Abstract
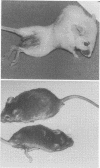
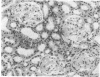
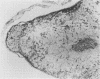
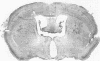
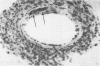
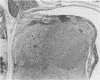
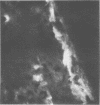
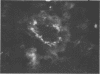
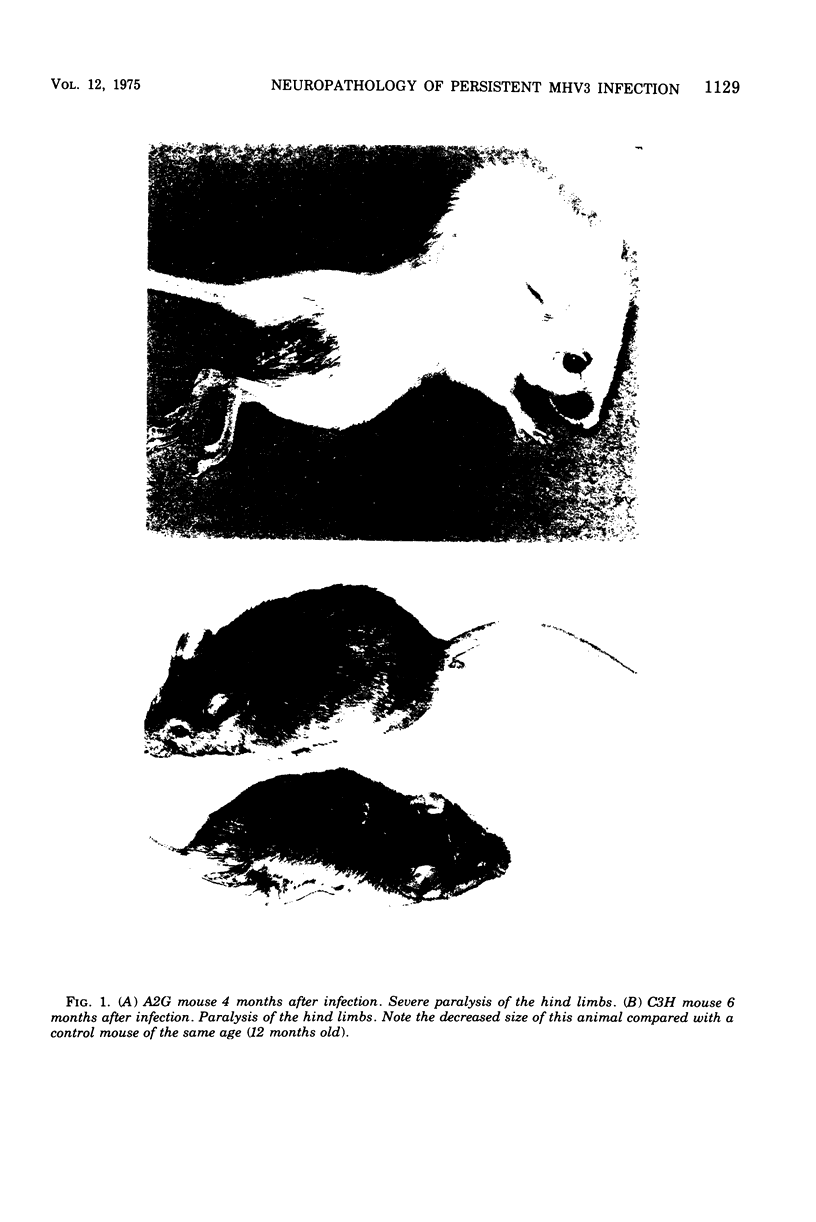
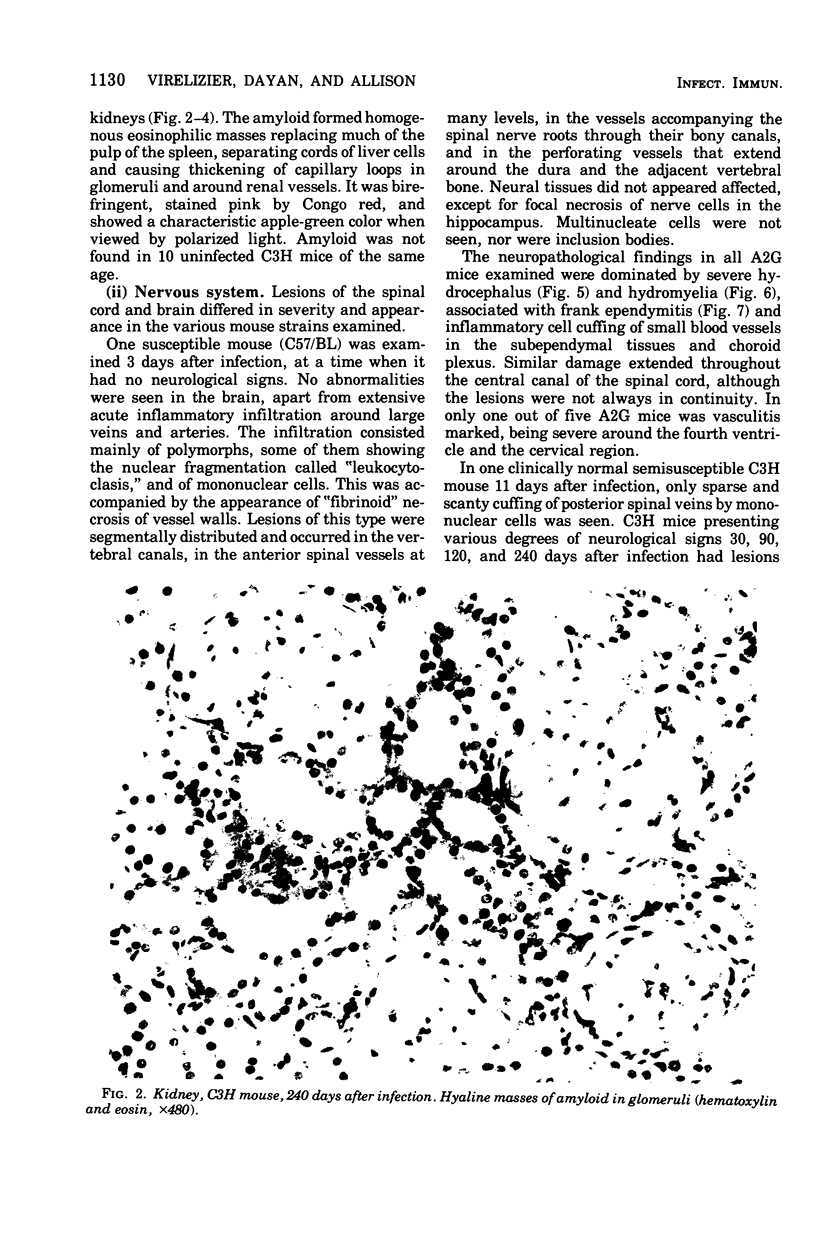
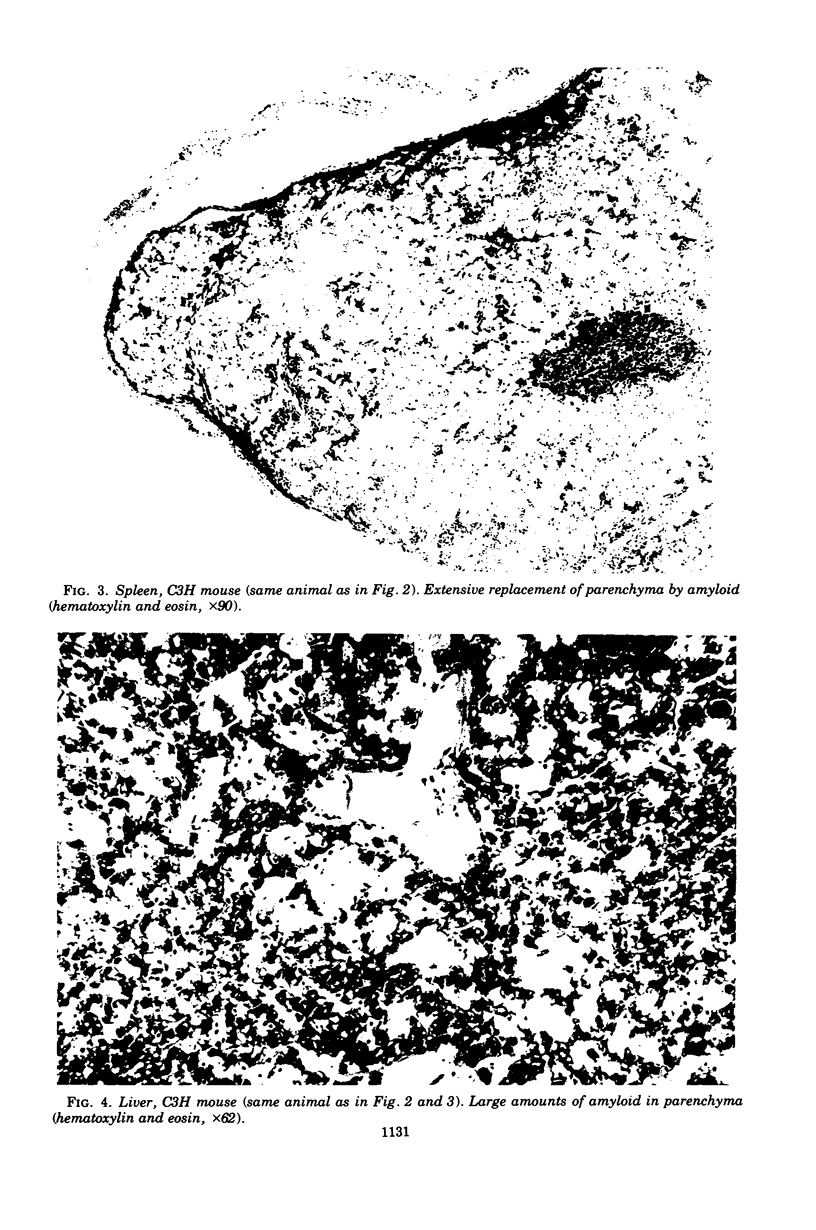
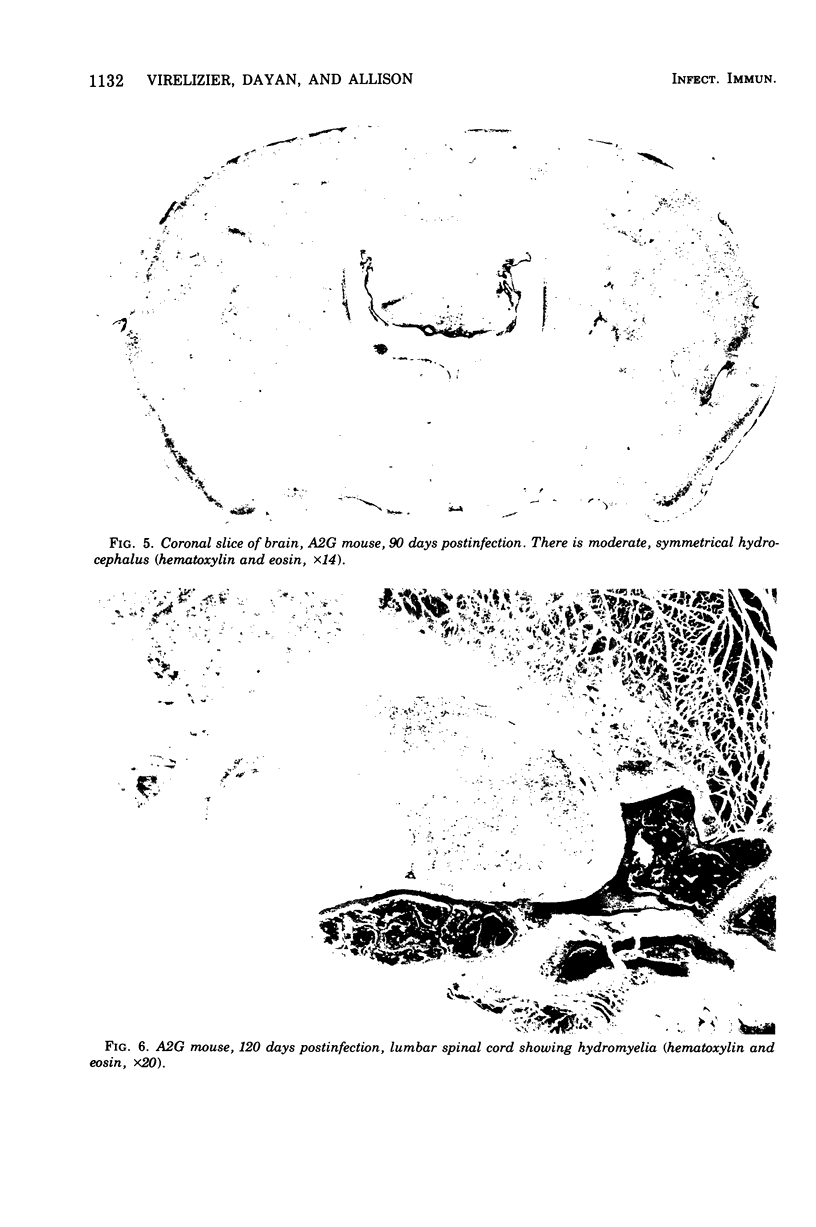
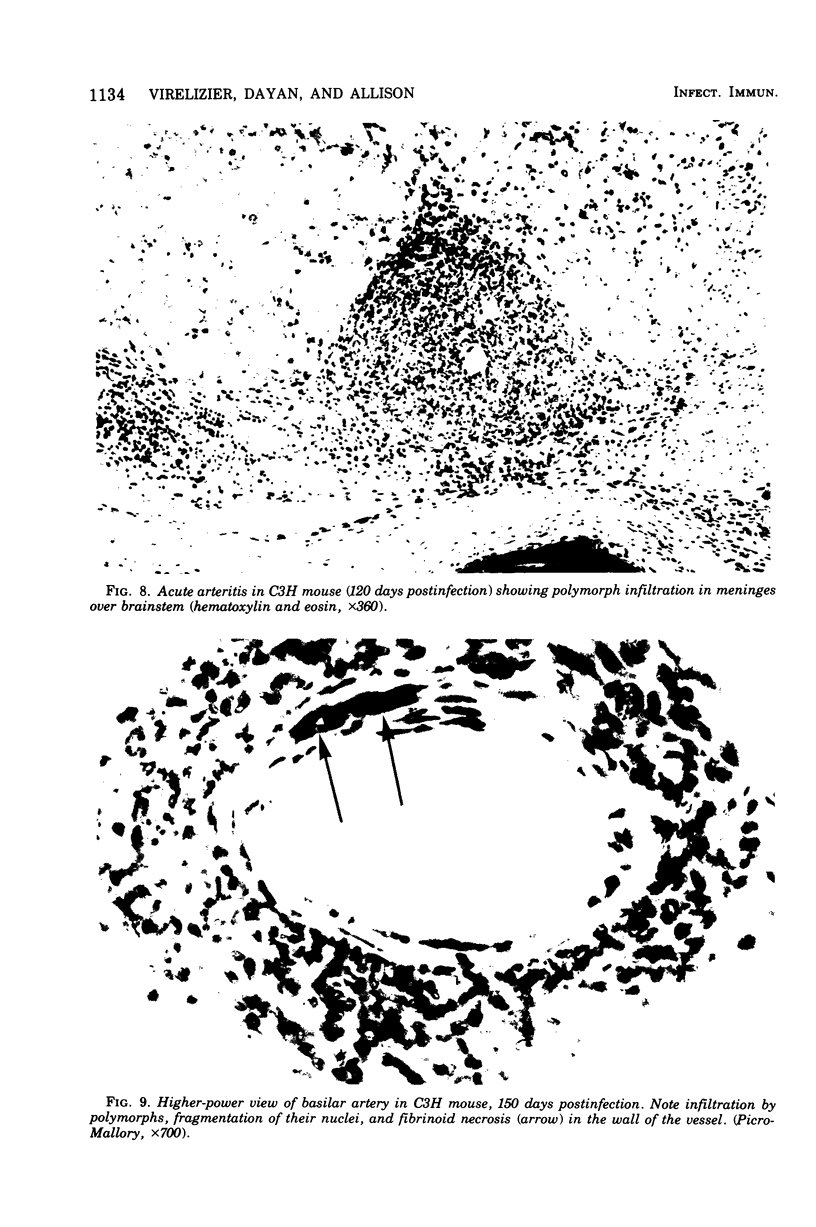
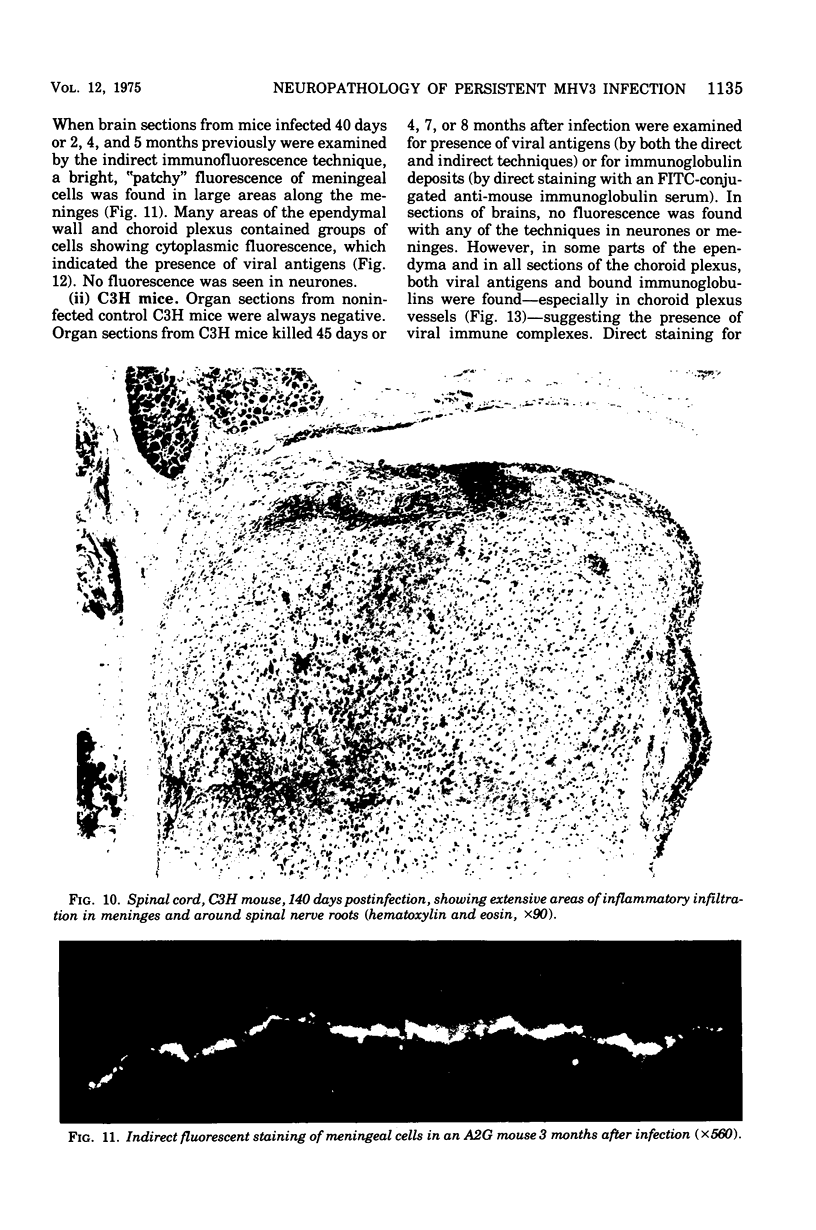
