Abstract
Testosterone (T) stimulates erythropoiesis and regulates iron homeostasis. However, it remains unknown whether the (type II) 5α-reduction of T to dihydrotestosterone (DHT) mediates these androgenic effects, as it does in some other tissues. Our purpose was to determine whether inhibition of type II 5α-reductase (via finasteride) alters red blood cell (RBC) production and serum markers of iron homeostasis subsequent to testosterone-enanthate (TE) administration in older hypogonadal men. Sixty men aged ≥60 yr with serum T <300 ng/dl or bioavailable T <70 ng/dl received treatment with TE (125 mg/wk) vs. vehicle paired with finasteride (5 mg/day) vs. placebo using a 2 × 2 factorial design. Over the course of 12 mo, TE increased RBC count 9%, hematocrit 4%, and hemoglobin 8% while suppressing serum hepcidin 57% (P < 0.001 for all measurements). Most of the aforementioned changes occurred in the first 3 mo of treatment, and finasteride coadministration did not significantly alter any of these effects. TE also reduced serum ferritin 32% (P = 0.002) within 3 mo of treatment initiation without altering iron, transferrin, or transferrin saturation. We conclude that TE stimulates erythropoiesis and alters iron homeostasis independently of the type II 5α-reductase enzyme. These results demonstrate that elevated DHT is not required for androgen-mediated erythropoiesis or for alterations in iron homeostasis that would appear to support iron incorporation into RBCs.
Keywords: androgen, testosterone, finasteride, hypogonadal, hematocrit, hepcidin
iron metabolism and erythropoiesis are intrinsically interrelated because incorporation of iron into the heme group of erythrocytes is necessary for oxygen transport (38). It is well established that testosterone (T) regulates erythropoietic activity in men (32, 33). Clinically, this remains an important concept given the five- to 13-fold higher prevalence of anemia in hypogonadal men compared with their eugonadal counterparts (14) and by the ability of T replacement therapy (TRT) to elevate hematocrit (HCT) and hemoglobin (HGB) in androgen-deficient men (4, 12, 25). In this regard, classical studies have established that androgen-stimulated erythropoiesis is mediated by erythropoietin (EPO) (32, 33), as evidenced by the complete inhibition of erythropoiesis in androgen-treated animals receiving anti-EPO antibody (15, 31). However, androgens may also indirectly support erythropoiesis by altering iron homeostasis (5) via the suppression of hepcidin (3, 4), a negative regulator of the iron transporter ferroportin (38). Hepcidin binds to and internalizes ferroportin within cells, limiting transport of intracellular iron into the circulation (28). Elevated hepcidin underlies anemia of chronic disease (38), and androgen-induced hepcidin suppression increases splenic ferroportin expression, which effectively increases iron absorption and iron incorporation into red blood cells (RBCs) in mice (17). Interestingly, androgen-induced hepcidin suppression occurs in a dose-dependent manner within 7 days of TRT initiation in men (4), preceding the time frame in which HGB is elevated subsequent to androgen administration (4). Additionally, the androgen-induced suppression of hepcidin appears independent of EPO, given that T-stimulated hepcidin suppression persists in mice administered anti-EPO antibody (17). However, the mechanisms underlying this effect require further elucidation.
In addition, many of the biological effects of T are mediated by the type II 5α-reductase enzyme (22) that converts T to dihydrotestosterone (DHT), a more potent and longer-acting androgen (37). This is an important clinical concept given the increasing prevalence of TRT in older hypogonadal men and our recent findings that pharmacological 5α-reductase inhibition prevents prostate enlargement (a primary clinical concern associated with TRT) without inhibiting the musculoskeletal or lipolytic benefits of this therapy (10). Interestingly, hepatocytes (the predominant source of circulating hepcidin) express 5α-reductase (27), and human liver extracts actively convert T to DHT (16), indicating that 5α-reductase influences hepatic androgen metabolism. Additionally, 5α-reductase is thought to be involved in erythropoiesis in mammals (23). However, few studies have examined the influence of type II 5α-reductase on iron homeostasis or androgen-stimulated erythropoiesis in humans. The primary purpose of this study was to determine whether type II 5α-reductase activity influences androgen-stimulated erythropoiesis in elderly hypogonadal men. A secondary purpose was to determine the role of type II 5α-reductase in regulating iron homeostasis subsequent to androgen administration.
METHODS
Study design.
The 52-wk, double-blind, randomized controlled trial (RCT) involved men aged ≥60 yr with a serum T concentration of ≤300 ng/dl or bioavailable fractions of T (BioT) of ≤70 ng/dl. Participants were randomized to receive one of four treatments: 1) vehicle-placebo, 2) vehicle-finasteride, 3) T-enanthate (TE)-placebo, or 4) TE-finasteride using a 2 × 2 factorial design. Treatment lasted for 12 mo and consisted of Proscar (5 mg/day po finasteride), placebo and Delatestryl (125 mg/wk im TE), or vehicle. Both drugs were administered in FDA-approved doses. Proscar and matching placebo were donated by Merck, Delatestryl was donated by Novartis, and matching vehicle was prepared by WestLab Pharmacy (Gainesville, FL). The study was approved by the Institutional Review Board at the University of Florida. All participants provided written, informed consent.
