Abstract
Physiological calcium (Ca2+) signals within the pancreatic acinar cell regulate enzyme secretion, whereas aberrant Ca2+ signals are associated with acinar cell injury. We have previously identified the ryanodine receptor (RyR), a Ca2+ release channel on the endoplasmic reticulum, as a modulator of these pathological signals. In the present study, we establish that the RyR is expressed in human acinar cells and mediates acinar cell injury. We obtained pancreatic tissue from cadaveric donors and identified isoforms of RyR1 and RyR2 by qPCR. Immunofluorescence staining of the pancreas showed that the RyR is localized to the basal region of the acinar cell. Furthermore, the presence of RyR was confirmed from isolated human acinar cells by tritiated ryanodine binding. To determine whether the RyR is functionally active, mouse or human acinar cells were loaded with the high-affinity Ca2+ dye (Fluo-4 AM) and stimulated with taurolithocholic acid 3-sulfate (TLCS) (500 μM) or carbachol (1 mM). Ryanodine (100 μM) pretreatment reduced the magnitude of the Ca2+ signal and the area under the curve. To determine the effect of RyR blockade on injury, human acinar cells were stimulated with pathological stimuli, the bile acid TLCS (500 μM) or the muscarinic agonist carbachol (1 mM) in the presence or absence of the RyR inhibitor ryanodine. Ryanodine (100 μM) caused an 81% and 47% reduction in acinar cell injury, respectively, as measured by lactate dehydrogenase leakage (P < 0.05). Taken together, these data establish that the RyR is expressed in human acinar cells and that it modulates acinar Ca2+ signals and cell injury.
Keywords: tritiated ryanodine binding, acinar cell, acinar cell injury, ryanodine receptor
the pancreatic acinar cell is the main parenchymal cell of the exocrine pancreas and the site for the initiation of acute pancreatitis (13). Cellular Ca2+ homeostasis is governed by the interaction of several Ca2+ transporters, including Ca2+ release channels, pumps, and exchangers (3). In the acinar cell, the initial rise in intracellular Ca2+ concentration is dependent on the release of Ca2+ from intracellular stores (58). This process is predominantly controlled by two major Ca2+ release channels present on the endoplasmic reticulum (ER): the inositol 1,4,5-trisphosphate receptor (IP3R) and the ryanodine receptor (RyR). The IP3R is activated by inositol 1,4,5-trisphosphate and is primarily localized to the apical region of acinar cells, where digestive enzymes are normally secreted (28). The second major intracellular Ca2+ release channel is the RyR, which has structural similarity to the IP3R (11).
RyR Ca2+ release in rodent pancreatic acinar cells was first implicated with the use of the RyR activator caffeine (57). In these early studies, Wakui and colleagues described the globalization of acinar cell Ca2+ signals with 1 mM caffeine. The results suggested the presence of a RyR Ca2+ pool that is triggered by IP3R-Ca2+ release. Later, Thorn et al. (55) also described a primary role for the RyR in the generation of Ca2+ spikes (55). Whereas physiological stimuli provoke low-amplitude, oscillatory Ca2+ signals in acinar cells, pathological insults (which cause pancreatic injury and pancreatitis in vivo) induce high-amplitude, nonoscillatory Ca2+ signals. These latter aberrant Ca2+ signals are associated with acinar pathology, such as intra-acinar protease activation (26, 46), vacuole formation (50), mitochondrial depolarization (46), and acinar cell leakage (23). In addition, various agonists have been shown to induce such outcomes in an RyR-dependent manner; they include cholecystokinin (24), carbachol (24), ethanol (16, 23), fatty acid ethyl esters (17), and bile acids (23).
In mammals, although three RyR isoforms are transcribed from separate genes, they have remarkably similar biophysical properties and share ∼70% homology (11). RyR1 is the dominant Ca2+ release channel in skeletal muscle (53), RyR2 in cardiac muscle (35), and RyR3 is primarily enriched in brain (20). Cytosolic Ca2+ is the most potent activator of the RyR, which makes the RyR a prototypic Ca2+-induced Ca2+-release (CICR) channel. Other activators include calmodulin (during low Ca2+ concentrations) (1, 19, 54), cyclic adenosine diphosphate ribose (37, 38, 60), and caffeine (7, 24, 57).
In this study, we sought to demonstrate the presence of RyR in human acinar cells and determine its role in cell injury. We found that 1) by qPCR, immunofluorescence, and [3H] ryanodine binding studies, the RyR is expressed in human acinar cells; 2) acinar cell Ca2+ signals are modulated by the RyR; and 3) RyR inhibition attenuates acinar cell injury.
MATERIALS AND METHODS
Reagents and animals.
All reagents were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Male Swiss Webster mice weighing 20–25 g (Charles River, Wilmington, MA) were fed standard laboratory chow and given free access to water. All animal experiments were performed using a protocol approved by the University of Pittsburgh Institutional Animal Care and Use Committee, and the use of human pancreatic acinar cells from healthy cadaveric donors was approved by the Institutional Review Board.
RT-PCR.
Total RNA was extracted and reverse transcribed from human pancreas using an RNeasy kit (Qiagen, Valencia, CA). RT-PCR was performed with SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen, San Diego, CA). The mix was supplemented with the following forward and reverse oligonucleotide primers specific for human RyR isoforms: hRyR1: (f) 5′-CAAGGTCCTGGACAAACATGGG-3′, (r) 5′-TCGCTCTTGTTGTAGAACTTGCGG-3′; hRyR2: (f) 5′-GAATCAGTGAATTACTTGGCATGG-3′, (r) 5′-TTGGTCTCTTAGTTCTCCAAAAGC-3′; hRyR3: (f) 5′-CCTTCGCTATCAACTTCATCCTGC-3′, (r) 5′-TCTTCTACTGGGCTAAAGTCAAGA-3′. The amplified products were electrophoresed on an agarose gel, stained with ethidium bromide, and visualized by UV illumination.
qRT-PCR.