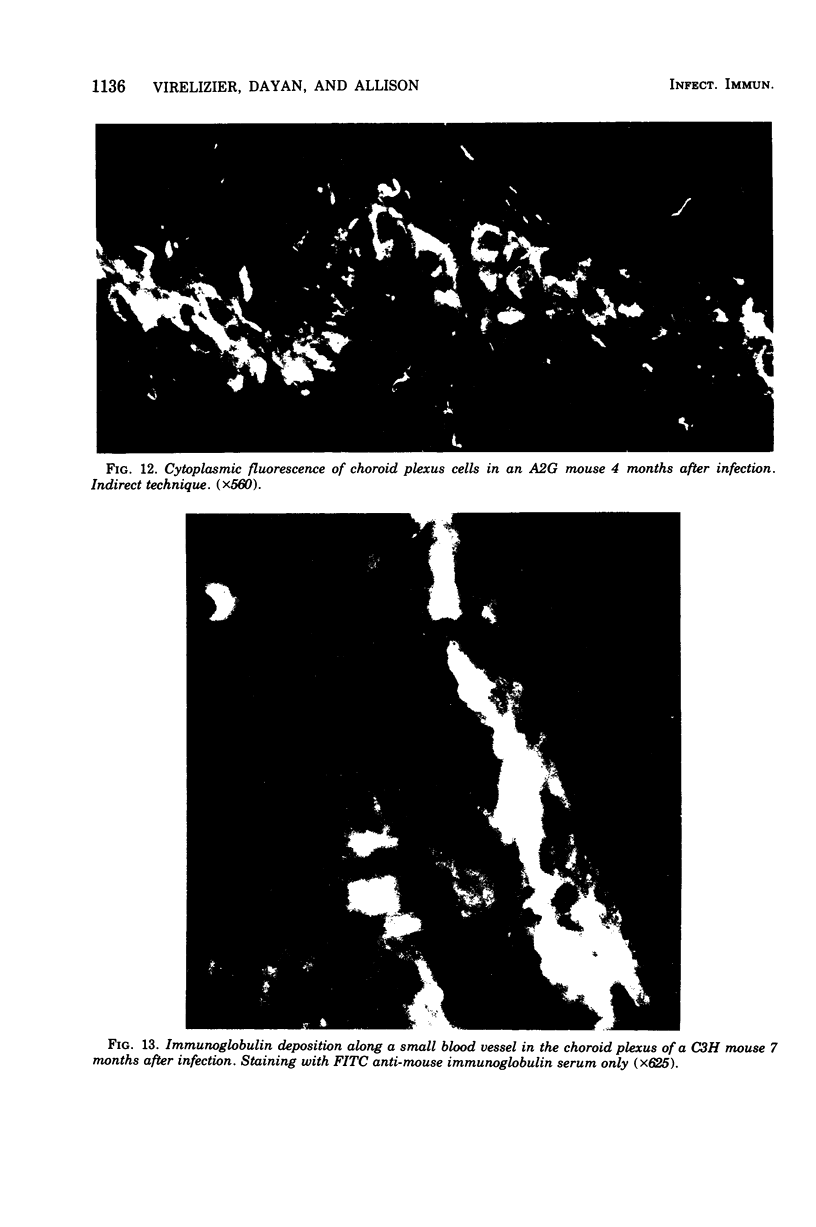
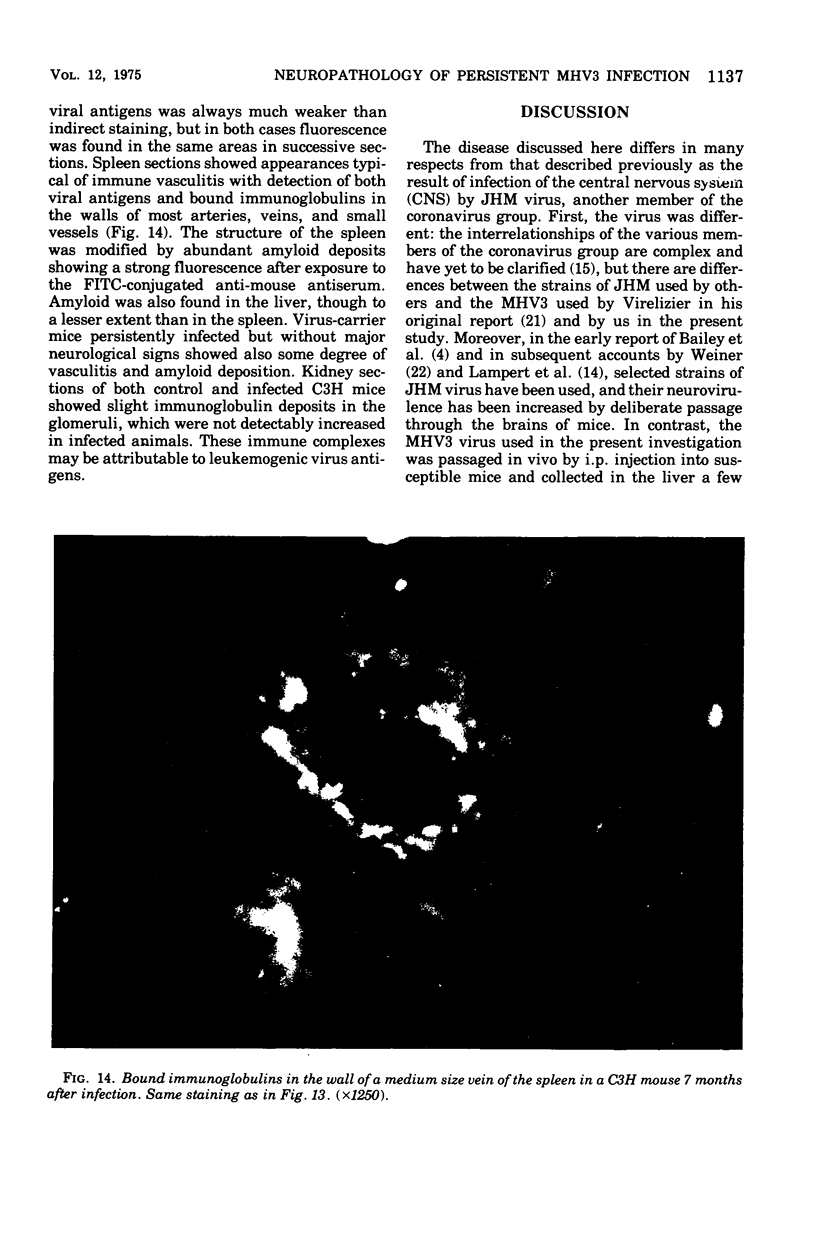
Mouse hepatitis virus (MHV3) can persist for months in strains of mice with genetically controlled "semisusceptibility" to this virus. The pathology of the chronic neurological disease induced in these animals has been investigated by conventional histology and immunofluorescence. A2G mice develop a chronic choroidoependymitis and meningitis leading to severe hydrocephalus and hydromyelia. In C3H mice a widespread vasculitis was observed, with both viral antigens and bound immunoglobulins in