Abstract
The forkhead box transcription factor FoxM1, a positive regulator of the cell cycle, is required for β-cell mass expansion postnatally, during pregnancy, and after partial pancreatectomy. Up-regulation of full-length FoxM1, however, is unable to stimulate increases in β-cell mass in unstressed mice or after partial pancreatectomy, probably due to the lack of posttranslational activation. We hypothesized that expression of an activated form of FoxM1 could aid in recovery after β-cell injury. We therefore derived transgenic mice that inducibly express an activated version of FoxM1 in β-cells (RIP-rtTA;TetO-hemagglutinin (HA)-Foxm1ΔNRD mice). This N-terminally truncated form of FoxM1 bypasses 2 posttranslational controls: exposure of the forkhead DNA binding domain and targeted proteasomal degradation. Transgenic mice were subjected to streptozotocin (STZ)-induced β-cell ablation to test whether activated FoxM1 can promote β-cell regeneration. Mice expressing HA-FoxM1ΔNRD displayed decreased ad libitum–fed blood glucose and increased β-cell mass. β-Cell proliferation was actually decreased in RIP-rtTA:TetO-HA-Foxm1NRD mice compared with that in RIP-rtTA mice 7 days after STZ treatment. Unexpectedly, β-cell death was decreased 2 days after STZ treatment. RNA sequencing analysis indicated that activated FoxM1 alters the expression of extracellular matrix and immune cell gene profiles, which may protect against STZ-mediated death. These studies highlight a previously underappreciated role for FoxM1 in promoting β-cell survival.
Type 1 diabetes and type 2 diabetes are both characterized by a decrease in β-cell mass. Therefore, augmenting β-cell proliferation and decreasing β-cell death are goals for new therapeutic approaches for both forms of diabetes. In mice, a single high-dose administration of streptozotocin (STZ) induces β-cell apoptosis and necrosis within 24 to 72 hours (1–4). After high-dose STZ-treatment, both degranulated and insulin-expressing β-cells show increased proliferation compared with that of β-cells in control mice, but this enhanced proliferation is not sufficient to increase β-cell mass, probably due to ongoing β-cell death (5, 6).
FoxM1 is a forkhead box transcription factor that promotes progression through the cell cycle in multiple cell types (7–10). FoxM1 is also required for normal β-cell proliferation and β-cell mass expansion in mice postweaning (11). Both male and female mice lacking Foxm1 specifically in the pancreas (Pdx1-Cre;Foxm1flox/flox mice) exhibit decreased β-cell mass by 4 weeks of age, and males exhibit glucose intolerance or overt diabetes by 9 weeks (11). Female Pdx1-Cre;Foxm1flox/flox (Foxm1Δpanc) mice display impaired β-cell mass expansion during pregnancy (12) or after 60% partial pancreatectomy (13), and they also demonstrate a greater impairment in glucose tolerance after high-fat diet feeding (14). Interestingly, Foxm1Δpanc mice display no change in β-cell apoptosis but do display an increase in β-cell necrosis (11).
FoxM1 is required for regeneration not only in β-cells but also in other cell types such as hepatocytes and lung endothelial cells; in addition, FoxM1 overexpression enhances regeneration in the lung, liver, and endothelial cells (15–19). Although mice overexpressing the human isoform FOXM1B under the control of the ubiquitous ROSA26 promoter display no overt abnormal phenotype when unstressed, these mice show enhanced recovery from a variety of insults (7, 20). For example, when subjected to partial hepatectomy, ROSA26-FOXM1B mice recover liver mass more swiftly than their control counterparts, and aged ROSA26-FOXM1B mice are able to regenerate hepatocytes as efficiently as young animals, which is not the case for control mice (16). The difference in the ability of overexpressed full-length FOXM1 to potentiate proliferation in stressed tissues while having little effect in unstressed tissues is in part conferred by posttranslational control of FoxM1 activity. Relief of intramolecular inhibition by an internal N-terminal repressor domain (NRD; amino acids 1–230) (21–23), subcellular localization (20, 24), protein degradation (25), and recruitment of coactivators such as p300 (26) are all controlled by phosphorylation events. One example of the control exerted on FoxM1 activity is demonstrated by the partial hepatectomy experiment mentioned above (20). Before partial hepatectomy, endogenous and exogenous forms of FoxM1 are sequestered in the cytoplasm of hepatocytes, but 15 minutes after partial hepatectomy, both are translocated to the nucleus due to phosphorylation by the MAPK pathway (20).
