [DOI] [PubMed] [Google Scholar]
- 9.Kumar V, Sharma A. Adenosine: an endogenous modulator of innate immune system with therapeutic potential. Eur J Pharmacol. 2009;616(1-3):7–15. doi: 10.1016/j.ejphar.2009.05.005. [DOI] [PubMed] [Google Scholar]
- 10.Le Feuvre RA, Brough D, Iwakura Y, Takeda K, Rothwell NJ. Priming of macrophages with lipopolysaccharide potentiates P2X7-mediated cell death via a caspase-1-dependent mechanism, independently of cytokine production. J Biol Chem. 2002;277(5):3210–8. doi: 10.1074/jbc.M104388200. [DOI] [PubMed] [Google Scholar]
- 11.Perregaux D, Gabel CA. Interleukin-1 beta maturation and release in response to ATP and nigericin. Evidence that potassium depletion mediated by these agents is a necessary and common feature of their activity. J Biol Chem. 1994;269(21):15195–203. [PubMed] [Google Scholar]
- 12.Dinarello CA. Biologic basis for interleukin-1 in disease. Blood. 1996;87(6):2095–147. [PubMed] [Google Scholar]
- 13.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–8. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 14.Chung KF. Cytokines in chronic obstructive pulmonary disease. Eur Respir J Suppl. 2001;34:50s–59s. [PubMed] [Google Scholar]
- 15.Joos L, McIntyre L, Ruan J, Connett JE, Anthonisen NR, Weir TD, et al. Association of IL-1beta and IL-1 receptor antagonist haplotypes with rate of decline in lung function in smokers. Thorax. 2001;56(11):863–6. doi: 10.1136/thorax.56.11.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marieb E.N. San Francisco: Pearson Education; 2004. Human Anatomy & Physiology; pp. 388–423. [Google Scholar]
- 17.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2(3):165–74. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 18.Fields RD, Burnstock G. Purinergic signalling in neuron-glia interactions. Nat Rev Neurosci. 2006;7(6):423–36. doi: 10.1038/nrn1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mortaz E, Folkerts G, Nijkamp FP, Henricks PA. ATP and the pathogenesis of COPD. Eur J Pharmacol. 2010;638(1-3):1–4. doi: 10.1016/j.ejphar.2010.04.019. [DOI] [PubMed] [Google Scholar]
- 20.Polosa R, Blackburn MR. Adenosine receptors as targets for therapeutic intervention in asthma and chronic obstructive pulmonary disease. Trends Pharmacol Sci. 2009;30(10):528–35. doi: 10.1016/j.tips.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stojilkovic S.S. Purinergic Regulation of Hypothalamo-Pituitary Functions. 2009;20(9):460–468. doi: 10.1016/j.tem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khakh BS, North RA. P2X receptors as cell-surface ATP sensors in health and disease. Nature. 2006;442(7102):527–32. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- 23.Rang H.P. SL: Elsevier; 2007. Pharmacology; pp. 27–35. [Google Scholar]
- 24.Khakh BS. Molecular physiology of P2X receptors and ATP signalling at synapses. Nat Rev Neurosci. 2001;2(3):165–74. doi: 10.1038/35058521. [DOI] [PubMed] [Google Scholar]
- 25.Pochet S, Garcia-Marcos M, Seil M, Otto A, Marino A, Dehaye JP. Contribution of two ionotropic purinergic receptors to ATP responses in submandibular gland ductal cells. Cell Signal. 2007;19(10):2155–64. doi: 10.1016/j.cellsig.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 26.Lamkanfi M, Kanneganti TD, Franchi L, Núñez G. Caspase-1 inflammasomes in infection and inflammation. J Leukoc Biol. 2007;82(2):220–5. doi: 10.1189/jlb.1206756. [DOI] [PubMed] [Google Scholar]