Total RNA from human skeletal muscle and human brain were purchased from Clontech (Mountain View, CA) and Biochain (Newark, CA), respectively. Total RNA from human heart was a kind gift of Dr. Charles McTiernan (University of Pittsburgh). Human pancreatic acinar cell cDNA was obtained using the μMACS mRNA isolation system (Miltenyi Biotec, San Diego, CA). Total RNA samples were used to generate cDNA using the iScript advanced cDNA synthesis kit (Bio-Rad, Hercules, CA).
qRT-PCR was performed to determine the relative expression of RyR isoforms in human acinar cells compared with human skeletal muscle, human heart, and human brain samples. The primer pairs for each human RyR isoform as well as 18S rRNA control sample were obtained as part of the PrimePCR-PreAMP SYBR Green Assay (Bio-Rad). qRT-PCR reactions were carried out in 20-μl volume reactions using the SsoAdvanced Universal Supermix SYBR Green system (Bio-Rad). The reactions contained 1× SsoAdvanced Universal SYBR Green Supermix, 300 nM forward primer, 300 nM reverse primer, and 100 ng cDNA. qRT-PCR conditions were 95°C for 10 s and 60°C for 30 s for 35 cycles on a Bio-Rad CFX96 Touch thermocycler (Bio-Rad). Results for the expression of mRNA were normalized to expression of 18S rRNA and are represented relative to expression levels for each of the control groups.
Immunofluorescence.
Sections from pancreas and skeletal muscle (used as a positive control) were fixed in cold acetone for 5 min and incubated with a rabbit polyclonal RyR antibody (1327) at a 1:25 dilution (kind gift of Dr. Andy Marks). Specimens were imaged on a Zeiss 510 laser-scanning confocal microscope.
Preparation of human acinar cells.
Pancreatic tissue was harvested from human cadaveric donors as described by Bottino et al. (4). Briefly, specimens were transported in cold preservation fluid (histidine-tryptophan-ketoglutarate) and had a minimum cold ischemia time of 12 h. Fat, connective tissue, and blood vessels were trimmed away. The pancreas was washed in a cocktail of antibiotics and then cut at the level of the neck to reveal the pancreatic duct. Catheters were placed in both sides of the transected duct, and a blend of exogenous enzymes, including collagenases and neutral proteases (Serva, Heidelberg, Germany), were freshly dissolved in Hanks's balanced saline solution prewarmed to 28–30°C, and injected intraductally. The pancreas was then transferred to a Ricordi digestion chamber, and the tissue was mechanically disrupted, as described (48). Pancreatic cells were washed several times in cold RPMI medium and supplemented with 2.5% human serum albumin. In this process, islets and ducts were separated by centrifugation, and the resultant cellular pool consisted primarily of acinar cells. Studies by Houbracken et al. (21) have shown that cells obtained using a similar isolation method are viable and display a distinct acinar cell phenotype and express multiple acinar cell markers including chymotrypsin, amylase, lipase, and carboxypeptidase within 1 day of cell culture, which was our time frame for usage of the cells.
Preparation of RyR-enriched microsomal fractions.
We obtained RyR-enriched microsomal fractions using a modification of a protocol for ER fractions (6, 59). Pancreatic tissues or mouse brain (used as a positive control) were excised and immediately placed in 7.5 times the volume of ice-cold homogenization buffer per weighted volume of tissue. Homogenization buffer contained 0.3 M sucrose, 10 mM HEPES-NaOH, 2 mM DTT, 1 mM benzamidine, 0.5 mM PMSF, 5 mM NaF, 1 mM NaVO4, and a protease inhibitor cocktail (1 tablet per 30 ml; Roche, Nutley, NJ). All subsequent steps were performed on ice or at 4°C. Using sterile scissors, the tissue was diced into small pieces and loaded into a 10-ml dounce homogenizer. After 15 strokes of homogenization, samples were sonicated at a 50% setting for a total of 2 min using 15-s bursts followed by a 5-s recovery. The tissue homogenate was then spun at 800 g for 5 min. The resulting supernatant was then removed and again spun at 12,000 g for 10 min. The resulting solid fraction, (called pellet no. 2, or P2) was used in subsequent experiments to examine ryanodine binding.
For lysis of isolated acinar cell preparations, cells were spun down at 800 g for 5 min. The supernatant was discarded, and the pelleted cells were resuspended in 10 times the volume of ice-cold homogenization buffer. The acinar cells were then sonicated on ice at a 50% setting for 4 min using 15-s bursts followed by a 10-s recovery. The resulting cell lysate was then used to obtain RyR-enriched microsomal fractions as described above. Western blots of the enriched fractions were probed with a pan-RyR antibody at a titer of 1:1,000 (34C; Iowa Hybridoma Bank, Iowa City, IA).
Ryanodine binding.
Tritiated [3H] ryanodine was purchased from Perkin Elmer (Boston, MA), and binding studies were performed as previously described (9, 25, 31). Mouse brain was assayed as a positive control. Briefly, 100-μg aliquots of P2 fractions from brain, mouse, or human were incubated for 1 h at room temperature in buffer containing 10 mM K-HEPES (at pH 7.4), 1 M KCl, 25 μM CaCl2, and 2.5–20 nM [3H] ryanodine. [3H] ryanodine binding without proteins was included as a negative control. Free ligand was separated from bound by depositing the protein fractions onto Whatman GFB filters that were presoaked with 1% polyethylenimine. The filters were rinsed three times with deionized water, and then radioactivity was measured by liquid scintillation counting. [3H] ryanodine binding was expressed as counts per minute per milligram protein. Nonspecific binding was measured as the radioactivity for each sample condition in the presence of excess (40 μM) cold, or nonradiolabeled, ryanodine.
Cell injury assays.
Acinar cell injury was measured using a cytotoxicity assay for lactate dehydrogenase (LDH) leakage (Promega, Madison, WI) as previously described (39). Absorbance was measured at 492 nm. Results were expressed as a percentage of LDH released into the media.
Preparation of mouse pancreatic acini for Ca2+ imaging.
Groups of pancreatic acinar cells were isolated as previously described (24, 39), with minor modifications. Briefly, the pancreas was removed and then minced for 5 min in buffer containing 20 mM HEPES (pH 7.4), 95 mM NaCl, 4.7 mM KCl, 0.6 mM MgCl2, 1.3 mM CaCl2, 10 mM glucose, and 2 mM glutamine, plus 1% BSA, 1× MEM nonessential amino acids (GIBCO, Grand Island, NY), 200 U/ml type-4 collagenase (Worthington, Freehold, NJ), and 1 mg/ml soybean trypsin inhibitor. The tissue was incubated for 30 min at 37°C with shaking at 90 revolution/min. The digest was transferred to a 15-ml conical tube and washed with collagenase-free buffer. The suspension was vigorously shaken for 15–20 s to separate the cells into smaller clusters.