Contrary to what is observed in the liver, endogenous FoxM1 is normally located in the nuclei of β-cells (11). Despite this lack of requirement for translocation to the nucleus in β-cells, ROSA26-FOXM1B mice subjected to partial pancreatectomy do not regenerate β-cell mass more effectively than control mice (13), raising the possibility that pathways that normally stimulate FoxM1 function are not sufficiently present or activated within β-cells, even after a partial pancreatectomy. Recently, another group derived a transgenic mouse carrying a doxycycline (Dox)-inducible, green fluorescent protein (GFP)-tagged human FOXM1B with the NRD removed (GFP-FOXM1ΔNRD) (27). Deletion of the NRD removes not only a domain responsible for blocking transcriptional activity of FOXM1 but also sequences responsible for targeted FOXM1 degradation, creating a protein more stable than full-length FOXM1 (27). When expressed in the postnatal lung epithelium, GFP-FOXM1ΔNRD induces increased epithelial proliferation and an expansion of secretory Clara cells (27). These phenotypes are not observed in ROSA26-FOXM1B mice, indicating that the NRD does indeed function in vivo to block FOXM1 activation.
To determine whether an activated form of FoxM1 can promote β-cell regeneration, we derived novel transgenic mice in which activated FoxM1 expression can be induced at will and strikingly found that FoxM1 activation enhanced β-cell survival after STZ treatment, resulting in improved recovery from β-cell injury. This novel FoxM1 function suggests that FoxM1 could be targeted for the prevention of β-cell loss under conditions of β-cell stress, as occurs in individuals with diabetes.
Materials and Methods
Mice
RIP-rtTA mice have been described previously (28). For the derivation of TetO-HA-Foxm1ΔNRD mice, full-length mouse Foxm1 cDNA was transferred from pYX-Asc-Foxm1 (Thermo Scientific) into pcDNA3.1 (Invitrogen) using the EcoRI and NotI restriction sites. The coding region of Foxm1 corresponding to amino acids 231–747 and including the 3′ untranslated region was PCR amplified from pcDNA3.1-Foxm1. Primers used to amplify Foxm1 also contained an hemagglutinin (HA)-tag (5′) and sequences to add EcoRV restriction sites both 5′ and 3′. The PCR product was ligated into the EcoRV site of pTetO(7)/CMV/BGH.polyA/PBSIISK(Asci), a gift from Dr Timothy Blackwell (Vanderbilt University, Nashville, Tennessee). The region of interest was liberated from the plasmid backbone using AscI, gel purified, and injected into C57BL/6 blastocysts by the Vanderbilt Transgenic Core/ES Cell Shared Resource. The resulting mice were screened for the presence of the transgene using PCR. The 5′ primer annealed to the cytomegalovirus (CMV) minimal promoter, whereas the 3′ primer annealed to sequences within Foxm1 cDNA (5′-GGAGGTCTATATAAGCAGAGCTCG-3′ and 5′-CTCTCAGTGCTGTTGATGGCA-3′). Two founders were obtained and mated to B6/D2 mice. Offspring were genotyped for the transgene using PCR. Homozygous RIP-rtTA mice were mated to hemizygous TetO-HA-Foxm1ΔNRD mice. Experimental mice contained 1 allele each of RIP-rtTA and TetO-HA-Foxm1ΔNRD, whereas control mice contained 1 allele of RIP-rtTA. RIP-rtTA mice from both lines were combined for controls. Mice were maintained on a mixed C57BL6J/DBA2J background. Only male mice were used, except for Western blot analysis and experiments in which islets were treated with cytokines; for these, islets from both sexes were used. For analysis of protein expression, 2% Dox was administered in drinking water with Splenda; drinking water was replaced 3 times per week. For assessment of activated FoxM1 expression, 6-week-old mice were treated with Dox and examined at 8 weeks of age. For regeneration experiments, mice were treated with STZ at 8 weeks of age and immediately started on Dox-containing water for 1 week. Mice were kept in a 12-hour light/dark cycle and allowed to feed ad libitum. All mice were maintained and experiments conducted in accordance with protocols approved by the Vanderbilt Institutional Animal Care and Use Committee under the supervision of the Division of Animal Care.