Individuals underwent screening to determine eligibility, including structured medical history, blood acquisition (performed twice between 0800 and 1000, separated by ≥30 min), and a physical exam, as reported previously by us (10). Two blood samples were obtained during screening as per the Endocrine Society's recommendations (6). To ensure participant safety, we excluded individuals who failed the Mini-Cog test indicating dementia, those with a history of prostate or breast cancer, those with severe benign prostatic hyperplasia (BPH), and those with an American Urological Association/International Prostate Symptom Score (AUA/IPSS) of ≥25, class 3 or 4 congestive heart failure, sleep apnea, HCT >49%, serum prostate-specific antigen of ≥2.6 ng/ml, body mass index >35, and certain orthopedic limitations. Additionally, we excluded individuals who had received TRT within the previous 4 wk or finasteride/dutasteride within 6 mo or who were taking Coumadin (a contraindication for im injections). Participants were advised to maintain their current level of physical activity.
Participants underwent thorough health screenings (including blood acquisitions) at baseline and after 3, 6, 9, and 12 mo of treatment, as reported previously (10). Participants were removed from the study if any of the following occurred: HCT ≥54%, serum PSA ≥4.0 ng/ml, an increase in AUA/IPSS of ≥4 points, or if gynecomastia or peripheral edema were noted at physical exam. One subject in the Vehicle-Finasteride group and one in the T group were removed for elevated PSA, one subject in the T-placebo group was removed for sleep apnea, and one subject in the T-finasteride group was removed for urinary symptoms.
Serum biochemistry.
The Pathology and Laboratory Medicine Service, Malcom Randall VAMC, assessed complete blood count (including HCT, HGB, and RBC count), iron, transferrin, ferritin, and total T by automated Cobas electrochemoluminescence immunoassays, which is the Veterans Health Administration (VHA) clinical standard. Additional serum samples were stored at −80°C and analyzed for DHT by liquid chromatography-mass spectrometry/mass spectrometry (Laboratory Corporation of America, Calabasas Hills, CA) and for total estradiol (E2) by ELISA (American Laboratory Products, Salem, NH). The BioT and bioavailable fractions of E2 (BioE2) were assessed by ammonium sulfate precipitation of samples spiked with 3H-T or 3H-E2 (34). The bioactive 25-amino acid residue form of hepcidin was evaluated using a commercially available high-sensitivity ELISA (Bachem, UK) with a reported range of 0–25 ng/ml. Serum samples were diluted 1:10 according to assay instructions and run in duplicate. In our hands, the assay had an intra-assay coefficient of variance (CV) of 11.55% and an interassay CV of 23.7%.
Statistical analyses.
The effects of TE (groups 3 and 4) and finasteride (groups 1 and 2) on RBC, HCT, HGB, and hepcidin were analyzed by the following statistical models that fit the data with three covariance structures considered [i.e., compound symmetry, autoregressive (1), and unstructured], as shown in the equation yijk = α0 + αtrt I(I = 1) + α1I(k = 3) + α2I(k = 6) + α3I(k = 9) + α4 I(I = 1) I(k = 3) + α5 I(I = 1) I(k = 6) + α6 I(I = 1) I(k = 9) + uij + εijk yijk = α0 + αtrt I(I = 1) + α1I(k = 3) + α2I(k = 6) + α3I(k = 9) + uij + εijk, where yijk is the response (i.e., hepcidin change, etc.) of subject j measured at time point k with respect to the change from the baseline under treatment i. I(x = value) is the indicator function (i.e., = 1 if x = value and 0 otherwise). uij is the cluster effect due to subject j under treatment i and assumed to follow N(0,∑), with ∑ being the covariance matrix of 4 × 4 dimension. Three model selection criteria were used to select the best fit model among the six candidate models for the data, i.e., AIC, corrected AIC, and BIC. εijk is the random measurement error following normal distribution. A two-sample t-test was used to compare the changes (baseline: 3 mo) between the groups administered vehicle (groups 1 and 2) vs. TE (groups 3 and 4) or the groups administered finasteride (groups 2 and 4) vs. placebo (groups 1 and 3). Pearson correlation coefficients were used to determine potential associations between variables. Baseline measurements are reported as means ± SE with actual two-sided P values where applicable.
RESULTS
The primary outcomes from this RCT, including musculoskeletal and prostate findings, sex hormone concentrations, and clinical laboratory values, have been reported previously (10). Briefly, TE administration (i.e., the combined effects of groups 3 and 4) elevated nadir T and BioT 1.8- and 2.2-fold, respectively, over baseline. TE also elevated E2 and BioE2, representing 1.7- and 2.2-fold increases, respectively, over baseline. Finasteride coadministration did not significantly affect the aforementioned increases. TE also elevated serum DHT 2.4-fold, and finasteride administration (i.e., the combined effects of treatments 2 and 4) lowered DHT by 65%.