Detection and analysis of cellular Ca2+ signals from mouse and human acini.
Acinar cells were loaded at room temperature with the high-affinity Ca2+-sensing dye Fluo-4 AM (Kd = 300 nM; Invitrogen). Acinar cells were plated on acid-washed glass coverslips and then mounted on a perfusion chamber. Thereupon, they were stimulated at room temperature with the bile acid taurolithocholic acid-3 sulfate (TLCS; 500 μM) or the muscarinic agonist carbachol (1 mM) in the presence or absence of the RyR inhibitor ryanodine (100 μM). A Zeiss LSM710 laser-scanning confocal microscope was used with a ×40, 1.4 numerical aperture objective. The dye was excited at 488-nm wavelength, and emission signals of >515 nm were collected every 2 s. Fluorescence from individual acinar cells was recorded. Analysis of recordings was performed using ImageJ software (NIH, Bethesda, MD), and mean fluorescence over time in each region was graphed.
Statistical analysis.
Data were expressed as means ± SD unless otherwise stated. Statistical analysis was performed using a Student's t-test. Statistical significance was defined as a P value ≤0.05.
RESULTS
RyR is expressed in the human pancreas.
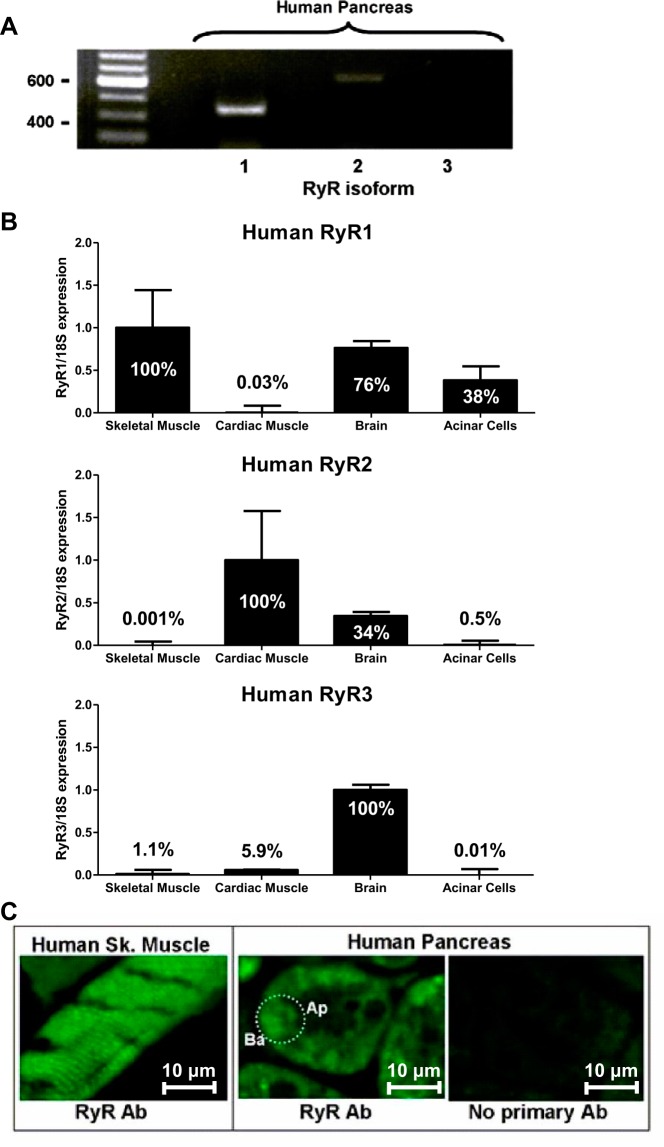
Most studies have examined the role of the RyR in acinar pathobiology using rodent models (15, 18, 22–24), but, to our knowledge, none have studied the RyR in human acinar cells. Using total RNA extracted from cadaveric pancreas specimens and RyR isoform-specific primers, we found that RyR1 and trace amounts of RyR2, but not RyR3, were expressed in the human pancreas (Fig. 1A). Similar results were observed for RyR isoform expression by qPCR from acinar cells relative to expression of RyR in human skeletal muscle, human cardiac muscle, and human brain. Each qPCR reaction was normalized to the expression of 18S rRNA (Fig. 1B). Among isoforms, by qPCR, RyR1 was most highly expressed (38% of skeletal muscle), followed by small amounts of RyR2 (0.5% of cardiac muscle), and RyR3 expression was negligible (0.001% of brain). By contrast, in mouse pancreas only the RyR1 gene transcript was identified (41). In rat acini, however, RyR1, RyR2, and RyR3 have all been reported (12, 29). We also show in human pancreas tissue that the RyR is distributed in the basal region of human pancreatic acinar cells (Fig. 1B), which is consistent with both mouse and rat RyR distribution (12, 29, 41, 51). In skeletal muscle (our positive control), RyRs are located at the triadic junction between sarcoplasmic reticulum terminal cisternae and sarcolemmal T-tubules, yielding a “striated” pattern (30).
Fig. 1.
The ryanodine receptor (RyR) is expressed in the human pancreas. A: RT-PCR shows RyR1 and trace RyR2 expression in the human pancreas. B: qPCR was performed to determine the relative expression levels of the RyR isoforms in human acinar cells compared with human skeletal muscle, human heart, and human brain. 18S rRNA served as the endogenous housekeeping transcript in all experiments. Each sample was run twice in triplicate. Data are expressed as means ± SE. C: human skeletal (sk) muscle (positive control) shows a typical striated pattern for RyR labeling. In the human pancreatic acinar cell, the RyR is primarily distributed in the basal (ba) region but excluded from the apical (ap). An individual acinar cell is delineated by the dotted oval. A control section without primary antibody, but the same confocal microscopy settings, was used to gauge background labeling.
Enrichment for RyR in isolated microsomal fractions.