Immunohistochemistry, immunofluorescence, and quantification of immunolabeling
The preparation of tissue and sections and the general protocols have been described previously (14, 29). Paraffin sections (14) were used for most immunolabeling experiments, but frozen sections (29) were used for brain and liver sections as well as immunolabeling for macrophages and B and T cells. Antigen retrieval in 10 mM citric acid buffer, pH 6.0, was required for anti-Ki67 and anti-HA immunolabeling. Alkaline phosphatase–conjugated secondary antibodies were visualized using Alkaline Phosphatase Substrate II (Vector Laboratories), and biotinylated secondary antibodies were visualized with 3,3′-diaminobenzidine (DAB) substrate (Vector) according to the manufacturer's instructions. Eosin Y (Thermo Fisher Scientific) was used as a counterstain when no alkaline phosphatase substrate was used. All slides were scanned with a 20× objective (300×) using a ScanScope CS or FL (Aperio; Leica). For β-cell mass assessment, approximately 2% of the pancreas was immunolabeled and analyzed (5–10 sections per animal, each separated by 250 μm). An algorithm developed from a Genie macro within Spectrum (Aperio) was used to identify β-cells and other tissue (30). The DAB+ percentage was multiplied by the pancreas weight to obtain β-cell mass. For each assay, 500 to 12 000 cells were counted for each mouse, since fewer cells were able to be counted for STZ-treated mice. For HA-positive cells, proliferating cells, or terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL)-positive cells, approximately 1% to 2% of the pancreas was immunolabeled and analyzed by manual counting using MetaMorph (Molecular Devices). Cell size was calculated by dividing the total insulin+ area (Aperio macro) by the number of insulin+ nuclei (manually counted). Antibodies and staining kits used were the following: mouse anti-HA (1:100; Cell Signaling); mouse anti-Ki67 (1:500; BD Biosciences); rabbit anti-Ki67 (1:500; Abcam); guinea pig anti-insulin (1:1000; Dako); rat anti-F4/80 (1:100; Millipore); rat-anti CD3 (1:100, BD Pharmingen); rat-anti CD45 (1:100; BD Pharmingen); rat-anti B220 (1:100; BD Pharmingen); and the ApoAlert DNA fragmentation assay (Clontech).
Islet isolation, RNA isolation, RT-quantitative PCR (qPCR), and TaqMan low-density array (TLDA)
Islet isolation and RNA extraction were performed by the Vanderbilt Islet Procurement and Analysis Core, and RNA quality assessment was performed by Vanderbilt Technology for Advanced Genomics (VANTAGE). qRT-PCR was performed as described previously (14), as was qPCR for determining the transgene copy number (31). For assessing total Foxm1 mRNA levels, primers were designed at the 3′ end of mRNA (12). For endogenous Foxm1, primers within the NRD coding region were used (F: 5′-CTCCAAGGCAAAGACAGGAG-3′ and R: 5′-GCCCGTCAGAACTCATCTTT-3′). For assessing the copy number, the following primers were used: 5′-CTGTGGCTCAGAGGAAGAATG-3′ and 5′-AGGCTCCCTGGTACGAGATAC-3′. The TLDA and analysis were performed according to the manufacturer's instructions (Applied Biosystems).
Protein extraction and immunoblotting
Islets were lysed in radioimmunoprecipitation assay lysis buffer (Sigma-Aldrich) with complete protease inhibitors (Roche Diagnostics) using mechanical disruption. Protein (20 μg) was loaded onto a NuPAGE 4% to 12% Bis-Tris gel (Life Technologies). Transfer was performed using standard techniques. HA-FoxM1ΔNRD was detected using a mouse-anti-HA antibody (1:2000; Cell Signaling) or rabbit-anti-FoxM1 antibody (1:250; Cell Signaling). Loading was examined with a rabbit-anti-β-tubulin antibody (1:5000–1:10 000; Cell Signaling). Band intensity was analyzed with ImageJ software (National Institutes of Health).