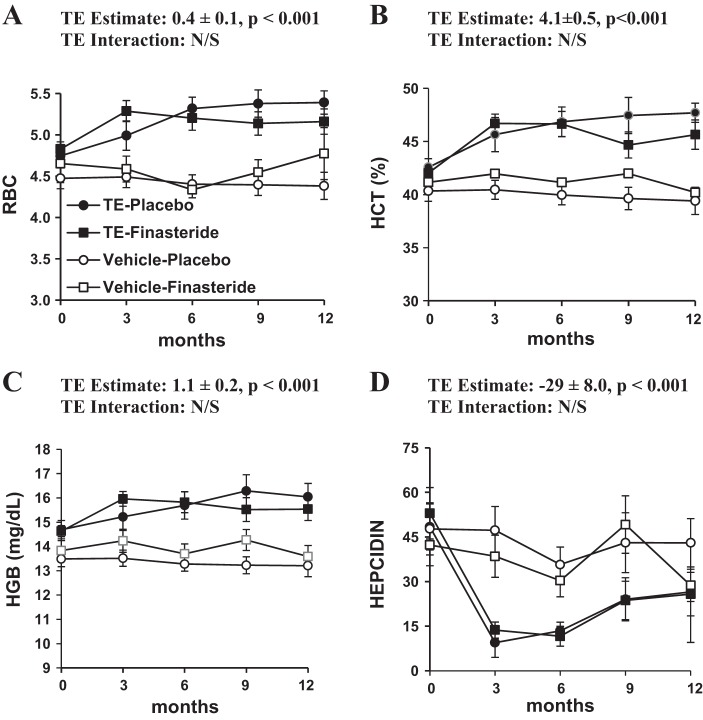
Baseline values for this study are reported in Table 1 and the 12-mo treatment effects in Tables 2 and 3. TE administration increased RBC count 9% (P < 0.001), HCT 4% (P < 0.001), and HGB 8% (P < 0.001) and reduced serum hepcidin 57% (P < 0.001) over 12 mo, with the vast majority of these changes occurring in the first 3 mo of treatment (Fig. 1, A–D). Finasteride coadministration did not significantly alter any of the aforementioned variables. A small increase in HGB that likely resulted from the increase present in the TE-finasteride group was also observed in the finasteride-treated groups (P = 0.034), but no significant effect was seen in the placebo-finasteride group (interaction P = 0.065, trend). Finasteride treatment did not significantly alter any other variables.
Table 1.
Baseline characteristics for participants receiving vehicle-placebo, vehicle-finasteride, TE-placebo, or TE-finasteride
| Variable | Vehicle Placebo | Vehicle Finasteride | TE Placebo | TE Finasteride |
|---|---|---|---|---|
| Age, yr | 70.8 ± 9.7 | 69.5 ± 9.2 | 69.2 ± 8.0 | 64.2 ± 4.8 |
| BMI, kg/m2 | 30.5 ± 3.4 | 28.8 ± 3.9 | 29.5 ± 4.6 | 31.1 ± 2.5 |
| Testosterone, ng/dl | 264 ± 92 | 241 ± 112 | 246 ± 73 | 243 ± 147 |
| BioT, ng/dl | 46 ± 22 | 41 ± 17 | 46 ± 17 | 43 ± 20 |
| DHT, ng/dl | 25 ± 15 | 19 ± 13 | 21 ± 6 | 22 ± 23 |
| RBC, cells/mcl | 4.5 ± 0.5 | 4.7 ± 0.8 | 4.7 ± 0.4 | 4.8 ± 0.4 |
| HCT, % | 40 ± 3.9 | 41 ± 3.9 | 43 ± 3.0 | 42 ± 2.8 |
| HGB, g/dl | 13.5 ± 1.3 | 13.8 ± 1.5 | 14.7 ± 1.4 | 14.6 ± 0.9 |
| Hepcidin, ng/ml | 48 ± 35 | 42 ± 22 | 53 ± 26 | 48 ± 35 |
| Iron, μg/dl | 88 ± 32 | 83 ± 38 | 82 ± 33 | 96 ± 35 |
| Transferrin, μg/dl | 247 ± 37 | 250 ± 25 | 249 ± 31 | 277 ± 36 |
| Transferrin saturation, % | 36 ± 3.3 | 34 ± 4.8 | 34 ± 4.2 | 35 ± 2.89 |
| Ferritin, ng/ml | 142 ± 76 | 154 ± 69 | 186 ± 118 | 222 ± 271 |
Values are means ± SD.
