Abstract
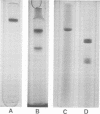
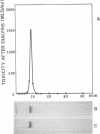
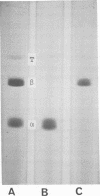
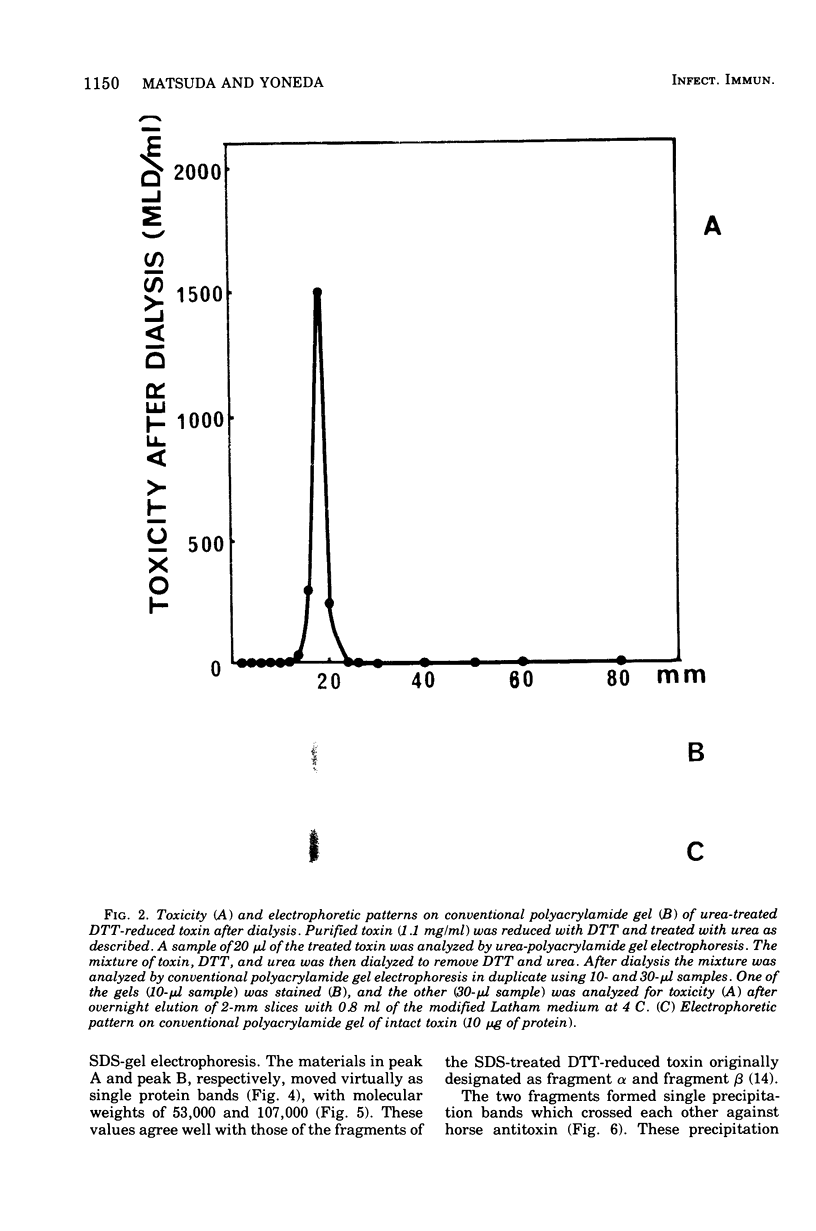
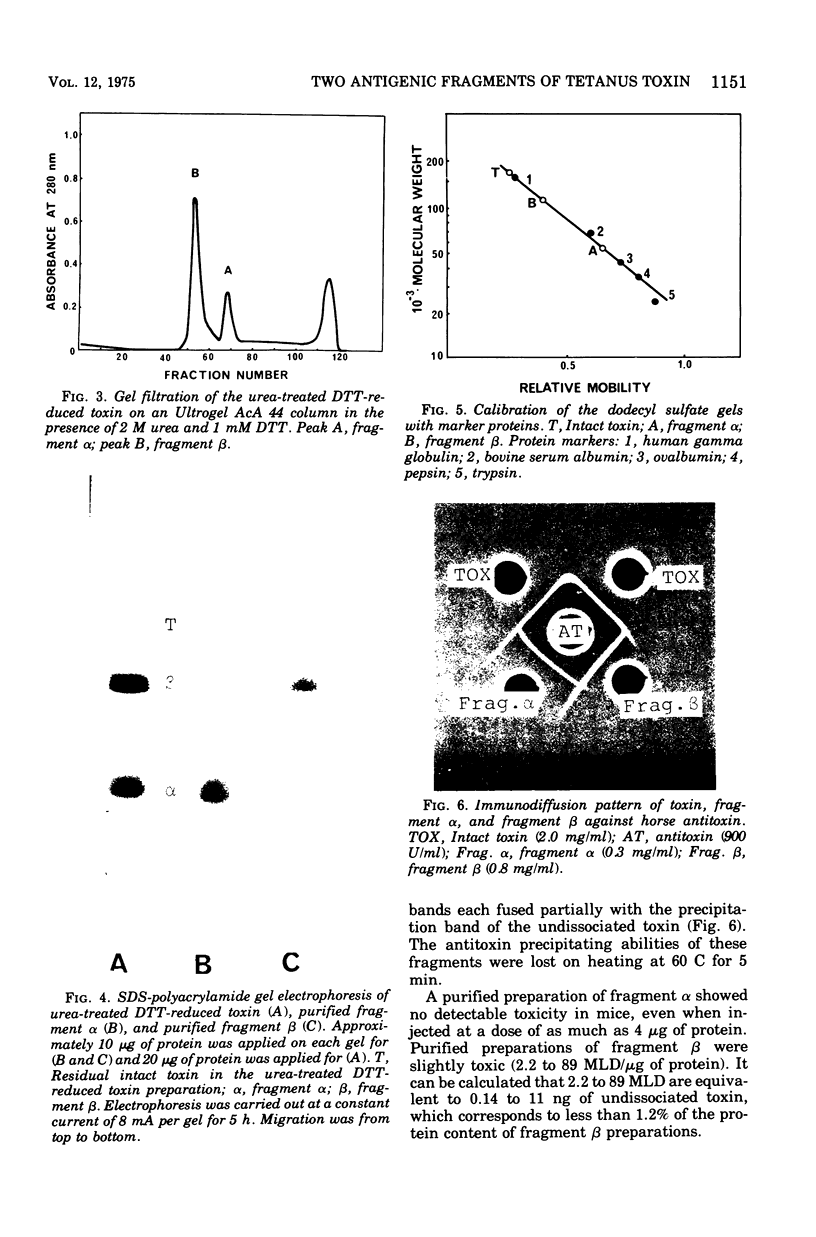
Tetanus neurotoxin (molecular weight approximately 160,000) was purified from bacterial extracts (intracellular toxin) and mildly trypsinized and from culture filtrates (extracellular toxin). Both purified preparations could be dissociated reversibly into two polypeptide chains, with molecular weights of 53,000 (fragment alpha) and 107,000 (fragment beta), by treatment with 100 mM dithiothreitol (DTT) and 4 M urea with concomitant loss of toxicity. Upon removal of DDT and urea from the dissociated toxin preparation by dialysis, these fragments reassociated, forming the whole toxin. The two fragments were isolated and purified from the dissociated toxin by gel filtration on an Ultrogel AcA 44 column equilibrated with buffer containing 2 M urea and 1 mM DTT. The preparation of fragment alpha was nontoxic whereas that of fragment beta was slightly toxic. Immunodiffusion analyses, using horse antitoxin, showed that the antigenicities of fragment alpha and fragment beta were distinct from each other but were partially identical with that of undissociated toxin. The abilities of these fragments to precipitate antitoxin were lost on heating at 60 C for 5 min. The molecular substructure of tetanus neurotoxin is discussed on the basis of these findings.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bizzini B., Raynaud M. Etude immunologique et biologique de sous-unités de la toxine tétanique. C R Acad Sci Hebd Seances Acad Sci D. 1974 Dec 2;279(23):1809–1812. [PubMed] [Google Scholar]
