INTRODUCTION
Lung cancer is the leading cause of cancer deaths in both men and women in the USA. The incidence of lung cancer is higher in men than in women, but at present the incidence is increasing in women.
Lung cancer is expected to account for 26% of all female cancer deaths in 2011 and has been the leading cause of cancer death in women since 1987 (1).
Smoking tobacco is the primary risk factor for lung cancer (2–4). Heavy smoking is associated with a 20 to 30-fold increase in lung cancer risk compared to nonsmokers. Cigarette smoking is the cause of lung cancer in %90 of cases (2).
Squamous cell carcinomas, small cell lung carcinoma, large cell carcinoma, and to a lesser extent adenocarcinoma have an increased incidence with increased number of cigarettes smoked per day.
Radon, a radioactive gas, is the second cause of lung cancer in general population (5), and is the main cause of lung cancer in nonsmokers. Second-hand smoke is the third leading cause of lung cancer (6, 7).
The WHO divided lung cancer into 2 major classes: Non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Non-small cell lung cancer accounts for more than 85% of lung cancer cases and has 2 major subtypes: Squamous cell carcinoma and non squamous cell carcinoma.
In general, 30% of lung cancer cases are squamous cell carcinoma which is strongly associated with smoking. Cavitation is more common in this type than the other types of lung cancer. About two third of squamous cell carcinomas arise within the main bronchus and lead to atelectasis and consolidation. In one third of cases, SCC can be seen in the periphery as a lung nodule or mass.
Adenocarcinoma is the most common type of lung cancer and accounts for 35% of cases. It is most commonly seen in women and nonsmokers. The periphery of the lung especially the upper lobe is the dominant area of localization. The margins of tumor are speculated and irregular.
Large cell carcinoma accounts for 10% of lung cancers and typically presents as a large peripheral mass with early metastasis and poor prognosis.
In total, 15% of lung cancer cases are small cell lung cancer (SCLC). When compared, SCLC has a more rapid doubling time in comparison to NSCLC and earlier development of metastases. Most patients with SCLC have hematogenous metastases, and limited disease confined to the chest is seen in only one third of cases. Other types present small percentage of all lung carcinomas.
IMAGING IN LUNG CANCER
The primary role of imaging is initial staging (8). Accurate staging is very important for management strategies and evaluation of prognosis. The management strategies for NSCLC and SCLC are significantly different. In the early stages of NSCLC, surgery provides the best chance of cure either alone or in combination with chemotherapy or radiotherapy. But most patients have metastatic disease at the time of diagnosis. Small cell lung cancer initially responds well to chemotherapy and radiation and surgery should be considered only for stage I (T1-T2 N0)(9).
Computed tomography (CT) and magnetic resonance imaging have been used for NSCLC staging. CT provides morphologic information about the tumor size and the extent of disease (10) but has limited ability to differentiate between benign and malignant lesions.
PET, Positron Emission Tomography, also called PET imaging or a PET scan, is a type of nuclear medicine imaging. Nuclear medicine is a branch of medical imaging that uses small amounts of radioactive material to diagnose or treat a variety of diseases, including many types of cancers. Radionuclide imaging procedures are noninvasive and with the exception of intravenous injections, are usually painless medical tests. These imaging scans use radioactive materials called radiopharmaceuticals or radionuclide.
The radionuclide positron emitter is injected into a vein and accumulates in the target organ. After a positron is emitted, it travels a short distance in tissue and losing almost all kinetic energy, it annihilates with an electron. Annihilation produces a pair of photon in opposite directions, each with energy of 511kev (Figure 1).
Figure 1.
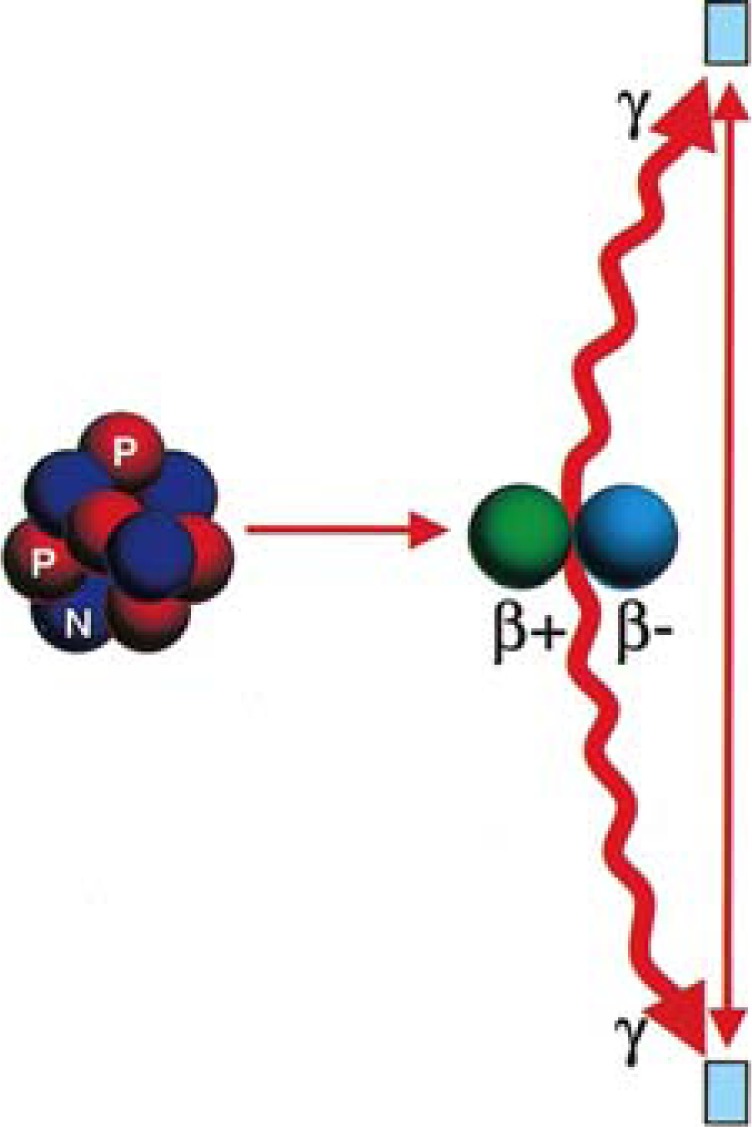
Annihilation reaction (Kapoor V et al. Radiographics 2004;24:523-543)
These photons are detected by a device called a PET scanner. A PET scan measures important body functions, such as blood flow, oxygen use, and glucose metabolism, to evaluate function of organs and tissues.