BMI, body mass index; TE, testosterone-enanthate; BioT, bioavailable fractions of testosterone; DHT, dihydrotestosterone; RBC, red blood cells; HCT, hematocrit; HGB, hemoglobin. To convert values for testosterone to nmol/l, multiply by 0.0347. To convert values for DHT to nmol/l, multiply by 0.0344.
Table 2.
Change in RBC count, HCT, HGB, and hepcidin over 12 mo of treatment as a result of TE treatment [i.e., combined effects of treatments 1 and 2 (no TE) vs. 3 and 4 (with TE), respectively]
| Estimate | P Value | Interaction (P Value) | |
|---|---|---|---|
| 12 mo ΔRBC, cells/mcl | 0.4 ± 0.1 | <0.001 | 0.969 |
| 12 mo ΔHCT, % | 4.1 ± 0.5 | <0.001 | 0.458 |
| 12 mo ΔHGB, g/dl | 1.1 ± 0.2 | <0.001 | 0.242 |
| 12 mo ΔHepcidin, ng/ml | −29 ± 8.0 | <0.001 | 0.224 |
Values represent means ± SE. The interaction test was performed only when a significant effect was observed.
Table 3.
Change in RBC count, HCT, HGB, and hepcidin over 12 mo as a result of finasteride treatment [i.e., combined effects of treatments 1 and 3 (no finasteride) vs. 2 and 4 (with finasteride), respectively]
| Estimate | P Value | Interaction (P Value) | |
|---|---|---|---|
| 12-Mo ΔRBC, cells/mcl | 0.2 ± 0.1 | NS | |
| 12-Mo ΔHCT, % | 1.1 ± 0.7 | NS | |
| 12-Mo ΔHGB, g/dl | 0.5 ± 0.2 | 0.034 | 0.065, Trend |
| 12-Mo Δhepcidin, ng/ml | 0.2 ± 8.9 | NS |
Values represent means ± SE. NS, not significant. The interaction test was performed only when a significant effect was observed.
Fig. 1.
Twelve-month change in red blood cell (RBC) count, hematocrit (HCT), hemoglobin (HGB), and hepcidin in those receiving vehicle-placebo, vehicle-finasteride, testosterone enanthate (TE)-placebo, and TE-finasteride. Values represent means ± SE. Some error bars are within range of the symbols. N/S, not significant.
Given the large changes in RBCs and hepcidin occurring primarily within the first 3 mo of TE treatment, we limited subsequent analyses to this time frame. Within the first 3 mo of treatment, TE reduced serum ferritin 32% (P = 0.002), and finasteride coadministration did not significantly alter this effect (Table 4). Neither TE nor finasteride significantly altered serum iron, transferrin, or transferrin saturation. No associations were present between baseline hepcidin and the magnitude of HCT/HGB change or between the 3-mo change in hepcidin and the magnitude of HCT/HGB change in groups receiving TE. The baseline to 3-mo change in E2 was significantly and positively correlated to the changes in RBC count and HGB (see Table 5). However, changes in T, BioT, DHT, and bioestradiol did not show similarly significant correlations.
Table 4.
Change in serum ferritin, iron, and transferrin over the intial 3 mo of treatment as a result of TE treatment [i.e., combined effects of treatments 1 and 2 (no TE) vs. 3 and 4 (with TE), respectively]
| Estimate | P Value | Interaction (P Value) | |
|---|---|---|---|
| 3-Mo Δferritin, ng/ml | −132 ± 32 | 0.002 | 0.450 |
| 3-Mo Δiron, μg/dl | −3 ± 11 | NS | |
| 3-Mo Δtransferrin, mg/dl | 11 ± 8 | NS | |
| 3-Mo Δtransferrin saturation, % | −1.0 ± 4.6 | NS |
Values represent means ± SE. The interaction test was performed only when a significant effect was observed.
Table 5.
Three-month change from baseline in hormones (ChbioT, ChE2, and ChBioE2) to change in blood characteristics (ChRBC, ChHCT, and ChHGB) correlation among TE treatment group
| ChRBC, Pearson Coefficient (P Value) | ChHCT, Pearson Coefficient (P Value) | ChHGB, Pearson Coefficient (P Value) | |
|---|---|---|---|
| ChT | −0.043 (0.846); n = 25 | 0.125 (0.569); n = 24 | 0.110 (0.617); n = 24 |
| ChBioT | 0.109 (0.612); n = 24 | 0.253 (0.244); n = 23 | 0.063 (0.776); n = 23 |
| ChDHT | 0.057 (0.786); n = 25 | 0.183 (0.393); n = 24 | 0.091 (0.673); n = 24 |
| ChE2 | 0.398 (0.049); n = 25 | 0.191 (0.372); n = 24 | 0.415 (0.044); n = 24 |
| ChBioE2 | 0.339 (0.097); n = 25 | 0.227 (0.286); n = 24 | 0.388 (0.061); n = 24 |
T, testosterone; E2, estradiol; BioE2, bioavailable fractions of E2; n = no. of observations.