STZ administration and glucose measurements
STZ was reconstituted in cold 100 mM citric acid buffer, pH 4.5. On day (D) 0, 220 mg/kg STZ was administered ip after a 4-hour fast. Immediately after STZ administration, mice were treated with Dox and killed on D2 or D7. Ad libitum–fed glucose was measured daily with an Accu-Chek glucometer (Roche). Any mouse whose blood glucose did not increase by >100 mg/dL from pre-STZ treatment to D7 of STZ treatment was excluded from the experiment. This criterion excluded 4 of 37 mice for analysis. For D2, mice whose blood glucose did not increase by 100 mg/dL and whose β-cell mass did not differ from untreated controls were excluded (3 of 14).
Tissue culture and luciferase assays
INS-1 (832/13) cells were grown in RPMI 1640 medium supplemented with 10% fetal bovine serum (FBS) plus antibiotics. Medium was replaced with medium containing 10% Tet approved FBS (Clontech) 24 hours before transfections. Cells were transfected with pCMV-rtTA, pTetO-HA-Foxm1ΔNRD, and a plasmid containing luciferase downstream of a 6×-multimerized promoter fragment of Cdx2 (27). pCdx2-luciferase was a gift from Dr Vladamir Kalinichenko (University of Cincinnati, Cincinnatti, Ohio). Transfections were performed with Lipofectamine 2000 according to the manufacturer's directions (Life Technologies). Dox was added to the medium at a final concentration of 1 μg/mL. Luciferase assays were performed 24 hours after transfections and were conducted with the Dual-Luciferase Reporter Assay System (Promega).
For cytokine treatment of islets, mice were treated with Dox for 2 weeks before islet isolation. Islets were cultured in RPMI 1640 medium, 5.6 mM glucose, 10% FBS, and 1 μg/mL Dox overnight before treatment with human TNFα (20 ng/mL; Sigma-Aldrich), mouse interferon (IFN)-γ (10 ng/mL; Sigma-Aldrich), and mouse IL-1β. Islets were incubated for 48 hours before dispersal in 0.025% trypsin in 2 mM EDTA-1× PBS at 37°C for 6 minutes with manual disruption. Dispersion was halted with RPMI 1640 medium before islets were cytospun onto charged slides.
RNA sequencing (RNA-Seq)
Islets were isolated from 8-week-old RIP-rtTA;TetO-HA-Foxm1ΔNRD mice after 2 weeks of Dox administration. RNA was isolated using an RNAqueous kit according to the manufacturer's directions (Life Technologies). RNA-Seq was performed by VANTAGE. Alignment and basic transcript quantification were performed using RUM software (32), after which a script that uses edgeR was employed for calculating differential expression (33). Ingenuity (QIAGEN) and GOFFA (34) software were used to analyze changes in defined pathways and gene ontology (GO) functions, respectively. Ingenuity analysis was performed with help from Vanderbilt Technology for Advanced Genomics and Research Design.
Statistics
Other than RNA-Seq, data were analyzed with a two-tailed Student t test (Figures 1C and 2B), one-way ANOVA (Figures 1B, 2E, and 4A), or two-way ANOVA (Figures 3, A and B, 4, B and G, 5C, and 7L) with the Bonferroni post test or regression analysis (Figures 3, G–J, and 4, E and F) using GraphPad Prism (GraphPad Software).
Figure 1.