Now, manufacturers are making positron emission tomography/computed tomography (PET/CT) units that are able to perform both imaging studies at the same time.
In these new systems, the PET and CT images are fused and provide combined physiologic information as well as exact anatomic localization in a single examination. FDG-PET uses 18 F-fluorodeoxyglucose, which is a short-lived radioactive compound that localizes in tumor cells. Tumoral cells have a high metabolic activity and use more glucose; 18 F-fluorodeoxyglucose is a glucose analog that after entering the cell, has the similar metabolism pathway as glucose. After phosphorylation, FDG-6-Po4 is not further metabolized and does not diffuse out of the cell, thus remain trapped which is ideal for imaging.
Following IV injection, F-18 FDG rapidly distributes into the body and kidneys are the main route of excretion.
The half life of F-18 is 109.7 minutes and the optimal time for imaging is 60 minutes after injection.
Serum glucose level competes with FDG and decreases its uptake by tumoral cells; thus fasting for 4 hours is strongly recommended prior to PET-scan. For this reason special prepration is needed for diabetic patients to control glucose.
STAGING OF NON-SMALL CELL LUNG CANCER
Tumor staging by the TNM system provides information about the extent of disease and determines the best therapy and prognosis (11). Resectability is the most important decision made by using TNM system.
T status describes the extent of the primary tumor by its size and its invasion to the pleura, bronchovascular structures, diaphragm, and mediastinum. Presence of mediastinal fat planes or lung between the tumor and the mediastinum means no direct extension into the mediastinum.
Lymph node (N) status is an important factor for determining the resectability; N describes the presence or absence and localization of regional lymph node metastasis. The location of the primary tumor determines the lymphatic pathway and localization of regional lymph node involvement (12). A tumor in the right lung metastasizes to the right hilar lymph node and then to the right paratracheal lymph nodes. Only in 4% of cases, contralateral mediastinal lymph nodes are involved by metastasis. Primary tumor in the left upper lobe metastasizes to the aortopulmonary window and left paratracheal nodes. T Lung cancer in the both lower lobes and right middle lobe may send early metastasis to subcarinal nodes. Initial involvement of left hilar lymph nodes may be seen in left upper and lower lobe tumors.
N1 means metastasis to lymph nodes in the ipsilateral peribronchial or hilar region.
N2 is defined as a metastasis to ipsilateral mediastinal or subcarinal lymph nodes and represents at least IIIA.
N3 is defined as a metastasis to contralateral hilar, contralateral mediastinal, ipsilateral or contralateral scalene, or supraclavicular lymph nodes and represents at least IIIB.
The CT evaluation of mediastinal lymph nodes has extremely variable sensitivity and specificity: %60 and 77%, respectively (13). CT criteria for abnormal size of lymph node is more than 1cm in the short axis (14). Micrometastases may be seen in normal-size lymph nodes in %15 of patients with stage I, thus size alone is not a reliable criteria (15, 16). Fatty hilum in lymph node is another morphologic sign that emphasizes its benignity (17).
Distant metastasis (M status) is a critical factor for the resectability of a tumor. M defines tumoral involvement of distant lymph node or organs. Common sites of metastases are brain, bone, liver, adrenal glands (M1b) and contralateral lung (M1a) (18).
The frequency of occult metastasis at the time of presentation is 30% in patients with adenocarcinoma or large cell carcinoma and 15% in squamous cell carcinoma of the lung (19). The most common sites of distant occult metastases are adrenal gland and liver. The frequency of incidental nonfunctioning cortical adenomas is 5% in general population and in 10% of patients with lung cancer adrenal mass is seen on CT (20, 21). If the HU of adrenal mass is less than 10 on unenhanced CT, it is considered benign. F-FDG PET is sensitive for detection of adrenal metastases. Adrenal may be the only site for metastasis but liver is never the only site except in adenocarcinoma.
