Abstract
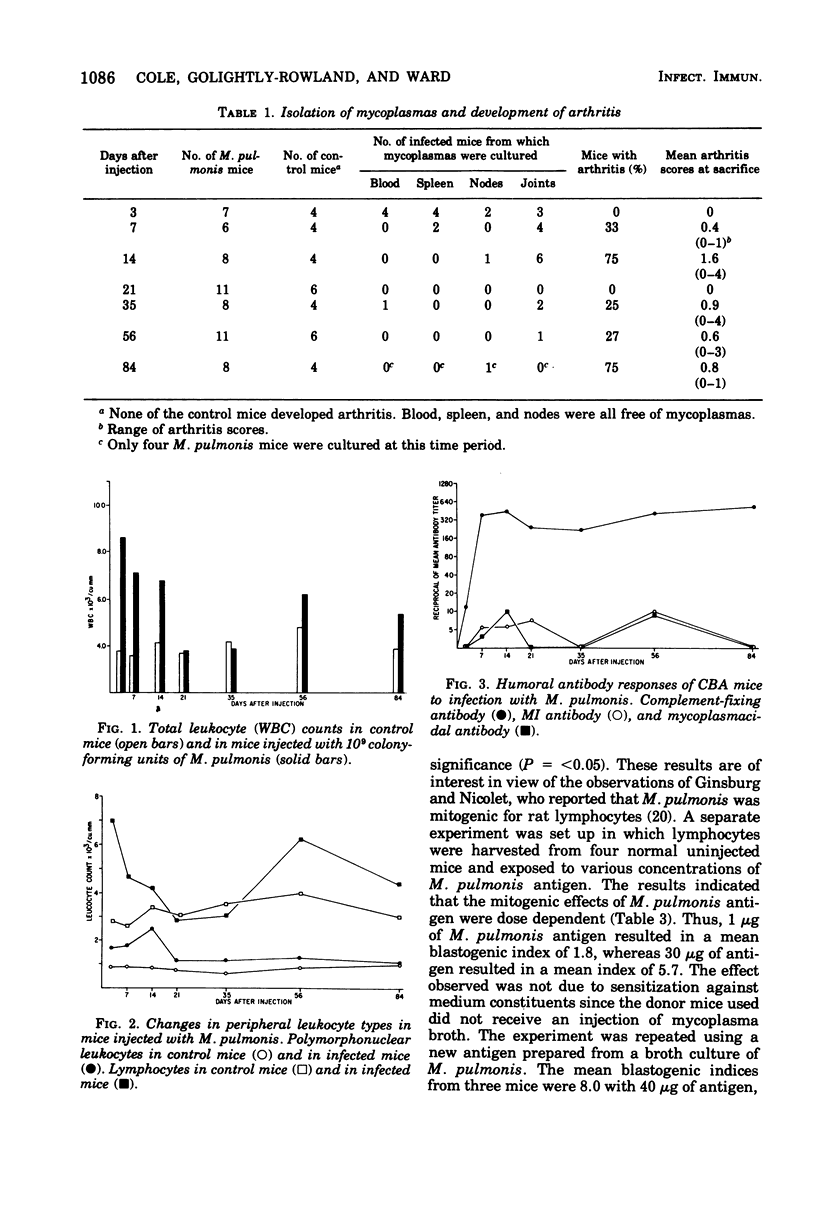
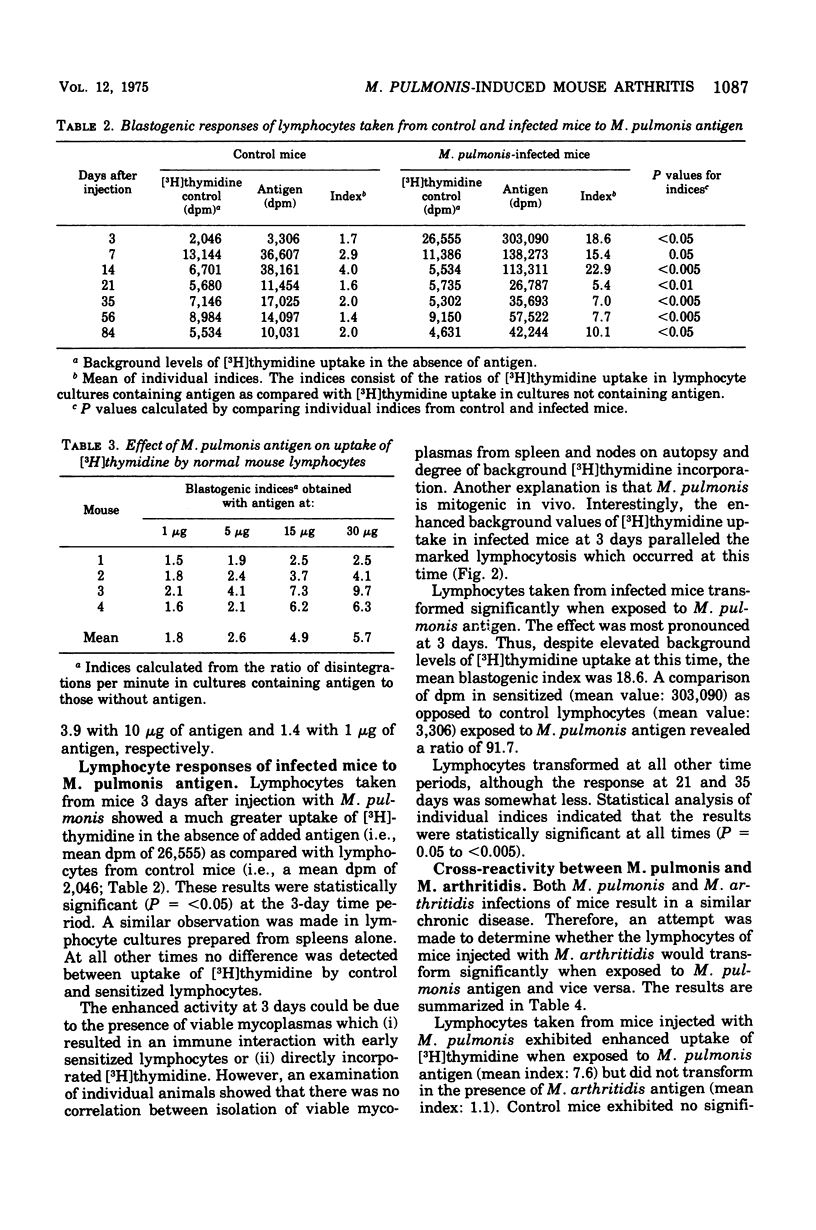
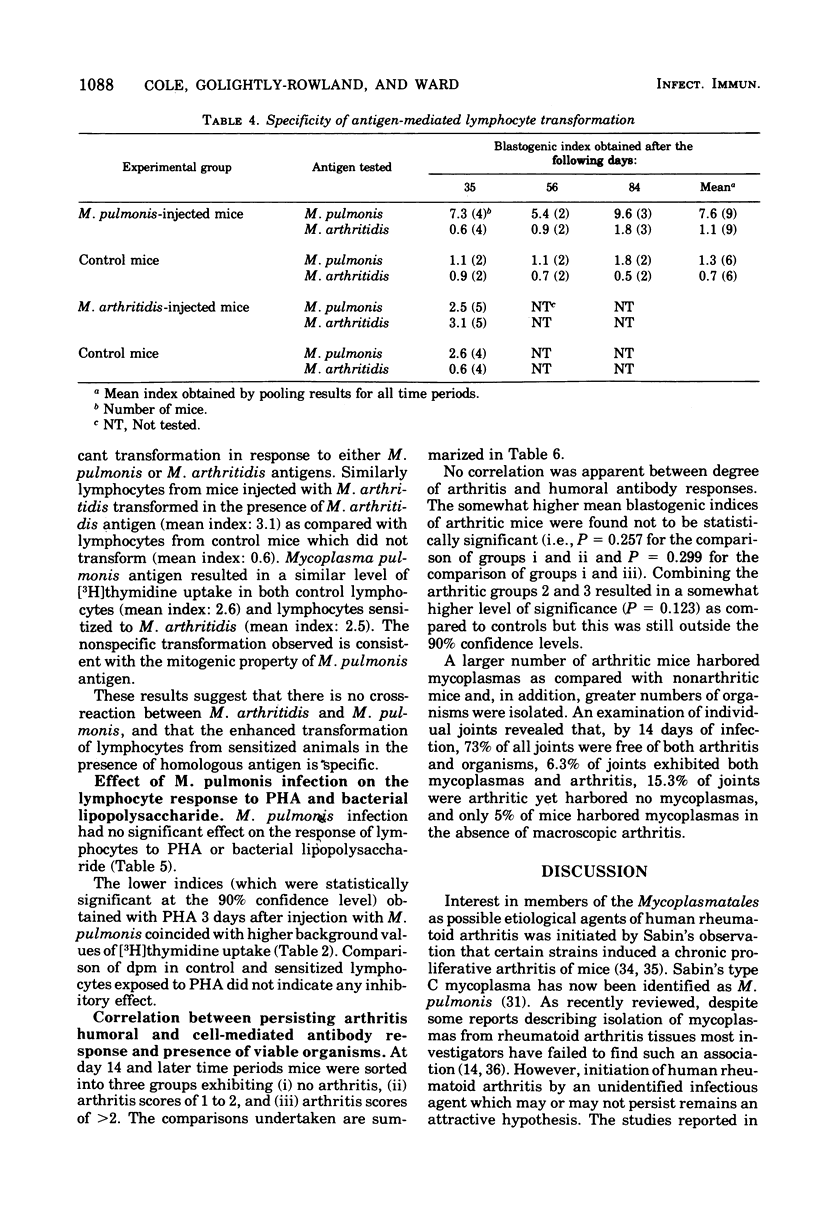
Peak arthritis occurred 14 days after intravenous injection of Mycoplasma pulmonis and persisted in some mice at low levels for 84 days. A marked lymphocytosis occurred during the first week of infection with only a slight increase in polymorphonuclear leukocytes. Complement-fixing antibodies appeared in low titer 3 days after infection and moderate levels persisted for 84 days. The metabolic-inhibiting and mycoplasmacidal antibody responses were absent or minimal. M. pulmonis appeared to be mitogenic for mouse lymphocytes as evidenced by (i) increased uptake of [3H]thymidine for normal lymphocytes exposed to various concentrations of nonviable M. pulmonis antigen, and (ii) a 13-fold increase in [3H]thymidine uptake in lymphocytes taken from mice 3 days after infection with M. pulmonis in the absence of added antigen. Lymphocytes taken from infected mice transformed significantly more at all time periods than control lymphocytes when exposed to M. pulmonis antigen. This response was maximal at 3 days and minimal at 21 to 35 days after infection. Lymphocytes sensitized to M. pulmonis did not transform when exposed to M. arthritidis antigen or vice versa. M. pulmonis infection had no effect upon the mitogenic responses of lymphocytes to phytohemagglutinin or lipopolysaccharide. There was no statistically significant correlation between persistence of arthritis and degree of humor antibody or lymphocyte responses. However, persisting arthritis was associated with a higher incidence of mycoplasma isolations.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson J., Sjöberg O., Möller G. Induction of immunoglobulin and antibody synthesis in vitro by lipopolysaccharides. Eur J Immunol. 1972 Aug;2(4):349–353. doi: 10.1002/eji.1830020410. [DOI] [PubMed] [Google Scholar]
