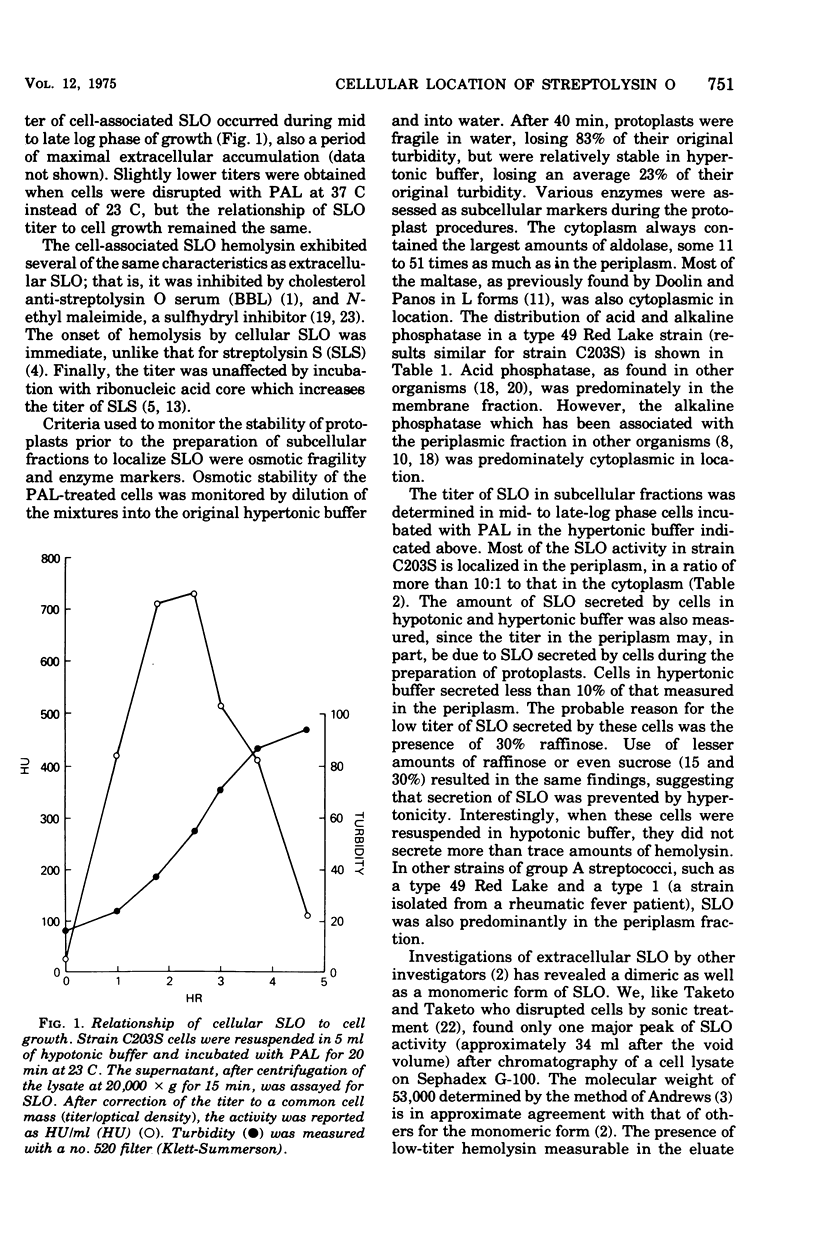
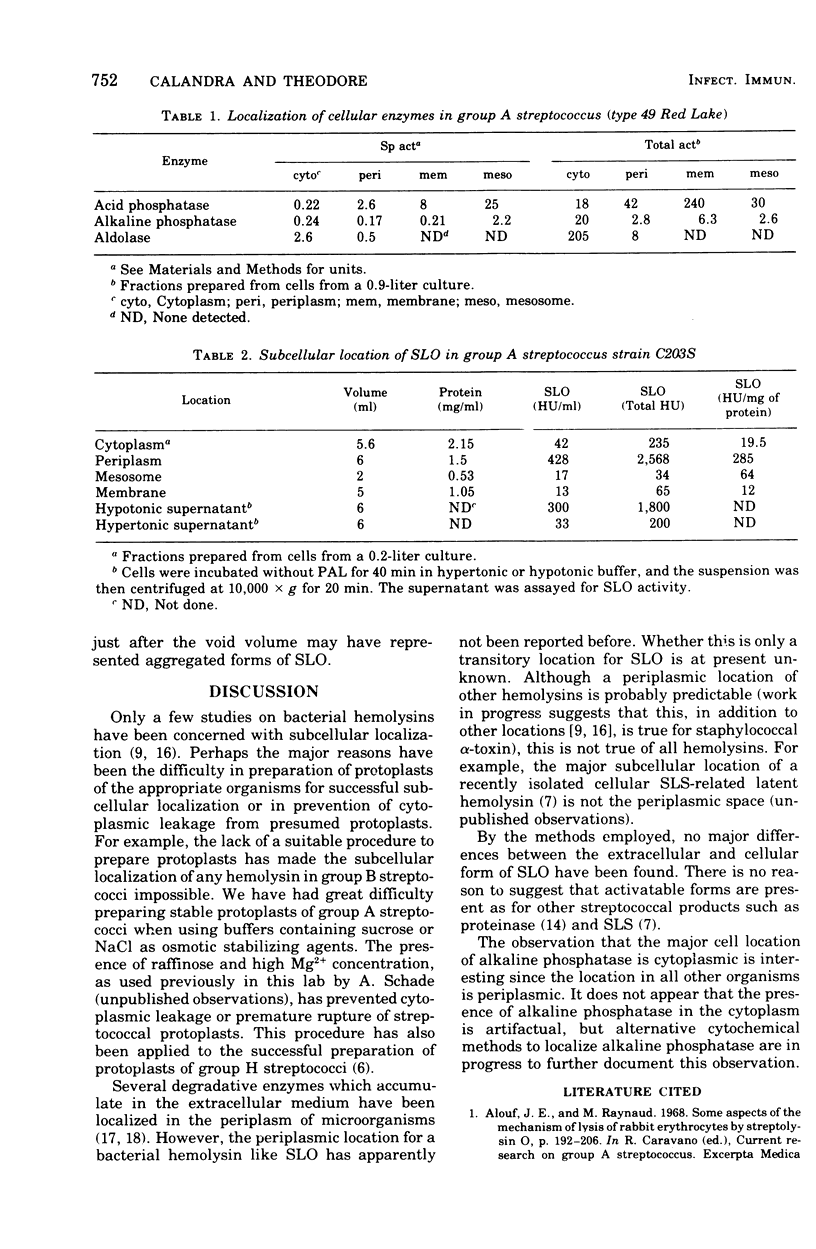
Abstract
Streptolysin O was measured in subcellular fractions of group A streptococci obtained after preparation of protoplasts in a hypertonic buffer containing raffinose. Most of the activity was located in the periplasm (the region between cell wall and membrane) and did not differ in several characteristics from that of extracellular streptolysin O. Of the enzymes used as subcellular markers, aldolase and maltase (cytoplasmic) and acid phosphatase (membrane associated) were in the same fractions as found in other bacteria. However, the location of alkaline phosphatase differed from that of other bacteria in the most of the activity was in cytoplasm rather than in the periplasm.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Raynaud M. Purification and some properties of streptolysin O. Biochimie. 1973;55(10):1187–1193. doi: 10.1016/s0300-9084(74)80322-6. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra G. B., Nugent K. M., Cole R. M. Preparation of protoplasts of group H streptococci (Streptococcus sanguis). Appl Microbiol. 1975 Jan;29(1):90–93. doi: 10.1128/am.29.1.90-93.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calandra G. B., Oginsky E. L. Cellular streptolysin S-related hemolysins of group A Streptococcus C203S. Infect Immun. 1975 Jul;12(1):13–28. doi: 10.1128/iai.12.1.13-28.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng K. J., Costerton J. W. Localization of alkaline phosphatase in three gram-negative rumen bacteria. J Bacteriol. 1973 Oct;116(1):424–440. doi: 10.1128/jb.116.1.424-440.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulter J. R., Mukherjee T. M. Electron microscopic localization of alpha toxin within the staphylococcal cell by ferritin-labeled antibody. Infect Immun. 1971 Nov;4(5):650–655. doi: 10.1128/iai.4.5.650-655.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day D. F., Ingram J. M. Purification and characterization of Pseudomonas aeruginosa alkaline phosphatase. Can J Microbiol. 1973 Oct;19(10):1225–1233. doi: 10.1139/m73-198. [DOI] [PubMed] [Google Scholar]