RyR expression in nonexcitable cells is low compared with excitable cell types, such as skeletal muscle or brain (2). To enrich for RyRs, we prepared microsomes from tissues and cells using a modified protocol (6, 59) that is outlined in materials and methods and schematized in Fig. 2A. Importantly, the fraction also contains the ER-specific markers binding immunoglobin protein and calreticulin, as demonstrated by Williams and colleagues (6, 59). Western blot analysis of mouse brain fractions (positive control) demonstrated that the RyR is enriched in the P2 fraction (Fig. 2, B and C). We subsequently used this fraction for ryanodine-binding studies. Because there is much lower expression of RyRs in pancreas compared with brain, pancreatic fractions failed to yield a band (data not shown).
Fig. 2.
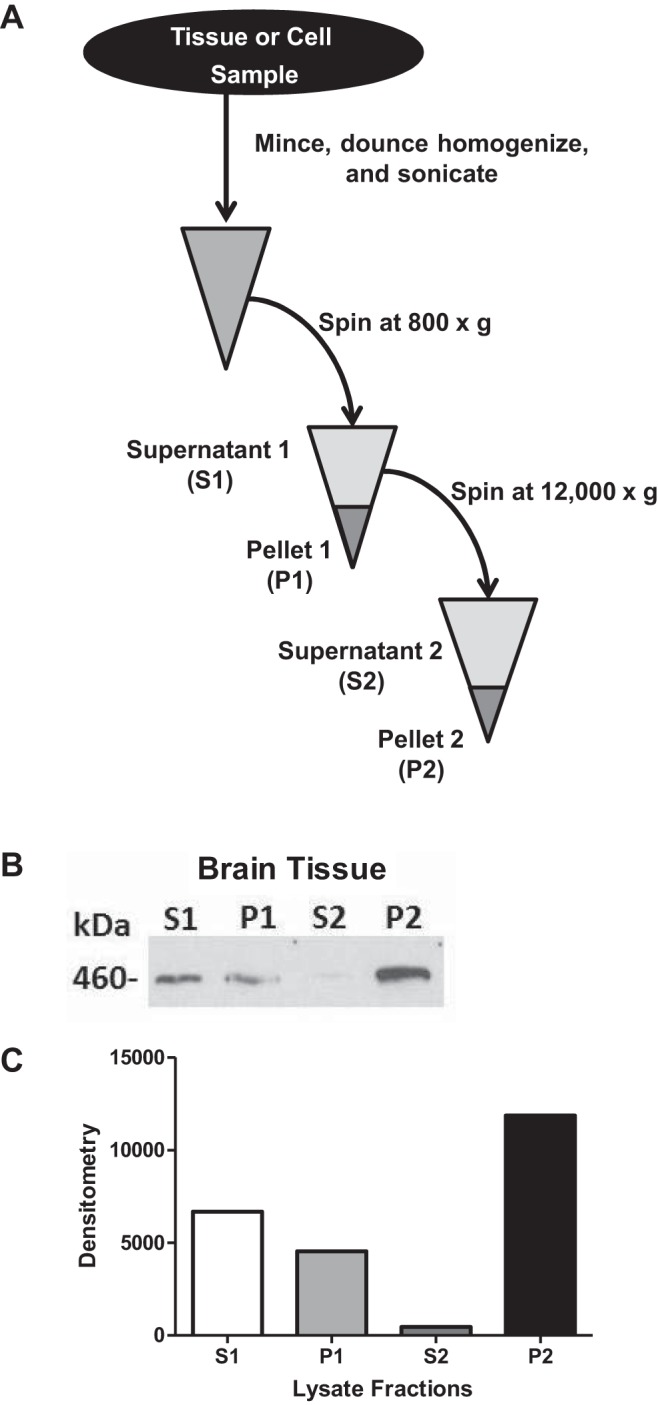
A schematic for enriching RyRs in microsomal fractions. A: methodology for the enrichment of the RyR in microsomal fractions from tissues or cells. B: representative Western blot using the 34C RyR antibody. C: its densitometry from a mouse brain fraction demonstrates enrichment of RyR in the P2 fraction.
RyR in human acinar cells by tritiated ryanodine binding.
All three RyR isoforms bind with high affinity (Kd < 50 nM) (43) to the plant alkaloid ryanodine. Low concentrations of ryanodine (in the nM range) increase the frequency of single RyR channel openings, whereas high concentrations (in the μM range) lock the channel in a closed configuration (43). We utilized [3H] ryanodine to confirm the presence of RyR in human acinar cells. Nonspecific binding was assessed by competition with excess amounts (40 μM) of nonradioactive (cold) ryanodine. In purified microsomes from mouse brain, mouse pancreas, and human acinar cells, there was marked binding of ryanodine above background and a concentration-dependent increase in ryanodine binding (Fig. 3).
Fig. 3.
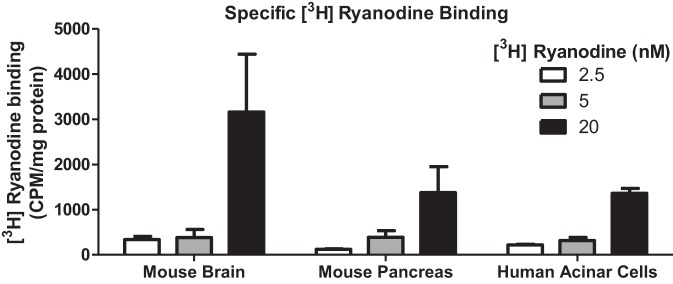
Tritiated ryanodine binding studies confirm the presence of RyR in mouse and human acinar cells. Specific ryanodine binding in mouse brain, mouse pancreas, and human acinar cells was calculated by subtracting samples incubated with excess cold ryanodine (40 μM) plus 2.5–20 nM [3H] ryanodine from samples incubated with [3H] ryanodine alone.