ROLE OF F-18 FDG-PET IN STAGING
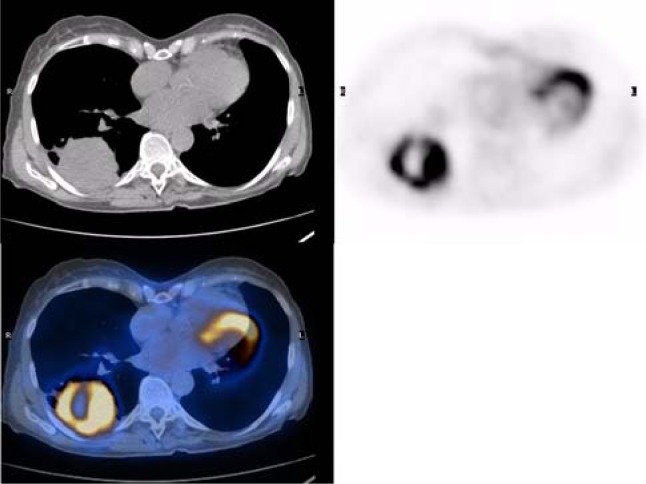
Integrated PET-CT is a useful method for the determination of invasion of lung cancer to the chest wall (Figure 2). Because of the exact F-18 FDG uptake correlation to anatomic finding in PET-CT images, the primary lung cancer precisely delinated which leads better determination of chest wall infiltration (potentially resectable T3a) and mediastinal invasion. F-18 FDG-PET can differentiate tumor from peritumoral atelectasis. This is especially important in radiotherapy planning to reduce the field of radiation.
Figure 2.
Non small cell Lung cancer.
Hypermetabolic cavitary lung mass is seen in right lower lobe. Abnormal mass in RLL is seen in CT that cannot be characterized. The PET study shows increased glucose uptake in the periphery of the mass indicating viable tumor tissue without chest wall infiltration and no uptake in the center means central necrosis; thus, exact localization of biopsy site is easy with PET/CT fusion image.
The value of addition of 18F-FDGPET to conventional radiology in NSCLC staging was the prevention of unnecessary surgery in 1 of 5 patients (22). In non-central tumors, PET has high negative predictive value for mediastinal lymph node involvement; thus, preventing unnecessary mediastinoscopy. The sensitivity and specificity of PET were 91% and 86%, respectively for detection of mediastinal lymph node metastasis (23).
Some medical centers accept negative results because of high negative predictive value of PET without pathologic confirmation and resection of tumor by surgery. However, controversy exists about the role of PET in mediastinal staging (24).
Size of the metastasis is very important. Takamochi et al. found false-negative PET results in lesions with 1 to 7.5mm in diameter (25). Misregistration from respiratory, cardiac, and body motions leads to mediastinal activity as another source of error.
If the disease is stage I (T1ab, N0 lesions) according to clinical examination with negative CT and PET result, because of the low prior probability of lymph node involvement, routine mediastinoscopy in these patients is excluded (26).
If the PET/CT scan is positive in the mediastinum, the lymph node status needs pathologic confirmation. EBUS-TNBA can be used to determine the situation(27, 28). However, in case of negative EBUS-TNBA findings, mediastinoscopy can be done to confirm the results (28, 29).
In patients with peripheral T2a, central T1ab, or T2-T3 lesions with negative PET/CT scans, there is high risk for mediastinal lymph node involvement and mediastinoscopy is recommended (30).
Gonzalez-Stawinski et al. showed the false-negative rates of mediastinoscopy and PET to be 3% and 11.7%, respectively in stage II and III diseases (31). Thus, for stage II and III diseases, mediastinoscopy will remain part of the standard protocol for staging.
Differentiation of stage IIIA from IIIB (suspected N3) diseases is a very important factor for using mediastinoscopy and to determine which patient needs curative resection (32, 33).
N status is not relevant in stage IV disease; thus, PET for mediastinal staging is less of an issue.
F-FDG shows metabolic activity and increased glucose uptake by activated macrophages and inflammatory cells leading to increased uptake of 18F-FDG in sites of inflammation. Thus, FDG-PET cannot distinguish malignancy from inflammation or infection (34).
FDG-PET has positive predictive value of 75% -95% for evaluation of the mediastinum (35, 36). The high rate of false-positive results revealed that we need mediastinoscopy for better staging of PET-positive mediastinal lymph nodes (37, 38).
The benefit of PET in this situation is localization of mediastinal lymph node for biopsy and selecting another invasive method for inaccessible lymph nodes by mediastinoscopy (VATS and thoracotomy).
FALSE-NEGATIVE AND FALSE-POSITIVE FINDINGS IN FDG-PET SCAN
F-18 FDG-PET results can be false negative in pulmonary carcinoid tumors that are highly differentiated and in BAC (39, 40). Lymph node metastasis smaller than 4–6 mm is another false negative result. Imaging modalities cannot detect micro-metastasis.
Tuberculosis, eosinophilic lung disease, histoplasmosis, aspergillosis, lung emphysema and occult lung infarction may have abnormal increased uptake of F-18 FDG leading to false positive findings (41). Chronic inflammatory lesions usually do not increase the uptake of FDG.