- Barden J. A., Tully J. G. Experimental arthritis in mice with Mycoplasma pulmonis. J Bacteriol. 1969 Oct;100(1):5–10. doi: 10.1128/jb.100.1.5-10.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. An extension of the 51Cr-release assay for the estimation of mouse cytotoxins. Transplantation. 1968 Sep;6(6):761–764. doi: 10.1097/00007890-196809000-00002. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M., HAYFLICK L., BARILE M. F. Growth on artificial medium of an agent associated with atypical pneumonia and its identification as a PPLO. Proc Natl Acad Sci U S A. 1962 Jan 15;48:41–49. doi: 10.1073/pnas.48.1.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill J. F., Cole B. C., Wiley B. B., Ward J. R. Role of Biological Mimicry in the Pathogenesis of Rat Arthritis Induced by Mycoplasma arthritidis. Infect Immun. 1971 Jan;3(1):24–35. doi: 10.1128/iai.3.1.24-35.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassell G. H., Lindsey J. R., Baker H. J. Immune response of pathogen-free mice inoculated intranasally with Mycoplasma pulmonis. J Immunol. 1974 Jan;112(1):124–136. [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis: demonstration of a cell-mediated immune response to mycoplasma antigens in vitro. Infect Immun. 1975 May;11(5):1159–1161. doi: 10.1128/iai.11.5.1159-1161.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Golightly-Rowland L., Ward J. R., Wiley B. B. Immunological response of rodents to murine mycoplasmas. Infect Immun. 1970 Oct;2(4):419–425. doi: 10.1128/iai.2.4.419-425.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Detection and characterization of defective mycoplasmacidal antibody produced by rodents against Mycoplasma arthritidis. Infect Immun. 1973 Aug;8(2):199–207. doi: 10.1128/iai.8.2.199-207.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R. Interaction of Mycoplasma arthritidis and other mycoplasmas with murine peritoneal macrophages. Infect Immun. 1973 May;7(5):691–699. doi: 10.1128/iai.7.5.691-699.