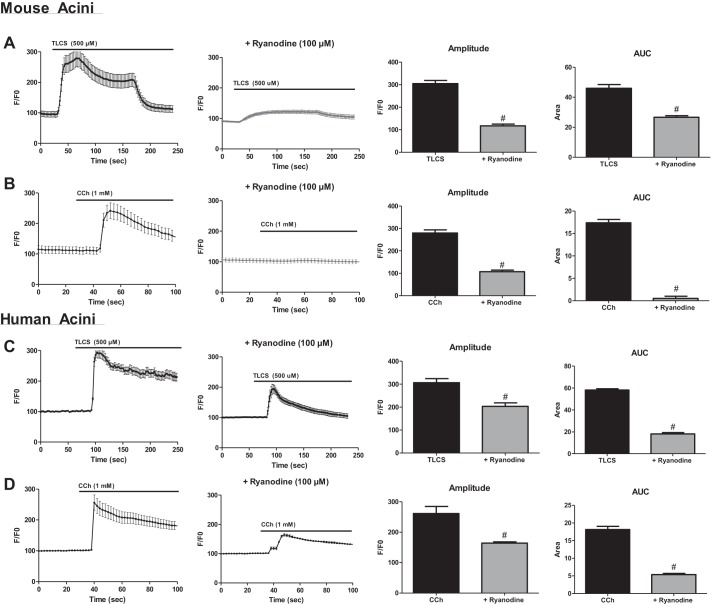
The RyR modulates TLCS- and carbachol-induced Ca2+ signals.
Previous studies from our group have demonstrated that the RyR mediates pathological Ca2+ signals induced by TLCS (23). We now complement these data in mice by showing that ryanodine reduced the amplitude of the acinar cell Ca2+ transient by 100% and the overall rise in cytosolic Ca2+ release by 50%, as measured by area under the curve (AUC; Fig. 4A, P < 0.05). Furthermore, carbachol-induced Ca2+ release was abrogated by RyR inhibition (Fig. 4B; P < 0.05). Importantly, similar experiments in human acinar cells demonstrated that the RyR is functionally active and regulates agonist-induced Ca2+ release in humans. TLCS-induced amplitude and AUC were reduced by 33% and 45%, respectively (Fig. 4C; P < 0.05), and the carbachol-induced amplitude and AUC were reduced by 30% and 42%, respectively (Fig. 4C; P < 0.05). The data demonstrate that human acinar cell RyRs are functionally active, and they modulate pathological acinar cell Ca2+ signals.
Fig. 4.
Bile acid- and carbachol (CCh)-induced Ca2+ signals in mouse and human acinar cells are modulated by the RyR. Ca2+ tracings from mouse (A and B) or human (C and D) acinar cells stimulated with taurolithocholic acid 3-sulfate (TLCS) (500 μM) or CCh (1 mM) in the presence or absence of inhibitory concentrations of ryanodine (100 μM). Quantification of amplitude and area under the curve (AUC) are shown on right (n = 20–30 cells per condition; all from 1 cadaveric donor). #P < 0.05.
The RyR modulates TLCS- and carbachol-induced human acinar cell injury.
We have previously shown that RyR-dependent Ca2+ transients are necessary for both bile acid- and muscarinic agonist-induced cell injury (15, 23, 41, 42, 56). To determine whether these findings translate to the human condition, live acinar cells were harvested from cadaveric donors and stimulated for 2 h with either TLCS or carbachol (Fig. 5). Each of these agents caused a concentration-dependent increase in LDH leakage to a similar extent as previously reported in mouse acinar cells (23, 32–34, 44, 47). A 30-min pretreatment with inhibitory concentrations of ryanodine (100 μM) prevented cell injury with TLCS (500 μM) by 81% (P < 0.05). With carbachol (1 mM), ryanodine reduced LDH leakage by 50%, but the results were not significant (P = 0.059). The data corroborate in human acinar cells previous findings from rodent models that the RyR modulates acinar cell injury.
Fig. 5.
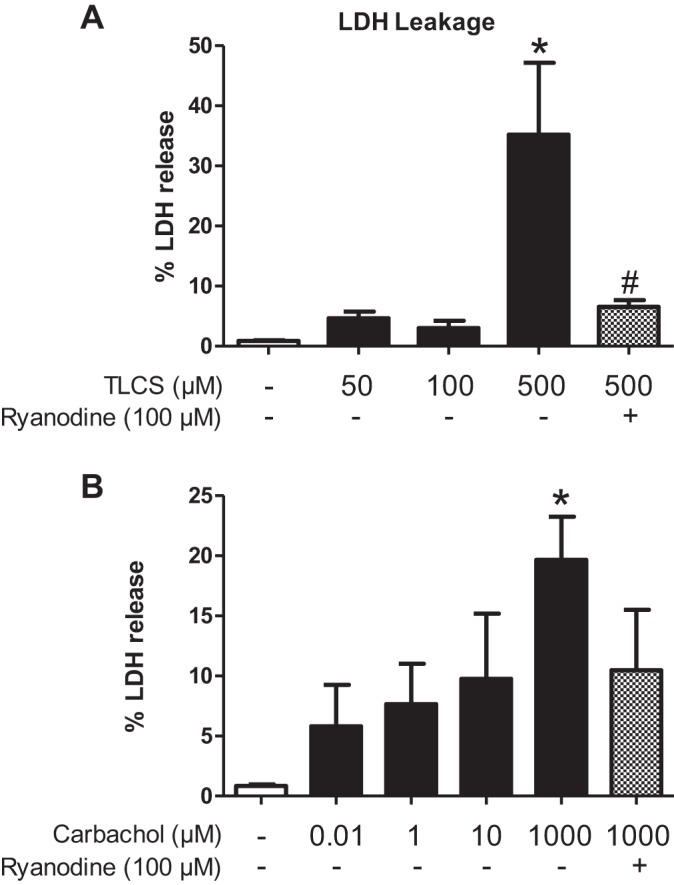
Bile acid- and carbachol-induced human acinar cell injury is modulated by the RyR. Human acinar cells were pretreated with inhibitory concentrations of ryanodine (100 μM) for 30 min and stimulated with varying concentrations of TLCS (A) and carbachol (B) for 2 h. Cell injury was measured as a percentage of lactate dehydrogenase (LDH) leakage (n = 3; groups of acinar cells from one cadaveric donor). *P < 0.05 compared with no agonist; #P < 0.05 compared with the condition causing maximal injury.
DISCUSSION
The three key findings of the current work are that the RyR 1) is expressed in human acinar cells and it modulates 2) acinar cell Ca2+ signals and 3) cell injury.
RyR isoforms are expressed in a wide range of tissues (27). In skeletal muscle and myocardium, the predominant isoforms are RyR1 and RyR2, respectively, where they function to cause the Ca2+ release that leads to the classic phenomenon of excitation-contraction coupling (10, 49). In the brain, all three RyR isoforms are expressed and are involved in a variety of Ca2+-signaling processes including neurotransmitter release and synaptic transmission efficiency (14).