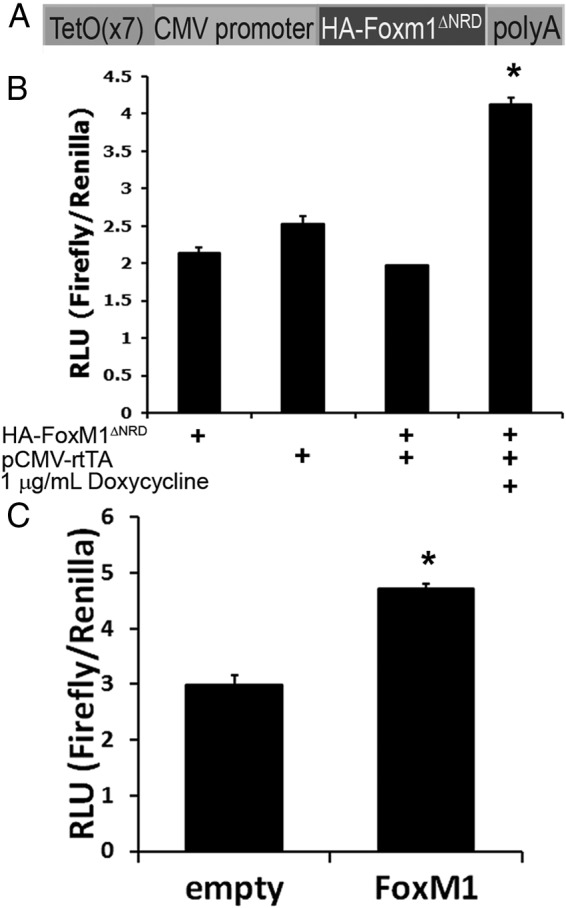
HA-tagged, activated FoxM1 functions normally in a β-cell line. A, An N-terminally HA-tagged FoxM1 construct with the N-terminal repressor domain removed was cloned into a plasmid containing 7 tetracycline operator repeats, a minimal CMV promoter, and a poly(A) tail. B, In INS-1 cells, combined addition of pTetO-HA-Foxm1ΔNRD, pCMV-rtTA, and Dox specifically activated a luciferase reporter plasmid containing a FoxM1-responsive promoter (n = 3, *, P < .0005 vs all controls). C, A plasmid encoding wild-type FoxM1 activates the same reporter as in B (n = 3; *, P < .0005). RLU, relative light units.
Figure 2.
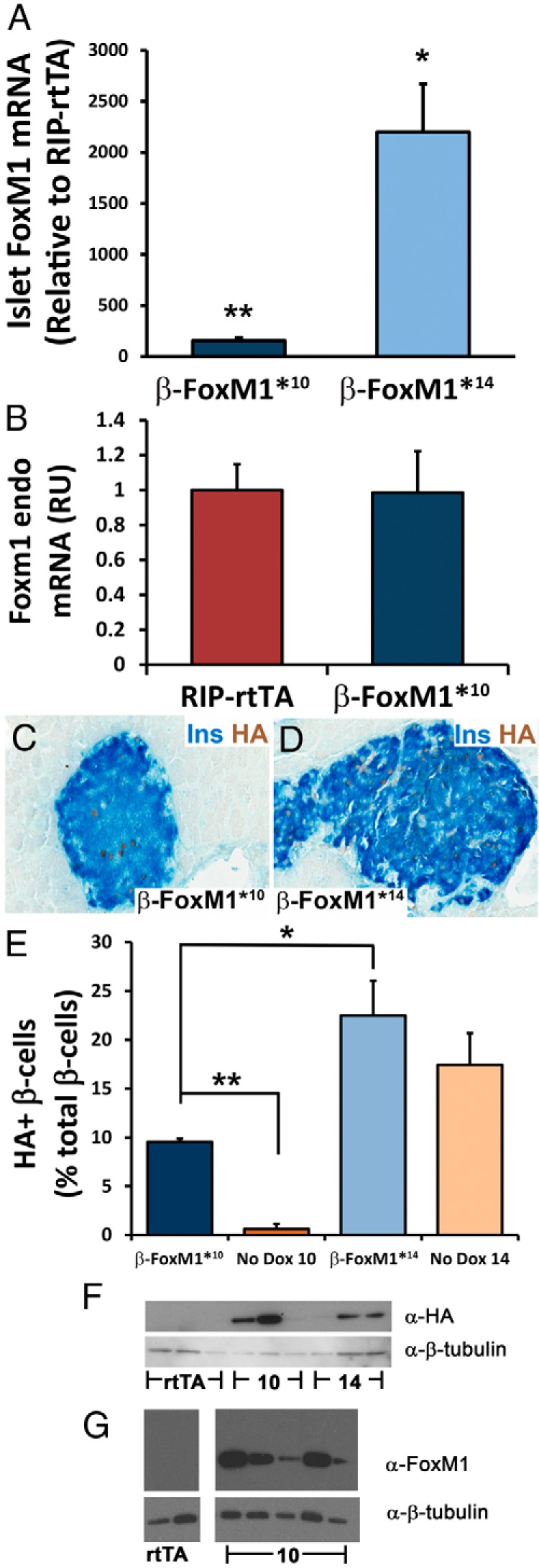
Characterization of FoxM1 expression in bitransgenic mice. A, After 2 weeks of Dox, total islet Foxm1 mRNA was up-regulated by approximately 150- or 2200-fold in the β-FoxM1*10 and β-FoxM1*14 lines, respectively, compared with that in littermate mice carrying the RIP-rtTA transgene alone (n = 3–5; *, P < .001; **, P < .0005). B, No change in endogenous Foxm1 expression was observed in β-FoxM1* mice. RU, relative units. C–E, Immunolabeling and assessment of HA-expressing insulin (Ins)-positive cells, with and without Dox treatment. In the presence of Dox, β-FoxM1*10 and β-FoxM1*14 mice express the transgene in approximately 10% and 22% of β-cells, respectively (n = 3; *, P < .05;**P < .00005). In the absence of