DISTANT METASTASIS
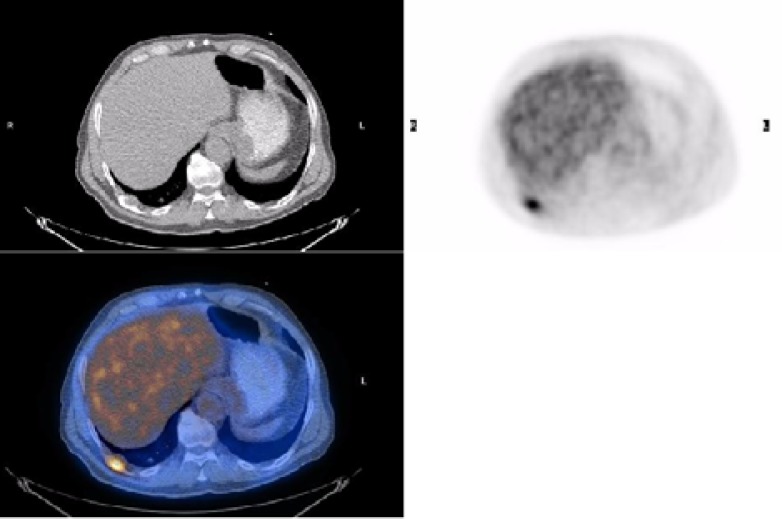
There is no need for curative surgical resection in Stage IV because of distant metastasis. An important advantage of PET-CT is the use of whole-body scanning to detect distant metastasis (Figure 3).
Figure 3.
The PET study shows unsuspected abnormal increased FDG uptake in posterior aspect of right chest, which is best localized in right dorsal rib in PET/CT fusion image.
Distant metastases commonly involve the adrenal glands, bones, liver, and brain (42). The mean frequency of extra-thoracic metastases by PET studies was 13%. The frequency of distant metastases was 7.5% in stage I, 18% in stage II, and 24% in stage III (43).The adrenal glands and liver are the most common sites of extra-thoracic metastases in lung cancer. At the time of presentation, up to 10% of patients have adrenal mass and two thirds of them are not malignant (44, 45). Sensitivity, specificity and accuracy of PET for detecting adrenal metastasis were 100%, 94%, and 96%; respectively (46).
Also, 18F-FDG PET has a sensitivity similar to that of bone scan but its specificity is more than bone scintigraphy. Fogelman et al. (47) in their study of breast cancer in patients with bone metastases showed that 18F-FDGPET has the advantage of detecting osteolytic lesions; whereas, bone scintigraphy can detect osteoblastic lesions (48). PET is less effective than CT or MRI for the detection of brain metastasis because of high FDG uptake by normal brain (49).
The routine use of magnetic resonance imaging (MRI) (to rule out asymptomatic brain metastases) and bone scans (to exclude bone metastases) is not recommended. Brain MRI is recommended for patients with stage II, III, and IV disease to rule out metastatic disease if aggressive combined-modality therapy is being considered (50).
The increased sensitivity of PET/CT scans, compared with other imaging methods, may identify additional metastases and, thus, spare some patients from unnecessary surgery. However, positive PET/CT scan findings need pathologic or other radiologic confirmation.
RESTAGING
Morphologic response usually occurs over several weeks to months after therapy. During the interim phase, approximately one-third of non-responder patients in the first line of chemotherapy are treated without any benefit from therapy bearing unacceptable side effects from several weeks of toxic and costly treatment. Morphologic evaluation may be incorrect because of edema and peri-tumoral scar tissue formation, which may mask tumor regression (51). Considering the above mentioned reasons, the morphologic response is not ideal.
Decreased metabolic activity, standardized uptake value (SUV) after one cycle of chemotherapy is closely correlated with effective chemotherapy (52). The treatment regimen may be changed to second-line therapy if there is no metabolic response; thereby, reducing the costs and morbidity from ineffective therapy. Patients with positive FDG PET results after first-line treatment, have a significantly worse prognosis than those with negative FDG PET results, with median survival of 12 months versus 34 months, respectively (53). PET is highly specific for the characterization of viable tumor and scar tissue after therapy (54).
Mass or symptoms suggestive of relapse after curative therapy are common and characterization of them is difficult.
Hicks et al. demonstrated early detection of relapse by PET that changed the management in 63% of patients from curative to palliative, from palliative to curative or active management in PET negative patients (55).
STAGING OF SMALL CELL LUNG CANCER (SCLC)
SCLC represents approximately 15% of all cases of lung cancer (56, 57). Nearly all cases are related to cigarette smoking. Two third of patients with SCLC present with hematogenous metastases, while the remainder of patients have limited disease confined to the chest.
SCLCs are typically located centrally with a large hilar mass and bulky mediastinal lymphadenopathy (57, 58) often with encasement of mediastinal structures and tracheobronchial compression (59, 60) that cause coughing and dyspnea. Frequently, patients present symptoms of metastatic disease, such as weight loss, bone pain, and neurologic problems. Solitary peripheral nodule without central adenopathy is an uncommon finding. Unlike the NSCLC, there is a 2-stage classification according to the growth pattern for SCLC:
Limited-stage disease is confined to the ipsilateral hemithorax, which can be safely included in a radiation field. Contralateral mediastinal and ipsilateral supraclavicular lymphadenopathies are classified as limited-stage disease, while contralateral hilar and supraclavicular lymphadenopathy is controversial.
Extensive-stage disease is beyond the ipsilateral hemithorax, including the malignant pleural or pericardial effusion or hematogenous metastases (61).
Patients with limited disease are given chemoradiation; whereas, patients with extensive disease are given chemotherapy alone.