vessal walls. No significant glomerulonephritis was found. Systemic amyloidosis was present in the spleen, liver, and kidneys. The virus was not detected in neural tissues, but brain and spinal cord lesions were found near inflammatory areas surrounding damaged vessels. It is suggested that viral persistance in ependymal cells is directly responsible for the lesions in A2G mice, whereas an immunopathological lesion of blood vessels of the central nervous system underlines the damage to mice of the C3H strain.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C. Genetic factors in resistance against virus infections. Arch Gesamte Virusforsch. 1965;17(2):280–294. doi: 10.1007/BF01267912. [DOI] [PubMed] [Google Scholar]
- Atkins C. J., Kondon J. J., Quismorio F. P., Friou G. J. The choroid plexus in systemic lupus erythematosus. Ann Intern Med. 1972 Jan;76(1):65–72. doi: 10.7326/0003-4819-76-1-65. [DOI] [PubMed] [Google Scholar]
- Bang F. B., Warwick A. MOUSE MACROPHAGES AS HOST CELLS FOR THE MOUSE HEPATITIS VIRUS AND THE GENETIC BASIS OF THEIR SUSCEPTIBILITY. Proc Natl Acad Sci U S A. 1960 Aug;46(8):1065–1075. doi: 10.1073/pnas.46.8.1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes P. C., Cheville N. F. The ultrastructure of vascular lesions in equine viral arteritis. Am J Pathol. 1970 Feb;58(2):235–253. [PMC free article] [PubMed] [Google Scholar]
- Franklin E. C., Zucker-Franklin D. Current concepts of amyloid. Adv Immunol. 1972;15:249–304. doi: 10.1016/s0065-2776(08)60687-2. [DOI] [PubMed] [Google Scholar]
- Johnson R. T., Johnson K. P. Hydrocephalus following viral infection: the pathology of aqueductal stenosis developing after experimental mumps virus infection. J Neuropathol Exp Neurol. 1968 Oct;27(4):591–606. [PubMed] [Google Scholar]
- Kilham L., Margolis G. Hydrocephalus in hamsters, ferrets, rats, and mice following inoculations with reovirus type I. I. Virologic studies. Lab Invest. 1969 Sep;21(3):183–188. [PubMed] [Google Scholar]
- Lampert P. W., Oldstone M. B. Pathology of choroid plexus in spontaneous immune complex disease and chronic viral infections. Virchows Arch A Pathol Anat Histol. 1974 May 27;363(1):21–32. doi: 10.1007/BF00432202. [DOI] [PubMed] [Google Scholar]
- Lampert P. W., Sims J. K., Kniazeff A. J. Mechanism of demyelination in JHM virus encephalomyelitis. Electron microscopic studies. Acta Neuropathol. 1973 Mar 30;24(1):76–85. doi: 10.1007/BF00691421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piazza M., Pane G., De Ritis F. The fate of murine hepatitis virus (MHV-3) after intravenous injection into susceptible mice. Arch Gesamte Virusforsch. 1967;22(3):472–474. doi: 10.1007/BF01242969. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner L. P. Pathogenesis of demyelination induced by a mouse hepatitis. Arch Neurol. 1973 May;28(5):298–303. doi: 10.1001/archneur.1973.00490230034003. [DOI] [PubMed] [Google Scholar]