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Jones R. S., Cahill J. F. Chronic proliferative arthritis of mice induced by Mycoplasma arthritidis. I. Induction of disease and histopathological characteristics. Infect Immun. 1971 Oct;4(4):344–355. doi: 10.1128/iai.4.4.344-355.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Martin C. H. Hemolysin and peroxide activity of Mycoplasma species. J Bacteriol. 1968 Jun;95(6):2022–2030. doi: 10.1128/jb.95.6.2022-2030.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole B. C., Ward J. R., Smith C. B. Studies on the infectious etiology of rheumatoid arthritis. Arthritis Rheum. 1973 Mar-Apr;16(2):191–198. doi: 10.1002/art.1780160209. [DOI] [PubMed] [Google Scholar]
- Colley D. G., DeWitt C. W. Mixed lymphocyte blastogenesis in response to multiple histocompatibility antigens. J Immunol. 1969 Jan;102(1):107–116. [PubMed] [Google Scholar]
- Colley D. G. Schistosomal egg antigen-induced lymphocyte blastogenesis in experimental murine Schistosoma mansoni infection. J Immunol. 1971 Nov;107(5):1477–1480. [PubMed] [Google Scholar]
- Deeb B. J., Kenny G. E. Characterizion of Mycoplasma pulmonis variants isolated from rabbits. II. Basis for differentiation of antigenic subtypes. J Bacteriol. 1967 Apr;93(4):1425–1429. doi: 10.1128/jb.93.4.1425-1429.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny F. W., Taylor-Robinson D., Allison A. C. The role of thymus-dependent immunity in Mycoplasma pulmonis infections of mice. J Med Microbiol. 1972 Aug;5(3):327–336. doi: 10.1099/00222615-5-3-327. [DOI] [PubMed] [Google Scholar]
- Dutton R. W., Mishell R. I. Cell populations and cell proliferation in the in vitro response of normal mouse spleen to heterologous erythrocytes. Analysis by the hot pulse technique. J Exp Med. 1967 Sep 1;126(3):443–454. doi: 10.1084/jem.126.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsburg H., Nicolet J. Extensive transformation of lymphocytes by a mycoplasma organism. Nat New Biol. 1973 Dec 5;246(153):143–146. doi: 10.1038/newbio246143a0. [DOI] [PubMed] [Google Scholar]