In the new TNM staging system, any T, any N and M0 is limited-stage SCLC. Extensive-stage is any T, any N, M1a/b, and T3-4 with multiple lung nodules (not fit in tolerable radiation field).
If limited-stage is suspected, PET–CT should assess distant metastases. PET scans can increase staging accuracy in patients with SCLC (62).
By PET, 15% of patients are up-staged from limited to extensive stage, and pathologic confirmation is required for PET/CT–detected lesions; while, only 5% are down-staged from extensive to limited stage.
PET/CT is superior to standard imaging for metastasis; however, PET/CT is inferior to MRI or CT for brain metastases (63).
Surgery is considered for 5% of patients with stage I (T1-2 N0) SCLC in whom biopsy and pathologic staging of mediatinum is required to confirm PET/CT findings and also in patients with clinical stage T1-2, N0 disease (64). In stage I disease, 5-year survival rate is 40%-60% (65, 66).
PET staging changed management in 16%-38% of patients mainly due to change of radiation field because of more accurate detection of intrathoracic sites (67, 68).
In brief, PET-CT scan can identify distant metastatic disease and may be applied to guide mediastinal evaluation, if not previously done.
CONCLUSION
One of the most important applications of FDG-PET is staging and restaging of lung cancer. PET is superior to conventional imaging modalities for detection of unsuspected mediastinal lymph node and distant metastasis. FDG-PET by accurate staging can guide the most appropriate therapy and by evaluation of therapy, reduce the morbidity and costs of ineffective treatment. Adequate information about the limitations of FDG-PET will also provide the most accurate interpretation of PET-scan.
REFERENCES
- 1.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61(4):212–36. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 2.The Health Consequences of Smoking: A Report of the Surgeon General. Atlanta (GA): U.S. Department of Health and Human Services. Centers for Disease Control and Prevention (US); 2004. ed 2010/07/30. [PubMed] [Google Scholar]
- 3.Secretan B, Straif K, Baan R, Grosse Y, El Ghissassi F, Bouvard V, et al. A review of human carcinogens--Part E: tobacco, areca nut, alcohol, coal smoke, and salted fish. Lancet Oncol. 2009;10(11):1033–4. doi: 10.1016/s1470-2045(09)70326-2. [DOI] [PubMed] [Google Scholar]
- 4.Doll R, Peto R. Mortality in relation to smoking: 20 years’ observations on male British doctors. Br Med J. 1976;2(6051):1525–36. doi: 10.1136/bmj.2.6051.1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omenn GS, Merchant J, Boatman E, Dement JM, Kuschner M, Nicholson W, et al. Contribution of environmental fibers to respiratory cancer. Environ Health Perspect. 1986;70:51–6. doi: 10.1289/ehp.867051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Taylor R, Najafi F, Dobson A. Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol. 2007;36(5):1048–59. doi: 10.1093/ije/dym158. [DOI] [PubMed] [Google Scholar]
- 7.Wald NJ, Nanchahal K, Thompson SG, Cuckle HS. Does breathing other people's tobacco smoke cause lung cancer? Br Med J (Clin Res Ed) 1986;293(6556):1217–22. doi: 10.1136/bmj.293.6556.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shreve P, Townsend DW. Clinical PET-CT in radiology. New York: Springer; 2011. p. 166. [Google Scholar]
- 9.Bradley J, Graham MV, Winter K, Purdy JA, Komaki R, Roa WH, et al. Toxicity and outcome results of RTOG 9311: a phase I-II dose-escalation study using three-dimensional conformal radiotherapy in patients with inoperable non-small-cell lung carcinoma. Int J Radiat Oncol Biol Phys. 2005;61(2):318–28. doi: 10.1016/j.ijrobp.2004.06.260. [DOI] [PubMed] [Google Scholar]
- 10.Antoch G, Stattaus J, Nemat AT, Marnitz S, Beyer T, Kuehl H, et al. Non-small cell lung cancer: dual-modality PET/CT in preoperative staging. Radiology. 2003;229(2):526–33. doi: 10.1148/radiol.2292021598. [DOI] [PubMed] [Google Scholar]
- 11.Mountain CF. The international system for staging lung cancer. Semin Surg Oncol. 2000;18(2):106–15. doi: 10.1002/(sici)1098-2388(200003)18:2<106::aid-ssu4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 12.Platt JF, Glazer GM, Gross BH, Quint LE, Francis IR, Orringer MB. CT evaluation of mediastinal lymph nodes in lung cancer: influence of the lobar site of the primary neoplasm. AJR Am J Roentgenol. 1987;149(4):683–6. doi: 10.2214/ajr.149.4.683. [DOI] [PubMed] [Google Scholar]