RyR expression has also been detected in nonexcitable cells including the cells from the stomach, kidney, lung, and pancreas (27). Pancreatic β-cells, which make up roughly 60% of islets, express both RyR2 and RyR3 (8, 52). β-Cell RyR has been shown to play a role in the Ca2+-dependent exocytosis of insulin granules in response to increased glucose levels (5). In acinar cells, although an initial report failed to see expression of RyRs in rat pancreatic acini by immunofluorescence (28), several subsequent studies observed a nonapical distribution (12, 24, 29, 51), and all three RyR isoforms were found to be expressed (12). In mouse acinar cells, only RyR1 was expressed (41). In the current study in human acinar cells, RyR1 and RyR2, but not RyR3, were expressed, which underscores species-related differences. Nonetheless, the distribution of RyR in human acinar cells was nonapical, as seen in murine pancreatic sections.
We used tritiated ryanodine to further verify the presence of RyR in human acinar cells. Ryanodine binds to all three RyR isoforms with high affinity (43). Although there was marked ryanodine binding compared with negative controls, both mouse pancreatic tissue and human acinar cell microsomes had 45–50% less binding compared with brain samples. Similarly low levels of ryanodine binding have been reported in other epithelial cells or cell lines compared with excitable cells (2, 28).
Physiological, low-amplitude, mostly oscillatory Ca2+ signals in acinar cells regulate pancreatic enzyme secretion (45), but aberrantly high-amplitude, peak-plateau Ca2+ signals are linked to intracellular protease activation (26, 46), vacuole formation (50), mitochondrial depolarization (46), and acinar cell leakage (23). IP3Rs control both the physiological and pathological Ca2+ signals, and they mediate both secretion and pathological events. RyRs, however, appear to amplify IP3R Ca2+ release through CICR. They do not affect enzyme secretion (24, 36), but they modulate injury attributable to cholecystokinin, carbachol, bile acids, and ethanol (16, 23, 24, 42). Using a limited supply of viable human acinar cells, we observed that bile-acid- and carbachol-induced LDH leakage was modulated by the RyR.
To our knowledge, this is the first demonstration of RyR expression and function in the human pancreas. The findings suggest that the RyR may serve as a viable therapeutic target for pancreatic injury.
GRANTS
This work was supported by National Institutes of Health Grants DK083327, DK093491, and DK03002 (to S. Husain).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.M.L., A.I.O., S.J., D.W., T.J., and S.Z.H. conception and design of research; C.M.L., A.I.O., S.J., D.W., K.A.M., A.U.S., A.M., R.B., and T.J. performed experiments; C.M.L., A.I.O., S.J., K.A.M., A.U.S., T.J., and S.Z.H. analyzed data; C.M.L., A.I.O., S.J., D.W., K.A.M., A.U.S., J.F.E., T.J., and S.Z.H. interpreted results of experiments; C.M.L. and A.I.O. prepared figures; C.M.L., A.I.O., and S.Z.H. drafted manuscript; C.M.L., A.I.O., D.W., K.A.M., J.F.E., A.M., T.J., and S.Z.H. edited and revised manuscript; C.M.L., A.I.O., S.J., D.W., K.A.M., A.U.S., J.F.E., A.M., R.B., T.J., and S.Z.H. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors acknowledge Dr. Rita Bottino and the Thomas Starzl Transplantation Institute at the University Of Pittsburgh School of Medicine for providing human samples.
REFERENCES
- 1.Ai X, Curran JW, Shannon TR, Bers DM, Pogwizd SM. Ca2+/calmodulin-dependent protein kinase modulates cardiac ryanodine receptor phosphorylation and sarcoplasmic reticulum Ca2+ leak in heart failure. Circ Res 97: 1314–1322, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Bennett DL, Cheek TR, Berridge MJ, De Smedt H, Parys JB, Missiaen L, Bootman MD. Expression and function of ryanodine receptors in nonexcitable cells. J Biol Chem 271: 6356–6362, 1996 [DOI] [PubMed] [Google Scholar]
- 3.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 4: 517–529, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bottino R, Bertera S, Grupillo M, Melvin PR, Humar A, Mazariegos G, Moser AJ, Walsh RM, Fung J, Gelrud A, Slivka A, Soltys K, Wijkstrom M, Trucco M. Isolation of human islets for autologous islet transplantation in children and adolescents with chronic pancreatitis. J Transplant 2012: 642787, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bruton JD, Lemmens R, Shi CL, Persson-Sjogren S, Westerblad H, Ahmed M, Pyne NJ, Frame M, Furman BL, Islam MS. Ryanodine receptors of pancreatic beta-cells mediate a distinct context-dependent signal for insulin secretion. FASEB J 17: 301–303, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Chen X, Sans MD, Strahler JR, Karnovsky A, Ernst SA, Michailidis G, Andrews PC, Williams JA. Quantitative organellar proteomics analysis of rough endoplasmic reticulum from normal and acute pancreatitis rat pancreas. J Proteome Res 9: 885–896, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chu A, Diaz-Munoz M, Hawkes MJ, Brush K, Hamilton SL. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol Pharmacol 37: 735–741, 1990 [PubMed] [Google Scholar]
- 8.Dixit SS, Wang T, Manzano EJ, Yoo S, Lee J, Chiang DY, Ryan N, Respress JL, Yechoor VK, Wehrens XH. Effects of CaMKII-mediated phosphorylation of ryanodine receptor type 2 on islet calcium handling, insulin secretion, and glucose tolerance. PLoS One 8: e58655, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eu JP, Meissner G. Detection of calcium release via ryanodine receptors. Methods Mol Biol 798: 373–382, 2012 [DOI] [PubMed] [Google Scholar]
- 10.Fabiato A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am J Physiol Cell Physiol 245: C1–C14, 1983 [DOI] [PubMed] [Google Scholar]
- 11.Fill M, Copello JA. Ryanodine receptor calcium release channels. Physiol Rev 82: 893–922, 2002 [DOI] [PubMed] [Google Scholar]