DISCUSSION
T induces direct biological effects via interactions with androgen receptors (ARs) and/or indirect effects via AR or estrogen receptor (ER) activation following 5α-reduction to DHT or aromatization to E2. In this regard, T functions as a hormone and as a prohormone for more potent androgenic and estrogenic sex steroids. As such, determining the mechanism(s) through which administered T produces tissue- and/or cell-specific effects remains biologically significant and clinically important. One of the well-established functions of T is the regulation of erythropoiesis (32, 33). However, it remains unknown whether the 5α-reduction of T to DHT mediates the effects of androgens on RBC production and iron homeostasis. The primary findings of this study are that finasteride (a type II 5α-reductase inhibitor) does not significantly inhibit T-induced erythropoiesis or androgen-mediated alterations in iron homeostasis. Specifically, TE administration elevated RBC and HGB production independently of finasteride, and finasteride (alone) did not significantly reduce HCT despite a >65% reduction in circulating DHT. In addition, we provide the first evidence demonstrating that type II 5α-reductase activity is not required for T-induced hepcidin suppression in elderly hypogonadal men. We also observed that TE administration resulted in suppressed serum ferritin without alterations in serum iron or transferrin. Collectively, these findings suggest that T regulates erythropoiesis and alters iron homeostasis in a manner that may not require action of the type II 5α-reductase enzyme or elevated systemic DHT.
Recently, our laboratory (10) and others (2, 7, 29) have demonstrated that the musculoskeletal and lipolytic benefits of TRT do not require action of the type II 5α-reductase enzyme or systemically elevated DHT. In contrast, prostate enlargement (2, 8–10, 26, 36) and other putative side effects resulting from androgen administration (e.g., male-pattern baldness or acne) (18) are mediated primarily by type II 5α-reductase. However, the role of the type II 5α-reductase enzyme in mediating androgen-induced erythropoiesis has received little attention in the literature. Herein, we report that 125 mg/wk TE (a supraphysiological TRT dose that is within the FDA-approved range) increases RBC production in older hypogonadal men, which supports meta-analysis data indicating an average HCT increase of 3.2% in men receiving a range of doses and various forms of TRT (13). Additionally, our results extend the above-mentioned findings by demonstrating that finasteride coadministration does not significantly inhibit TE-induced erythropoiesis in older hypogonadal men, which increases the clinical relevance of this combination pharmacological therapy because hypogonadal men present with an increased prevalence of anemia compared with age-matched eugonadal men (14) and because older men exhibit greater HCT elevations in response to TRT than younger men (12). However, TRT also increases the risk for polycythemia (i.e., HCT >54%), which is the most common side effect associated with this therapy (11) and which potentially increases stroke risk (35). Importantly, the incidence of polycythemia remained very low within the cohort of men receiving TE in our study despite the fact that we administered TE in a dose that produces transient supraphysiological T concentrations, and in all cases HCT returned to baseline following discontinuation of TRT.
Red blood cell production also requires adequate iron availability. However, the ability of T to suppress hepcidin (a negative regulator of the iron transporter ferroportin) was only recently identified as a mechanism through which androgens increase iron absorption and iron incorporation into RBCs (17, 24). Herein, we observed that TE suppressed serum hepcidin in a magnitude that was similar to previous clinical trials (3, 4). Additionally, our results are the first to indicate that finasteride does not significantly interfere with this effect, demonstrating that type II 5α-reductase is not a mediator of T-stimulated hepcidin suppression. Interestingly, we also observed a large reduction in serum ferritin that occurred within 3 mo of TE administration and corroborates the findings of others (4), suggesting that T increases iron utilization, likely as a result of increased erythropoiesis. Regardless, serum iron, transferrin, and transferrin saturation, common clinical markers of iron availability, were not altered significantly within this time frame.
Several recent clinical trials have attempted to identify serum markers, which when measured at baseline will predict the magnitude of subsequent T-induced increases in HCT/HGB. Such a marker would be valuable given that polycthemia is the most common adverse event in men undergoing TRT and is a predisposing factor for cerebrovascular events. In this regard, Bachman et al. (3) reported that men with the highest quartile of hepcidin change subsequent to TRT experienced the greatest risk for erythrocytosis. In contrast to these findings, we observed no associations between baseline hepcidin or the 3-mo change in hepcidin and the change in HCT/HGB in men receiving TE. As such, we are unaware of any marker that has consistently been shown to predict the magnitude of androgen-stimulated erythropoiesis prior to TRT initiation, which underlies the continued necessity to exclude men with elevated basal HCT (i.e., >50%) from TRT and the importance for regular HCT monitoring throughout the duration of TRT as recommended by the Endocrine Society Clinical Guidelines (6).