- 13.Lee JKT, Sagel SS, Stanley RJ, Heiken JP. Computed Body Tomography with MRI Correlation. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 1998. [Google Scholar]
- 14.Glazer GM, Gross BH, Quint LE, Francis IR, Bookstein FL, Orringer MB. Normal mediastinal lymph nodes: number and size according to American Thoracic Society mapping. AJR Am J Roentgenol. 1985;144(2):261–5. doi: 10.2214/ajr.144.2.261. [DOI] [PubMed] [Google Scholar]
- 15.Libshitz HI, McKenna RJ., Jr Mediastinal lymph node size in lung cancer. AJR Am J Roentgenol. 1984;143(4):715–8. doi: 10.2214/ajr.143.4.715. [DOI] [PubMed] [Google Scholar]
- 16.Verschakelen JA, Bogaert J, De Wever W. Computed tomography in staging for lung cancer. Eur Respir J Suppl. 2002;35:40s–48s. doi: 10.1183/09031936.02.00270802. [DOI] [PubMed] [Google Scholar]
- 17.Quint LE, Francis IR, Wahl RL, Gross BH, Glazer GM. Preoperative staging of non-small-cell carcinoma of the lung: imaging methods. AJR Am J Roentgenol. 1995;164(6):1349–59. doi: 10.2214/ajr.164.6.7754872. [DOI] [PubMed] [Google Scholar]
- 18.Klein JS, Webb WR. The radiologic staging of lung cancer. J Thorac Imaging. 1991;7(1):29–47. doi: 10.1097/00005382-199112000-00006. [DOI] [PubMed] [Google Scholar]
- 19.Sider L, Horejs D. Frequency of extrathoracic metastases from bronchogenic carcinoma in patients with normal-sized hilar and mediastinal lymph nodes on CT. AJR Am J Roentgenol. 1988;151(5):893–5. doi: 10.2214/ajr.151.5.893. [DOI] [PubMed] [Google Scholar]
- 20.Oliver TW, Jr, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984;153(1):217–8. doi: 10.1148/radiology.153.1.6473783. [DOI] [PubMed] [Google Scholar]
- 21.Sandler MA, Pearlberg JL, Madrazo BL, Gitschlag KF, Gross SC. Computed tomographic evaluation of the adrenal gland in the preoperative assessment of bronchogenic carcinoma. Radiology. 1982;145(3):733–6. doi: 10.1148/radiology.145.3.7146405. [DOI] [PubMed] [Google Scholar]
- 22.van Tinteren H, Hoekstra OS, Smit EF, van den Bergh JH, Schreurs AJ, Stallaert RA, et al. Effectiveness of positron emission tomography in the preoperative assessment of patients with suspected non-small-cell lung cancer: the PLUS multicentre randomised trial. Lancet. 2002;359(9315):1388–93. doi: 10.1016/s0140-6736(02)08352-6. [DOI] [PubMed] [Google Scholar]
- 23.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343(4):254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 24.Detterbeck FC, Falen S, Rivera MP, Halle JS, Socinski MA. Seeking a home for a PET, part 2: Defining the appropriate place for positron emission tomography imaging in the staging of patients with suspected lung cancer. Chest. 2004;125(6):2300–8. doi: 10.1378/chest.125.6.2300. [DOI] [PubMed] [Google Scholar]
- 25.Takamochi K, Yoshida J, Murakami K, Niho S, Ishii G, Nishimura M, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer. 2005;47(2):235–42. doi: 10.1016/j.lungcan.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Vansteenkiste JF. FDG-PET for lymph node staging in NSCLC: a major step forward, but beware of the pitfalls. Lung Cancer. 2005;47(2):151–3. doi: 10.1016/j.lungcan.2003.09.027. [DOI] [PubMed] [Google Scholar]
- 27.Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R, et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet. 2009;373(9674):1525–31. doi: 10.1016/S0140-6736(09)60569-9. [DOI] [PubMed] [Google Scholar]
- 28.Patel JD, Hensing TA, Rademaker A, Hart EM, Blum MG, Milton DT, et al. Phase II study of pemetrexed and carboplatin plus bevacizumab with maintenance pemetrexed and bevacizumab as first-line therapy for nonsquamous non-small-cell lung cancer. J Clin Oncol. 2009;27(20):3284–9. doi: 10.1200/JCO.2008.20.8181. [DOI] [PubMed] [Google Scholar]
- 29.Nadler E, Yu E, Ravelo A, Sing A, Forsyth M, Gruschkus S. Bevacizumab treatment to progression after chemotherapy: outcomes from a U.S. community practice network. Oncologist. 2011;16(4):486–96. doi: 10.1634/theoncologist.2010-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiller JH, Harrington D, Belani CP, Langer C, Sandler A, Krook J, et al. Comparison of four chemotherapy regimens for advanced non-small-cell lung cancer. N Engl J Med. 2002;346(2):92–8. doi: 10.1056/NEJMoa011954. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Stawinski GV, Lemaire A, Merchant F, O'Halloran E, Coleman RE, Harpole DH, et al. A comparative analysis of positron emission tomography and mediastinoscopy in staging non-small cell lung cancer. J Thorac Cardiovasc Surg. 2003;126(6):1900–5. doi: 10.1016/s0022-5223(03)01036-5. [DOI] [PubMed] [Google Scholar]