- Golightly-Rowland L., Cole B. C., Ward J. R., Wiley B. B. Effect of Animal Passage on Arthritogenic and Biological Properties of Mycoplasma arthritidis. Infect Immun. 1970 Jun;1(6):538–545. doi: 10.1128/iai.1.6.538-545.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwick H. J., Kalmanson G. M., Fox M. A., Guze L. B. Arthritis in mice due to infection with Mycoplasma pulmonis. I. Clinical and microbiologic features. J Infect Dis. 1973 Oct;128(4):533–540. doi: 10.1093/infdis/128.4.533. [DOI] [PubMed] [Google Scholar]
- Harwick H. J., Kalmanson G. M., Fox M. A., Guze L. B. Mycoplasmal arthritis of the mouse: development of cellular hypersensitivity to normal synovial tissue. Proc Soc Exp Biol Med. 1973 Nov;144(2):561–563. doi: 10.3181/00379727-144-37635. [DOI] [PubMed] [Google Scholar]
- Hayflick L. Tissue cultures and mycoplasmas. Tex Rep Biol Med. 1965 Jun;23(Suppl):285+–285+. [PubMed] [Google Scholar]
- Janossy G., Greaves M. F. Lymphocyte activation. I. Response of T and B lymphocytes to phytomitogens. Clin Exp Immunol. 1971 Oct;9(4):483–498. [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Hirsch J. G. The interaction in vitro of Mycoplasma pulmonis with mouse peritoneal macrophages and L-cells. J Exp Med. 1971 Feb 1;133(2):231–259. doi: 10.1084/jem.133.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones T. C., Yeh S., Hirsch J. G. Studies on attachment and ingestion phases of phagocytosis of Mycoplasma pulmonis by mouse peritoneal macrophages. Proc Soc Exp Biol Med. 1972 Feb;139(2):464–470. doi: 10.3181/00379727-139-36165. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemcke R. M., Forshaw K. A., Fallon R. J. The serological identity of Sabin's murine type C mycoplasma and Mycoplasma pulmonis. J Gen Microbiol. 1969 Sep;58(1):95–98. doi: 10.1099/00221287-58-1-95. [DOI] [PubMed] [Google Scholar]