- Doolin L. E., Panos C. The alpha-glucosidases of Streptococcus pyogenes and derived L form. Biochim Biophys Acta. 1969 Jul 30;184(2):271–280. doi: 10.1016/0304-4165(69)90029-4. [DOI] [PubMed] [Google Scholar]
- FREIMER E. H., KRAUSE R. M., McCARTY M. Studies of L forms and protoplasts of group A streptococci. I. Isolation, growth, and bacteriologic characteristics. J Exp Med. 1959 Dec 1;110:853–874. doi: 10.1084/jem.110.6.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LIU T. Y., ELLIOTT S. D. ACTIVATION OF STREPTOCOCCAL PROTEINASE AND ITS ZYMOGEN BY BACTERIAL CELL WALLS. Nature. 1965 Apr 3;206:33–34. doi: 10.1038/206033a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- McNiven A. C., Arbuthnott J. P. Cell-associated alpha-toxin from Staphylococcus aureus. J Med Microbiol. 1972 Feb;5(1):123–127. doi: 10.1099/00222615-5-1-123. [DOI] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. On the surface localization of enzymes in E. coli. Biochem Biophys Res Commun. 1964 Oct 14;17(3):215–219. doi: 10.1016/0006-291x(64)90386-9. [DOI] [PubMed] [Google Scholar]
- Nugent K. M., Huff E., Cole R. M., Theodore T. S. Cellular location of degradative enzymes in Staphylococcus aureus. J Bacteriol. 1974 Dec;120(3):1012–1016. doi: 10.1128/jb.120.3.1012-1016.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reaveley D. A., Rogers H. J. Some enzymic activities and chemical properties of the mesosomes and cytoplasmic membranes of Bacillus licheniformis 6346. Biochem J. 1969 Jun;113(1):67–79. doi: 10.1042/bj1130067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHWAB J. H. An intracellular hemolysin of group A Streptococci. I. influence of sonic energy and pH on hemolytic potency. J Bacteriol. 1956 Jan;71(1):94–99. doi: 10.1128/jb.71.1.94-99.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taketo A., Taketo Y. Biochemical studies on streptolysin S formation. 3. Intracellular streptolysins. J Biochem. 1965 Jun;57(6):787–792. doi: 10.1093/oxfordjournals.jbchem.a128145. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O II. Relationship of Sulfyhdryl Groups to Activity. Infect Immun. 1971 May;3(5):648–652. doi: 10.1128/iai.3.5.648-652.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]