- 12.Fitzsimmons TJ, Gukovsky I, McRoberts JA, Rodriguez E, Lai FA, Pandol SJ. Multiple isoforms of the ryanodine receptor are expressed in rat pancreatic acinar cells. Biochem J 351: 265–271, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frossard JL, Steer ML, Pastor CM. Acute pancreatitis. Lancet 371: 143–152, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Furuichi T, Furutama D, Hakamata Y, Nakai J, Takeshima H, Mikoshiba K. Multiple types of ryanodine receptor/Ca2+ release channels are differentially expressed in rabbit brain. J Neurosci 14: 4794–4805, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gerasimenko JV, Flowerdew SE, Voronina SG, Sukhomlin TK, Tepikin AV, Petersen OH, Gerasimenko OV. Bile acids induce Ca2+ release from both the endoplasmic reticulum and acidic intracellular calcium stores through activation of inositol trisphosphate receptors and ryanodine receptors. J Biol Chem 281: 40154–40163, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Gerasimenko JV, Lur G, Ferdek P, Sherwood MW, Ebisui E, Tepikin AV, Mikoshiba K, Petersen OH, Gerasimenko OV. Calmodulin protects against alcohol-induced pancreatic trypsinogen activation elicited via Ca2+ release through IP3 receptors. Proc Natl Acad Sci USA 108: 5873–5878, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gerasimenko JV, Lur G, Sherwood MW, Ebisui E, Tepikin AV, Mikoshiba K, Gerasimenko OV, Petersen OH. Pancreatic protease activation by alcohol metabolite depends on Ca2+ release via acid store IP3 receptors. Proc Natl Acad Sci USA 106: 10758–10763, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gerasimenko JV, Maruyama Y, Yano K, Dolman NJ, Tepikin AV, Petersen OH, Gerasimenko OV. NAADP mobilizes Ca2+ from a thapsigargin-sensitive store in the nuclear envelope by activating ryanodine receptors. J Cell Biol 163: 271–282, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guo T, Zhang T, Mestril R, Bers DM. Ca2+/calmodulin-dependent protein kinase II phosphorylation of ryanodine receptor does affect calcium sparks in mouse ventricular myocytes. Circ Res 99: 398–406, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Hakamata Y, Nakai J, Takeshima H, Imoto K. Primary structure and distribution of a novel ryanodine receptor/calcium release channel from rabbit brain. FEBS Lett 312: 229–235, 1992 [DOI] [PubMed] [Google Scholar]
- 21.Houbracken I, de Waele E, Lardon J, Ling Z, Heimberg H, Rooman I, Bouwens L. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology 141: 731–741; e731–e734, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Husain SZ, Grant WM, Gorelick FS, Nathanson MH, Shah AU. Caerulein-induced intracellular pancreatic zymogen activation is dependent on calcineurin. Am J Physiol Gastrointest Liver Physiol 292: G1594–G1599, 2007 [DOI] [PubMed] [Google Scholar]
- 23.Husain SZ, Orabi AI, Muili KA, Luo Y, Sarwar S, Mahmood SM, Wang D, Choo-Wing R, Singh VP, Parness J, Ananthanaravanan M, Bhandari V, Perides G. Ryanodine receptors contribute to bile acid-induced pathological calcium signaling and pancreatitis in mice. Am J Physiol Gastrointest Liver Physiol 302: G1423–G1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Husain SZ, Prasad P, Grant WM, Kolodecik TR, Nathanson MH, Gorelick FS. The ryanodine receptor mediates early zymogen activation in pancreatitis. Proc Natl Acad Sci USA 102: 14386–14391, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inui M, Saito A, Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem 262: 1740–1747, 1987 [PubMed] [Google Scholar]
- 26.Kruger B, Albrecht E, Lerch MM. The role of intracellular calcium signaling in premature protease activation and the onset of pancreatitis. Am J Pathol 157: 43–50, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lanner JT, Georgiou DK, Joshi AD, Hamilton SL. Ryanodine receptors: structure, expression, molecular details, and function in calcium release. Cold Spring Harb Perspect Biol 2: a003996, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee MG, Xu X, Zeng W, Diaz J, Wojcikiewicz RJ, Kuo TH, Wuytack F, Racymaekers L, Muallem S. Polarized expression of Ca2+ channels in pancreatic and salivary gland cells. Correlation with initiation and propagation of [Ca2+]i waves. J Biol Chem 272: 15765–15770, 1997 [DOI] [PubMed] [Google Scholar]
- 29.Leite MF, Dranoff JA, Gao L, Nathanson MH. Expression and subcellular localization of the ryanodine receptor in rat pancreatic acinar cells. Biochem J 337: 305–309, 1999 [PMC free article] [PubMed] [Google Scholar]
- 30.McGrew SG, Boucek RJ, Jr, McIntyre JO, Jung CY, Fleischer S. Target size of the ryanodine receptor from junctional terminal cisternae of sarcoplasmic reticulum. Biochemistry 26: 3183–3187, 1987 [DOI] [PubMed] [Google Scholar]
- 31.McGrew SG, Wolleben C, Siegl P, Inui M, Fleischer S. Positive cooperativity of ryanodine binding to the calcium release channel of sarcoplasmic reticulum from heart and skeletal muscle. Biochemistry 28: 1686–1691, 1989 [DOI] [PubMed] [Google Scholar]
- 32.Muili KA, Ahmad M, Orabi AI, Mahmood SM, Shah AU, Molkentin JD, Husain SZ. Pharmacological and genetic inhibition of calcineurin protects against carbachol-induced pathological zymogen activation and acinar cell injury. Am J Physiol Gastrointest Liver Physiol 302: G898–G905, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muili KA, Jin S, Orabi AI, Eisses JF, Javed TA, Le T, Bottino R, Jayaraman T, Husain SZ. Pancreatic acinar cell NF-kappaB activation due to bile acid exposure is dependent on calcineurin. J Biol Chem 288: 21065–21073, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Muili KA, Wang D, Orabi AI, Sarwar S, Luo Y, Javed TA, Eisses JF, Mahmood SM, Jin S, Singh VP, Ananthanaravanan M, Perides G, Williams JA, Molkentin JD, Husain SZ. Bile acids induce pancreatic acinar cell injury and pancreatitis by activating calcineurin. J Biol Chem 288: 570–580, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakai J, Imagawa T, Hakamat Y, Shigekawa M, Takeshima H, Numa S. Primary structure and functional expression from cDNA of the cardiac ryanodine receptor/calcium release channel. FEBS Lett 271: 169–177, 1990 [DOI] [PubMed] [Google Scholar]