- 32.Cohen MH, Cortazar P, Justice R, Pazdur R. Approval summary: pemetrexed maintenance therapy of advanced/metastatic nonsquamous, non-small cell lung cancer (NSCLC) Oncologist. 2010;15(12):1352–8. doi: 10.1634/theoncologist.2010-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fidias PM, Dakhil SR, Lyss AP, Loesch DM, Waterhouse DM, Bromund JL, et al. Phase III study of immediate compared with delayed docetaxel after front-line therapy with gemcitabine plus carboplatin in advanced non-small-cell lung cancer. J Clin Oncol. 2009;27(4):591–8. doi: 10.1200/JCO.2008.17.1405. [DOI] [PubMed] [Google Scholar]
- 34.Kubota R, Kubota K, Yamada S, Tada M, Ido T, Tamahashi N. Microautoradiographic study for the differentiation of intratumoral macrophages, granulation tissues and cancer cells by the dynamics of fluorine-18-fluorodeoxyglucose uptake. J Nucl Med. 1994;35(1):104–12. [PubMed] [Google Scholar]
- 35.Pieterman RM, van Putten JW, Meuzelaar JJ, Mooyaart EL, Vaalburg W, Koëter GH, et al. Preoperative staging of non-small-cell lung cancer with positron-emission tomography. N Engl J Med. 2000;343(4):254–61. doi: 10.1056/NEJM200007273430404. [DOI] [PubMed] [Google Scholar]
- 36.Vansteenkiste JF, Stroobants SG, De Leyn PR, Dupont PJ, Bogaert J, Maes A, et al. Lymph node staging in non-small-cell lung cancer with FDG-PET scan: a prospective study on 690 lymph node stations from 68 patients. J Clin Oncol. 1998;16(6):2142–9. doi: 10.1200/JCO.1998.16.6.2142. [DOI] [PubMed] [Google Scholar]
- 37.Takamochi K, Yoshida J, Murakami K, Niho S, Ishii G, Nishimura M, et al. Pitfalls in lymph node staging with positron emission tomography in non-small cell lung cancer patients. Lung Cancer. 2005;47(2):235–42. doi: 10.1016/j.lungcan.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Passlick B. Mediastinal staging (take home messages) Lung Cancer. 2004;45(Suppl 2):S85–7. doi: 10.1016/j.lungcan.2004.07.987. [DOI] [PubMed] [Google Scholar]
- 39.Higashi K, Ueda Y, Seki H, Yuasa K, Oguchi M, Noguchi T, et al. Fluorine-18-FDG PET imaging is negative in bronchioloalveolar lung carcinoma. J Nucl Med. 1998;39(6):1016–20. [PubMed] [Google Scholar]
- 40.Erasmus JJ, McAdams HP, Patz EF, Jr, Coleman RE, Ahuja V, Goodman PC. Evaluation of primary pulmonary carcinoid tumors using FDG PET. AJR Am J Roentgenol. 1998;170(5):1369–73\. doi: 10.2214/ajr.170.5.9574618. [DOI] [PubMed] [Google Scholar]
- 41.Strauss LG. Fluorine-18 deoxyglucose and false-positive results: a major problem in the diagnostics of oncological patients. Eur J Nucl Med. 1996;23(10):1409–15. doi: 10.1007/BF01367602. [DOI] [PubMed] [Google Scholar]
- 42.Pantel K, Izbicki J, Passlick B, Angstwurm M, Häussinger K, Thetter O, et al. Frequency and prognostic significance of isolated tumour cells in bone marrow of patients with non-small-cell lung cancer without overt metastases. Lancet. 1996;347(9002):649–53. doi: 10.1016/s0140-6736(96)91203-9. [DOI] [PubMed] [Google Scholar]
- 43.MacManus MP, Hicks RJ, Matthews JP, Hogg A, McKenzie AF, Wirth A, et al. High rate of detection of unsuspected distant metastases by pet in apparent stage III non-small-cell lung cancer: implications for radical radiation therapy. Int J Radiat Oncol Biol Phys. 2001;50(2):287–93. doi: 10.1016/s0360-3016(01)01477-8. [DOI] [PubMed] [Google Scholar]
- 44.Oliver TW, Jr, Bernardino ME, Miller JI, Mansour K, Greene D, Davis WA. Isolated adrenal masses in nonsmall-cell bronchogenic carcinoma. Radiology. 1984;153(1):217–8. doi: 10.1148/radiology.153.1.6473783. [DOI] [PubMed] [Google Scholar]
- 45.Ettinghausen SE, Burt ME. Prospective evaluation of unilateral adrenal masses in patients with operable non-small-cell lung cancer. J Clin Oncol. 1991;9(8):1462–6. doi: 10.1200/JCO.1991.9.8.1462. [DOI] [PubMed] [Google Scholar]
- 46.Yun M, Kim W, Alnafisi N, Lacorte L, Jang S, Alavi A. 18F-FDG PET in characterizing adrenal lesions detected on CT or MRI. J Nucl Med. 2001;42(12):1795–9. [PubMed] [Google Scholar]
- 47.Fogelman I, Cook G, Israel O, Van der Wall H. Positron emission tomography and bone metastases. Semin Nucl Med. 2005;35(2):135–42. doi: 10.1053/j.semnuclmed.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 48.Abe K, Sasaki M, Kuwabara Y, Koga H, Baba S, Hayashi K, et al. Comparison of 18FDG-PET with 99mTc-HMDP scintigraphy for the detection of bone metastases in patients with breast cancer. Ann Nucl Med. 2005;19(7):573–9. doi: 10.1007/BF02985050. [DOI] [PubMed] [Google Scholar]