Interestingly, we also observed that TE administration elevated circulating E2 and BioE2 and that the magnitude of change in E2 was correlated to the increases in RBC count and HGB. These findings raise the possibility that estrogens may mediate several of the effects we observed. In this regard, estrogen has been shown to suppress hepcidin, and an estrogen response element is present in the promoter region of the hepcidin gene (20, 21). However, we find it highly unlikely that E2 mediated the erythropoietic effects of T given that T administration results in elevated RBC count and HGB in aromatase-deficient men (30) and that RBC count and HGB are elevated following T plus letrozole (a potent aromatase inhibitor) treatment in boys with constitutional delay of puberty (19). Similarly, preclinical evidence from our laboratory indicates that trenbolone (a nonaromatizable and non-5α-reducible T analog) and TE elevate HGB in an identical manner in orchiectomized rats (26, 36). Together, these results demonstrate that aromatase activity is not necessary for androgen-stimulated erythropoiesis, although the possibility remains that the suppression of hepcidin was at least partially influenced by the elevated E2 following TE administration.
One limitation of our study is that we did not evaluate serum EPO. Several previous clinical trials have failed to detect alterations in serum EPO following TRT (25) even when T is administered well above the physiological range (12). However, others have reported that fluoxymesterone (an orally active synthetic androgen) produced a five- to 10-fold increase in urinary EPO within 4 days in both anemic and hypogonadal men (1), and a number of classical studies have demonstrated that anti-EPO antibody ablates androgen-stimulated erythropoiesis in animals (32, 33), which demonstrates that EPO is required for androgen-induced erythropoiesis. The inconsistencies in the aforementioned studies may be explained by the rather transient nature of androgen-stimulated EPO synthesis and by the high degree of variability in EPO between individuals. In this regard, Bachman et al. (4) recently observed elevated circulating EPO in older hypogonadal men 1 mo after TRT initiation, with values gradually declining toward baseline after several months. In contrast, we find it unlikely that androgen-stimulated EPO production mediates the hepcidin suppression resulting from TRT, because hepcidin is rapidly suppressed after T administration (3) and T-stimulated hepcidin suppression persists in mice administered anti-EPO antibody (17). Regardless, some controversy remains surrounding the mechanism(s) through which androgens initially stimulate and then subsequently maintain erythropoiesis, especially in the absence of continually elevated EPO.
In conclusion, we provide the first-ever evidence indicating that finasteride coadministration does not prevent T-stimulated erythropoiesis in older hypogonadal men and that elevated circulating DHT is not required for androgen-induced alterations in iron homeostasis. In this manner, our results provide further evidence supporting the viability of T plus finasteride coadministration as an alternative to traditional TRT, especially given the high prevalence of anemia in hypogonadal elderly men compared with their eugonadal counterparts. Future research focused on evaluating the mechanism(s) underlying androgen-stimulated erythropoiesis and androgen-induced alterations in iron homeostasis remains warranted and may provide insight into novel markers that predict the magnitude of androgen-stimulated erythropoiesis prior the to initiation of TRT.
GRANTS
This study was supported in part by the Merck-Investigator-Initiated Studies Program (Merck).
DISCLOSURES
The authors have nothing to disclose.
AUTHOR CONTRIBUTIONS
L.A.B., J.F.Y., J.R.M., D.T.B., M.M., and S.E.B. conception and design of research; L.A.B., J.F.Y., C.F.C., and D.T.B. performed experiments; L.A.B., J.F.Y., C.F.C., B.Z., J.J.S., and S.E.B. analyzed data; L.A.B., J.F.Y., J.J.S., and S.E.B. interpreted results of experiments; L.A.B., J.F.Y., and S.E.B. prepared figures; L.A.B., J.F.Y., C.F.C., J.R.M., D.T.B., M.M., B.Z., and J.J.S. edited and revised manuscript; L.A.B., J.F.Y., C.F.C., J.R.M., D.T.B., M.M., B.Z., J.J.S., and S.E.B. approved final version of manuscript; S.E.B. drafted manuscript.
ACKNOWLEDGMENTS
This work was supported by a Veteran's Health Administration Clinical Services Research & Development Merit Award to S. E. Borst and by grant 1UL1-TR-000064 from the National Center for Advancing Translational Science, National Institutes of Health.
REFERENCES
- 1.Alexanian R, Vaughn WK, Ruchelman MW. Erythropoietin excretion in man following androgens. J Lab Clin Med 70: 777–785, 1967 [PubMed] [Google Scholar]
- 2.Amory JK, Watts NB, Easley KA, Sutton PR, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone or testosterone with finasteride increases bone mineral density in older men with low serum testosterone. J Clin Endocrinol Metab 89: 503–510, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Bachman E, Feng R, Travison T, Li M, Olbina G, Ostland V, Ulloor J, Zhang A, Basaria S, Ganz T, Westerman M, Bhasin S. Testosterone suppresses hepcidin in men: a potential mechanism for testosterone-induced erythrocytosis. J Clin Endocrinol Metab 95: 4743–4747, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bachman E, Travison TG, Basaria S, Davda MN, Guo W, Li M, Connor Westfall J, Bae H, Gordeuk V, Bhasin S. Testosterone induces erythrocytosis via increased erythropoietin and suppressed hepcidin: evidence for a new erythropoietin/hemoglobin set point. J Gerontol A Biol Sci Med Sci 69: 725–735, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beggs LA, Borst SE, Yarrow JF. Testosterone regulates hepcidin. J Clin Invest 2013. e-letter: http://www.jci.org/eletters/view/67225#sec1.