Dox, β-FoxM1*10 and β-FoxM1*14 mice express the transgene in ∼0.5% and ∼17% of insulin-expressing cells, respectively. F and G, Western blots performed with total islet protein; β-tubulin is used as a loading control. F, An antibody against the HA-tag indicates that both lines (10 and 14) of mice express a transgenic protein of the predicted size of ∼60 kDa. G, Low levels of endogenous FoxM1 are undetectable, but higher levels of transgenic FoxM1 can be observed in the β-FoxM1*10 line.
Figure 4.
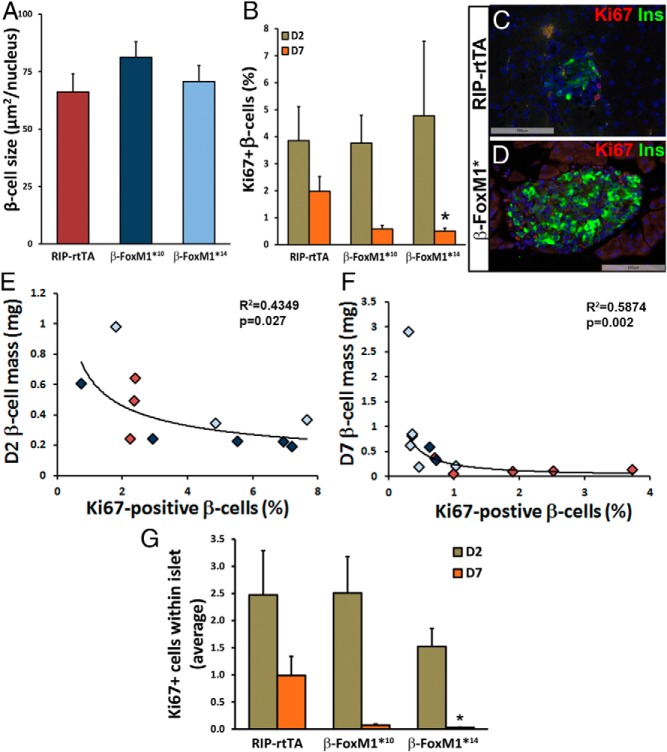
No increase in β-cell size or β-cell proliferation in β-FoxM1* mice. A, No change is observed in β-cell size at D7 (n = 3). B, No change in β-cell proliferation as measured by Ki67 colabeling is observed in β-FoxM1*10 or β-FoxM1*14 mice at D2, and by D7 after β-cell ablation, proliferation in insulin-positive cells is significantly decreased in β-FoxM1*14 mice (n = 3–5; *, P < .05). C and D, Representative images of Ki67 and insulin immunolabeling at D7 in RIP-rtTA (C) and β-FoxM1* (D) mice. E–H, The correlation between β-cell mass and percentages of Ki67-positive β-cells at D2 (E) and D7 (F) indicates that the diminished proliferation is due to an increase in β-cell mass. Red diamonds, RIP-rtTA mice; light blue diamonds, β-FoxM1*14 mice; dark blue diamonds, β-FoxM1*10 mice. G, Total proliferation within islets is diminished in β-FoxM1*14 mice at D7 (n = 3–5; *, P < .05).