- Manchee R. J., Taylor-Robinson D. Utilization of neuraminic acid receptors by mycoplasmas. J Bacteriol. 1969 Jun;98(3):914–919. doi: 10.1128/jb.98.3.914-919.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabin A. B. EXPERIMENTAL PROLIFERATIVE ARTHRITIS IN MICE PRODUCED BY FILTRABLE, PLEUROPNEUMONIA-LIKE MICROORGANISMS. Science. 1939 Mar 10;89(2306):228–229. doi: 10.1126/science.89.2306.228. [DOI] [PubMed] [Google Scholar]
- Singer S. H., Ford M., Kirschstein R. L. Respiratory diseases in cyclophosphamide-treated mice. I. Increased virulence of Mycoplasma pulmonis. Infect Immun. 1972 Jun;5(6):953–956. doi: 10.1128/iai.5.6.953-956.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. M., Duthie J. J., Mackay J. M., Marmion B. P., Alexander W. R. Mycoplasmas and rheumatoid arthritis. Ann Rheum Dis. 1974 Jul;33(4):346–352. doi: 10.1136/ard.33.4.346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor-Robinson D., Purcell R. H., Wong D. C., Chanock R. M. A colour test for the measurement of antibody to certain mycoplasma species based upon the inhibition of acid production. J Hyg (Lond) 1966 Mar;64(1):91–104. doi: 10.1017/s0022172400040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor G., Taylor-Robinson D., Slavin G. Effect of immunosuppression on arthritis in mice induced by Mycoplasma pulmonis. Ann Rheum Dis. 1974 Jul;33(4):376–384. doi: 10.1136/ard.33.4.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk A., Glade P. R., Chessin L. N. Blast-like transformation induced in peripheral blood lymphocytes by cellular injury: a comparison of sonication and phytohemagglutinin. Blood. 1969 Feb;33(2):329–340. [PubMed] [Google Scholar]
- Waner J. L., Weller T. H., Kevy S. V. Patterns of cytomegaloviral complement-fixing antibody activity: a longitudinal study of blood donors. J Infect Dis. 1973 May;127(5):538–543. doi: 10.1093/infdis/127.5.538. [DOI] [PubMed] [Google Scholar]