- 36.Nathanson MH, Padfield PJ, O'Sullivan AJ, Burgstahler AD, Jamieson JD. Mechanism of Ca2+ wave propagation in pancreatic acinar cells. J Biol Chem 267: 18118–18121, 1992 [PubMed] [Google Scholar]
- 37.Ogunbayo OA, Zhu Y, Rossi D, Sorrentino V, Ma J, Zhu MX, Evans AM. Cyclic adenosine diphosphate ribose activates ryanodine receptors, whereas NAADP activates two-pore domain channels. J Biol Chem 286: 9136–9140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Orabi AI, Muili KA, Javed TA, Jin S, Jayaraman T, Lund FE, Husain SZ. Cluster of differentiation 38 (CD38) mediates bile-acid induced acinar cell injury and pancreatitis through cyclic ADP ribose and intracellular calcium release. J Biol Chem 288: 27128–27137, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Orabi AI, Muili KA, Wang D, Jin S, Perides G, Husain SZ. Preparation of pancreatic acinar cells for the purpose of calcium imaging, cell injury measurements, and adenoviral infection. J Vis Exp 77: e50391, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orabi AI, Shah AU, Ahmad MU, Choo-Wing R, Parness J, Jain D, Bhandari V, Husain SZ. Dantrolene mitigates caerulein-induced pancreatitis in vivo in mice. Am J Physiol Gastrointest Liver Physiol 299: G196–G204, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Orabi AI, Shah AU, Muili K, Luo Y, Mahmood SM, Ahmad A, Reed A, Husain SZ. Ethanol enhances carbachol-induced protease activation and accelerates Ca2+ waves in isolated rat pancreatic acini. J Biol Chem 286: 14090–14097, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peracchia C. Handbook of Membrane Channels, Molecular and Cellular Physiology. San Diego, CA: Academic, 1994 [Google Scholar]
- 44.Perides G, Laukkarinen JM, Vassileva G, Steer ML. Biliary acute pancreatitis in mice is mediated by the G-protein-coupled cell surface bile acid receptor Gpbar1. Gastroenterology 138: 715–725, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Petersen OH, Ueda N. Pancreatic acinar cells: the role of calcium in stimulus-secretion coupling. J Physiol 254: 583–606, 1976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Raraty M, Ward J, Erdemli G, Vaillant C, Neoptolemos JP, Sutton R, Petersen OH. Calcium-dependent enzyme activation and vacuole formation in the apical granular region of pancreatic acinar cells. Proc Natl Acad Sci USA 97: 13126–13131, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reed AM, Husain SZ, Thrower E, Alexandre M, Shah A, Gorelick FS, Nathanson MH. Low extracellular pH induces damage in the pancreatic acinar cell by enhancing calcium signaling. J Biol Chem 286: 1919–1926, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ricordi C. Methods in cell transplantation. In: Methods in Cell Transplantation. Austin, TX: Landes, 1995 [Google Scholar]
- 49.Sandow A. Excitation-contraction coupling in muscular response. Yale J Biol Med 25: 176–201, 1952 [PMC free article] [PubMed] [Google Scholar]
- 50.Sherwood MW, Prior IA, Voronina SG, Barrow SL, Woodsmith JD, Gerasimenko OV, Petersen OH, Tepikin AV. Activation of trypsinogen in large endocytic vacuoles of pancreatic acinar cells. Proc Natl Acad Sci USA 104: 5674–5679, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Straub SV, Giovannucci DR, Yule DI. Calcium wave propagation in pancreatic acinar cells: functional interaction of inositol 1,4,5-trisphosphate receptors, ryanodine receptors, and mitochondria. J Gen Physiol 116: 547–560, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Takasawa S, Kuroki M, Nata K, Noguchi N, Ikeda T, Yamauchi A, Ota H, Itaya-Hironaka A, Sakuramoto-Tsuchida S, Takahashi I, Yoshikawa T, Shimosegawa T, Okamoto H. A novel ryanodine receptor expressed in pancreatic islets by alternative splicing from type 2 ryanodine receptor gene. Biochem Biophys Res Commun 397: 140–145, 2010 [DOI] [PubMed] [Google Scholar]
- 53.Takeshima H, Nishimura S, Matsumoto T, Ishida H, Kangawa K, Minamino N, Matsuo H, Ueda M, Hanaoka M, Hirose T, Numa S. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature 339: 439–445, 1989 [DOI] [PubMed] [Google Scholar]
- 54.Tanaka Y, Tashjian AH., Jr Calmodulin is a selective mediator of Ca2+-induced Ca2+ release via the ryanodine receptor-like Ca2+ channel triggered by cyclic ADP-ribose. Proc Natl Acad Sci USA 92: 3244–3248, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Thorn P, Gerasimenko O, Petersen OH. Cyclic ADP-ribose regulation of ryanodine receptors involved in agonist evoked cytosolic Ca2+ oscillations in pancreatic acinar cells. EMBO J 13: 2038–2043, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Voronina S, Longbottom R, Sutton R, Petersen OH, Tepikin A. Bile acids induce calcium signals in mouse pancreatic acinar cells: implications for bile-induced pancreatic pathology. J Physiol 540: 49–55, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wakui M, Osipchuk YV, Petersen OH. Receptor-activated cytoplasmic Ca2+ spiking mediated by inositol trisphosphate is due to Ca2+-induced Ca2+ release. Cell 63: 1025–1032, 1990 [DOI] [PubMed] [Google Scholar]
- 58.Wakui M, Potter BV, Petersen OH. Pulsatile intracellular calcium release does not depend on fluctuations in inositol trisphosphate concentration. Nature 339: 317–320, 1989 [DOI] [PubMed] [Google Scholar]
- 59.Williams J, Xuequn C. Isolation, purification and protein content of pancreatic endoplasmic reticulum. In: The Pancreapedia: Exocrine Pancreas Knowledge Base. 2011 [Google Scholar]
- 60.Zhao Y, Graeff R, Lee HC. Roles of cADPR and NAADP in pancreatic cells. Acta Biochim Biophys Sin (Shanghai) 44: 719–729, 2012 [DOI] [PubMed] [Google Scholar]