- 27.Elssner A, Duncan M, Gavrilin M, Wewers MD. A novel P2X7 receptor activator, the human cathelicidin-derived peptide LL37, induces IL-1 beta processing and release. J Immunol. 2004;172(8):4987–94. doi: 10.4049/jimmunol.172.8.4987. [DOI] [PubMed] [Google Scholar]
- 28.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X7 receptor. EMBO J. 2006;25(21):5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hazuda DJ, Strickler J, Kueppers F, Simon PL, Young PR. Processing of precursor interleukin 1 beta and inflammatory disease. J Biol Chem. 1990;265(11):6318–22. [PubMed] [Google Scholar]
- 30.Dinarello CA. The IL-1 family and inflammatory diseases. Clin Exp Rheumatol. 2002;20(5 Suppl 27):S1–13. [PubMed] [Google Scholar]
- 31.Wewers MD, Sarkar A. P2X(7) receptor and macrophage function. Purinergic Signal. 2009;5(2):189–95. doi: 10.1007/s11302-009-9131-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gudipaty L, Munetz J, Verhoef PA, Dubyak GR. Essential role for Ca2+ in regulation of IL-1beta secretion by P2X7 nucleotide receptor in monocytes, macrophages, and HEK-293 cells. Am J Physiol Cell Physiol. 2003;285(2):C286–99. doi: 10.1152/ajpcell.00070.2003. [DOI] [PubMed] [Google Scholar]
- 33.Andrei C, Margiocco P, Poggi A, Lotti LV, Torrisi MR, Rubartelli A. Phospholipases C and A2 control lysosome-mediated IL-1 beta secretion: Implications for inflammatory processes. Proc Natl Acad Sci U S A. 2004;101(26):9745–50. doi: 10.1073/pnas.0308558101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.MacKenzie A, Wilson HL, Kiss-Toth E, Dower SK, North RA, Surprenant A. Rapid secretion of interleukin-1beta by microvesicle shedding. Immunity. 2001;15(5):825–35. doi: 10.1016/s1074-7613(01)00229-1. [DOI] [PubMed] [Google Scholar]
- 35.Morelli A, Chiozzi P, Chiesa A, Ferrari D, Sanz JM, Falzoni S, et al. Extracellular ATP causes ROCK I-dependent bleb formation in P2X7-transfected HEK293 cells. Mol Biol Cell. 2003;14(7):2655–64. doi: 10.1091/mbc.02-04-0061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianco F, Pravettoni E, Colombo A, Schenk U, Möller T, Matteoli M, et al. Astrocyte-derived ATP induces vesicle shedding and IL-1 beta release from microglia. J Immunol. 2005;174(11):7268–77. doi: 10.4049/jimmunol.174.11.7268. [DOI] [PubMed] [Google Scholar]
- 37.Pellegatti P, Falzoni S, Pinton P, Rizzuto R, Di Virgilio F. A novel recombinant plasma membrane-targeted luciferase reveals a new pathway for ATP secretion. Mol Biol Cell. 2005;16(8):3659–65. doi: 10.1091/mbc.E05-03-0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeidel A, Beilin B, Yardeni I, Mayburd E, Smirnov G, Bessler H. Immune response in asymptomatic smokers. Acta Anaesthesiol Scand. 2002;46(8):959–64. doi: 10.1034/j.1399-6576.2002.460806.x. [DOI] [PubMed] [Google Scholar]
- 39.Lappalainen U, Whitsett JA, Wert SE, Tichelaar JW, Bry K. Interleukin-1beta causes pulmonary inflammation, emphysema, and airway remodeling in the adult murine lung. Am J Respir Cell Mol Biol. 2005;32(4):311–8. doi: 10.1165/rcmb.2004-0309OC. [DOI] [PubMed] [Google Scholar]
- 40.Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med. 2006;12(1):54–9. doi: 10.1097/01.mcp.0000199002.46038.cb. [DOI] [PubMed] [Google Scholar]
- 41.Eltom S, Stevenson CS, Rastrick J, Dale N, Raemdonck K, Wong S, et al. P2X7 receptor and caspase 1 activation are central to airway inflammation observed after exposure to tobacco smoke. PLoS One. 2011;6(9):24097. doi: 10.1371/journal.pone.0024097. [DOI] [PMC free article] [PubMed] [Google Scholar]