- 6.Bhasin S, Cunningham GR, Hayes FJ, Matsumoto AM, Snyder PJ, Swerdloff RS, Montori VM; Task Force, Endocrine Society. Testosterone therapy in men with androgen deficiency syndromes: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 95: 2536–2559, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Bhasin S, Travison TG, Storer TW, Lakshman K, Kaushik M, Mazer NA, Ngyuen AH, Davda MN, Jara H, Aakil A, Anderson S, Knapp PE, Hanka S, Mohammed N, Daou P, Miciek R, Ulloor J, Zhang A, Brooks B, Orwoll K, Hede-Brierley L, Eder R, Elmi A, Bhasin G, Collins L, Singh R, Basaria S. Effect of testosterone supplementation with and without a dual 5alpha-reductase inhibitor on fat-free mass in men with suppressed testosterone production: a randomized controlled trial. JAMA 307: 931–939, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Borst SE, Conover CF, Carter CS, Gregory CM, Marzetti E, Leeuwenburgh C, Vandenborne K, Wronski TJ. Anabolic effects of testosterone are preserved during inhibition of 5α-reductase. Am J Physiol Endocrinol Metab 293: E507–E514, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Borst SE, Lee JH, Conover CF. Inhibition of 5α-reductase blocks prostate effects of testosterone without blocking anabolic effects. Am J Physiol Endocrinol Metab 288: E222–E227, 2005 [DOI] [PubMed] [Google Scholar]
- 10.Borst SE, Yarrow JF, Conover CF, Nseyo U, Meuleman JR, Lipinska JA, Braith RW, Beck DT, Martin JS, Morrow M, Roessner S, Beggs LA, McCoy SC, Cannady DF, 2nd, Shuster JJ. Musculoskeletal and prostate effects of combined testosterone and finasteride administration in older hypogonadal men: a randomized, controlled trial. Am J Physiol Endocrinol Metab 306: E433–E442, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Calof OM, Singh AB, Lee ML, Kenny AM, Urban RJ, Tenover JL, Bhasin S. Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60: 1451–1457, 2005 [DOI] [PubMed] [Google Scholar]
- 12.Coviello AD, Kaplan B, Lakshman KM, Chen T, Singh AB, Bhasin S. Effects of graded doses of testosterone on erythropoiesis in healthy young and older men. J Clin Endocrinol Metab 93: 914–919, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Balsells MM, Murad MH, Lane M, Lampropulos JF, Albuquerque F, Mullan RJ, Agrwal N, Elamin MB, Gallegos-Orozco JF, Wang AT, Erwin PJ, Bhasin S, Montori VM. Clinical review 1: Adverse effects of testosterone therapy in adult men: a systematic review and meta-analysis. J Clin Endocrinol Metab 95: 2560–2575, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Ferrucci L, Maggio M, Bandinelli S, Basaria S, Lauretani F, Ble A, Valenti G, Ershler WB, Guralnik JM, Longo DL. Low testosterone levels and the risk of anemia in older men and women. Arch Intern Med 166: 1380–1388, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fried W, Gurney CW. The erythropoietic-stimulating effects of androgens. Ann NY Acad Sci 149: 356–365, 1968 [DOI] [PubMed] [Google Scholar]
- 16.Granata OM, Cocciadifero L, Campisi I, Miceli V, Montalto G, Polito LM, Agostara B, Carruba G. Androgen metabolism and biotransformation in nontumoral and malignant human liver tissues and cells. J Steroid Biochem Mol Biol 113: 290–295, 2009 [DOI] [PubMed] [Google Scholar]
- 17.Guo W, Bachman E, Li M, Roy CN, Blusztajn J, Wong S, Chan SY, Serra C, Jasuja R, Travison TG, Muckenthaler MU, Nemeth E, Bhasin S. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell 12: 280–291, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hartgens F, Kuipers H. Effects of androgenic-anabolic steroids in athletes. Sports Med 34: 513–554, 2004 [DOI] [PubMed] [Google Scholar]
- 19.Hero M, Wickman S, Hanhijarvi R, Siimes MA, Dunkel L. Pubertal upregulation of erythropoiesis in boys is determined primarily by androgen. J Pediatr 146: 245–252, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Hou Y, Zhang S, Wang L, Li J, Qu G, He J, Rong H, Ji H, Liu S. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene 511: 398–403, 2012 [DOI] [PubMed] [Google Scholar]