- Bizzini B., Turpin A., Raynaud M. Immunochemistry of tetanus toxin. The nitration of tyrosyl residues in tetanus toxin. Eur J Biochem. 1973 Nov 1;39(1):171–181. doi: 10.1111/j.1432-1033.1973.tb03115.x. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Turpin A., Raynaud M. On the structure of tetanus toxin. Naunyn Schmiedebergs Arch Pharmacol. 1973;276(3-4):271–288. doi: 10.1007/BF00499881. [DOI] [PubMed] [Google Scholar]
- Bizzini B., Turpin A., Raynaud M. Production et purification de la toxine tétanique. Ann Inst Pasteur (Paris) 1969 May;116(5):686–712. [PubMed] [Google Scholar]
- Cohen H., Nagel J., van der Veer M., Peetoom F. Studies on tetanus antitoxin. I. Isolation of two neutralizing antibodies from tetanus antitoxin. J Immunol. 1970 Jun;104(6):1417–1423. [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Helting T., Zwister O. Enzymatic breakdown of tetanus toxin. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1263–1270. doi: 10.1016/0006-291x(74)90832-8. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- LATHAM W. C., BENT D. F., LEVINE L. Tetanus toxin production in the absence of protein. Appl Microbiol. 1962 Mar;10:146–152. doi: 10.1128/am.10.2.146-152.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Matsuda M., Yoneda M. Dissociation of tetanus neurotoxin into two polypeptide fragments. Biochem Biophys Res Commun. 1974 Apr 23;57(4):1257–1262. doi: 10.1016/0006-291x(74)90831-6. [DOI] [PubMed] [Google Scholar]
- Nagel J., Cohen H. Studies on tetanus antitoxins. II. Demonstration of at least four antitoxins of different specificity in antitoxic sera. J Immunol. 1973 May;110(5):1388–1395. [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- RAYNAUD M. Extraction de la toxine tétanique a partir des corps microbiens. Ann Inst Pasteur (Paris) 1951 Apr;80(4):356–377. [PubMed] [Google Scholar]
- TSUNASHIMA I., SATO K., SHOJI K., YONEDA M., AMANO T. A REPRODUCIBLE METHOD FOR THE REMOVAL OF NON-SPECIFIC FLOCCULATING ANTIBODIES FROM TETANUS ANTITOXIN SERUM. Biken J. 1964 Dec;7:137–152. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]