- 49.Ludwig V, Komori T, Kolb D, Martin WH, Sandler MP, Delbeke D. Cerebral lesions incidentally detected on 2-deoxy-2-[18F]fluoro-D-glucose positron emission tomography images of patients evaluated for body malignancies. Mol Imaging Biol. 2002;4(5):359–62. doi: 10.1016/s1536-1632(02)00024-0. [DOI] [PubMed] [Google Scholar]
- 50.Paz-Ares LG, De Marinis F, Dediu M, et al. PARAMOUNT: Phase III study of maintenance pemetrexed (pem) plus best supportive care (BSC) versus placebo plus BSC immediately following induction treatment with pem plus cisplatin for advanced non squamous non-small cell lung cancer (NSCLC) [abstract] J Clin Oncol. 2011;29(Suppl 18) Abstract CRA7510. [Google Scholar]
- 51.Weber WA. Use of PET for monitoring cancer therapy and for predicting outcome. J Nucl Med. 2005;46(6):983–95. [PubMed] [Google Scholar]
- 52.Weber WA, Petersen V, Schmidt B, Tyndale-Hines L, Link T, Peschel C, et al. Positron emission tomography in non-small-cell lung cancer: prediction of response to chemotherapy by quantitative assessment of glucose use. J Clin Oncol. 2003;21(14):2651–7. doi: 10.1200/JCO.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 53.Patz EF, Jr, Connolly J, Herndon J. Prognostic value of thoracic FDG PET imaging after treatment for non-small cell lung cancer. AJR Am J Roentgenol. 2000;174(3):769–74. doi: 10.2214/ajr.174.3.1740769. [DOI] [PubMed] [Google Scholar]
- 54.Baum RP, Hellwig D, Mezzetti M. Position of nuclear medicine modalities in the diagnostic workup of cancer patients: lung cancer. Q J Nucl Med Mol Imaging. 2004;48(2):119–42. [PubMed] [Google Scholar]
- 55.Hicks RJ, Kalff V, MacManus MP, Ware RE, McKenzie AF, Matthews JP, et al. The utility of (18)F-FDG PET for suspected recurrent non-small cell lung cancer after potentially curative therapy: impact on management and prognostic stratification. J Nucl Med. 2001;42(11):1605–13. [PubMed] [Google Scholar]
- 56.Hollings N, Shaw P. Diagnostic imaging of lung cancer. Eur Respir J. 2002;19(4):722–42. doi: 10.1183/09031936.02.00280002. [DOI] [PubMed] [Google Scholar]
- 57.Filderman AE, Shaw C, Matthay RA. Lung cancer. Part I: Etiology, pathology, natural history, manifestations, and diagnostic techniques. Invest Radiol. 1986;21(1):80–90. doi: 10.1097/00004424-198601000-00014. [DOI] [PubMed] [Google Scholar]
- 58.Hansen M, Hansen HH, Dombernowsky P. Long-term survival in small cell carcinoma of the lung. JAMA. 1980;244(3):247–50. [PubMed] [Google Scholar]
- 59.Pearlberg JL, Sandler MA, Lewis JW, Jr, Beute GH, Alpern MB. Small-cell bronchogenic carcinoma: CT evaluation. AJR Am J Roentgenol. 1988;150(2):265–8. doi: 10.2214/ajr.150.2.265. [DOI] [PubMed] [Google Scholar]
- 60.Whitley NO, Fuks JZ, McCrea ES, Whitacre M, Masler JA, Whitley JE, et al. Computed tomography of the chest in small cell lung cancer: potential new prognostic signs. AJR Am J Roentgenol. 1984;142(5):885–92. doi: 10.2214/ajr.142.5.885. [DOI] [PubMed] [Google Scholar]
- 61.Henschke CI, Naidich DP, Yankelevitz DF, McGuinness G, McCauley DI, Smith JP, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer. 2001;92(1):153–9. doi: 10.1002/1097-0142(20010701)92:1<153::aid-cncr1303>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 62.Fossella FV, Putnam JB, Komaki R, editors. M.D. Anderson Cancer Care Series. New York: Springer; 2003. Lung Cancer; p. 316. [Google Scholar]
- 63.Eberhard DA, Johnson BE, Amler LC, Goddard AD, Heldens SL, Herbst RS, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. 2005;23(25):5900–9. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 64.Fraumeni JF., Jr Respiratory carcinogenesis: an epidemiologic appraisal. J Natl Cancer Inst. 1975;55(5):1039–46. doi: 10.1093/jnci/55.5.1039. [DOI] [PubMed] [Google Scholar]
- 65.Swanson SJ, Batirel HF. Video-assisted thoracic surgery (VATS) resection for lung cancer. Surg Clin North Am. 2002;82(3):541–59. doi: 10.1016/s0039-6109(02)00015-4. [DOI] [PubMed] [Google Scholar]
- 66.Alam N, Flores RM. Video-assisted thoracic surgery (VATS) lobectomy: the evidence base. JSLS. 2007;11(3):368–74. [PMC free article] [PubMed] [Google Scholar]
- 67.Cappuzzo F, Ligorio C, Toschi L, Rossi E, Trisolini R, Paioli D, et al. EGFR and HER2 gene copy number and response to first-line chemotherapy in patients with advanced non-small cell lung cancer (NSCLC) J Thorac Oncol. 2007;2(5):423–9. doi: 10.1097/01.JTO.0000268676.79872.9b. [DOI] [PubMed] [Google Scholar]
- 68.Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG, et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med. 2010;363(18):1693–703. doi: 10.1056/NEJMoa1006448. [DOI] [PMC free article] [PubMed] [Google Scholar]