- 21.Ikeda Y, Tajima S, Izawa-Ishizawa Y, Kihira Y, Ishizawa K, Tomita S, Tsuchiya K, Tamaki T. Estrogen regulates hepcidin expression via GPR30-BMP6-dependent signaling in hepatocytes. PLoS One 7: e40465, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jin Y, Penning TM. Steroid 5alpha-reductases and 3alpha-hydroxysteroid dehydrogenases: key enzymes in androgen metabolism. Best Pract Res Clin Endocrinol Metab 15: 79–94, 2001 [DOI] [PubMed] [Google Scholar]
- 23.Langlois VS, Zhang D, Cooke GM, Trudeau VL. Evolution of steroid-5alpha-reductases and comparison of their function with 5beta-reductase. Gen Comp Endocrinol 166: 489–497, 2010 [DOI] [PubMed] [Google Scholar]
- 24.Latour C, Kautz L, Besson-Fournier C, Island ML, Canonne-Hergaux F, Loreal O, Ganz T, Coppin H, Roth MP. Testosterone perturbs systemic iron balance through activation of epidermal growth factor receptor signaling in the liver and repression of hepcidin. Hepatology 59: 683–694, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Maggio M, Snyder PJ, Ceda GP, Milaneschi Y, Luci M, Cattabiani C, Masoni S, Vignali A, Volpi R, Lauretani F, Peachey H, Valenti G, Cappola AR, Longo D, Ferrucci L. Is the haematopoietic effect of testosterone mediated by erythropoietin? The results of a clinical trial in older men. Andrology 1: 24–28, 2013 [DOI] [PubMed] [Google Scholar]
- 26.McCoy SC, Yarrow JF, Conover CF, Borsa PA, Tillman MD, Conrad BP, Pingel JE, Wronski TJ, Johnson SE, Kristinsson HG, Ye F, Borst SE. 17β-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone 51: 667–673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meynard D, Babitt JL, Lin HY. The liver: conductor of systemic iron balance. Blood 123: 168–176, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemeth E, Tuttle MS, Powelson J, Vaughn MB, Donovan A, Ward DM, Ganz T, Kaplan J. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science 306: 2090–2093, 2004 [DOI] [PubMed] [Google Scholar]
- 29.Page ST, Amory JK, Bowman FD, Anawalt BD, Matsumoto AM, Bremner WJ, Tenover JL. Exogenous testosterone (T) alone or with finasteride increases physical performance, grip strength, and lean body mass in older men with low serum T. J Clin Endocrinol Metab 90: 1502–1510, 2005 [DOI] [PubMed] [Google Scholar]
- 30.Rochira V, Zirilli L, Madeo B, Maffei L, Carani C. Testosterone action on erythropoiesis does not require its aromatization to estrogen: Insights from the testosterone and estrogen treatment of two aromatase-deficient men. J Steroid Biochem Mol Biol 113: 189–194, 2009 [DOI] [PubMed] [Google Scholar]
- 31.Schooley JC. Inhibition of erythropoietic stimulation by testosterone in polycythemic mice receiving anti-erythropoietin. Proc Soc Exp Biol Med 122: 402–403, 1966 [DOI] [PubMed] [Google Scholar]
- 32.Shahani S, Braga-Basaria M, Maggio M, Basaria S. Androgens and erythropoiesis: past and present. J Endocrinol Invest 32: 704–716, 2009 [DOI] [PubMed] [Google Scholar]
- 33.Shahidi NT. Androgens and erythropoiesis. N Engl J Med 289: 72–80, 1973 [DOI] [PubMed] [Google Scholar]
- 34.Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab 84: 3666–3672, 1999 [DOI] [PubMed] [Google Scholar]
- 35.Vigen R, O'Donnell CI, Baron AE, Grunwald GK, Maddox TM, Bradley SM, Barqawi A, Woning G, Wierman ME, Plomondon ME, Rumsfeld JS, Ho PM. Association of testosterone therapy with mortality, myocardial infarction, and stroke in men with low testosterone levels. JAMA 310: 1829–1836, 2013 [DOI] [PubMed] [Google Scholar]
- 36.Yarrow JF, Conover CF, McCoy SC, Lipinska JA, Santillana CA, Hance JM, Cannady DF, VanPelt TD, Sanchez J, Conrad BP, Pingel JE, Wronski TJ, Borst SE. 17β-Hydroxyestra-4,9,11-trien-3-one (trenbolone) exhibits tissue selective anabolic activity: effects on muscle, bone, adiposity, hemoglobin, and prostate. Am J Physiol Endocrinol Metab 300: E650–E660, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yarrow JF, McCoy SC, Borst SE. Intracrine and myotrophic roles of 5alpha-reductase and androgens: a review. Med Sci Sports Exerc 44: 818–826, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao N, Zhang AS, Enns CA. Iron regulation by hepcidin. J Clin Invest 123: 2337–2343, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]