Figure 3.
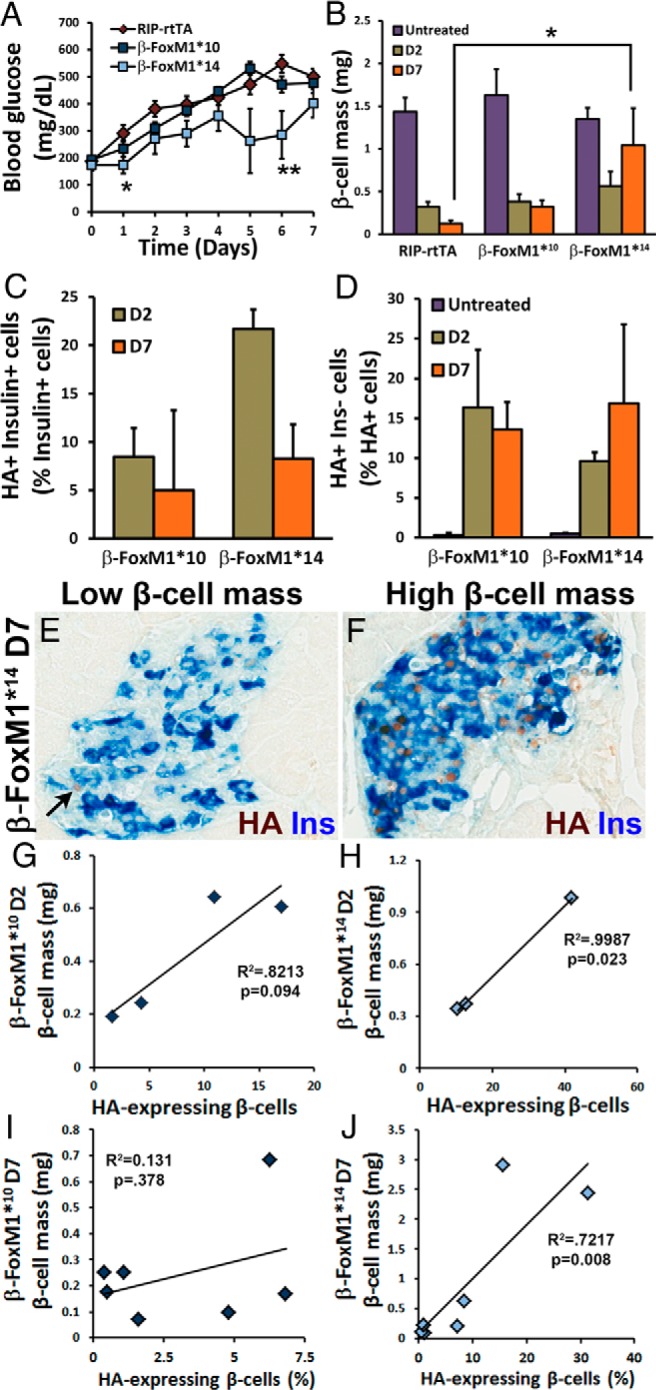
Pretreatment with activated FoxM1 protects against β-cell mass loss and aids in β-cell mass recovery after treatment with STZ. A, β-FoxM1*14 mice display decreased ad libitum blood glucose compared with that of RIP-rtTA mice in the week after STZ administration (n = 7–14; *, P < .05). B, No difference in β-cell mass is observed after 2 weeks of Dox in either β-FoxM1* line without STZ treatment. At D2, no significant difference is observed in β-cell mass between RIP-rtTA and either line of β-FoxM1* mice. RIP-rtTA mice continue to lose β-cell mass between D2 and D7, but β-cell mass in β-FoxM1*10 mice plateaus between D2 and D7. β-FoxM1*14 mice exhibit higher β-cell mass at D7 than RIP-rtTA mice, and β-cell mass is increased in this line compared with that at D2. β-Cell mass in β-FoxM1*14 mice is significantly higher than that in RIP-rtTA mice by D7 after STZ treatment. β-FoxM1*10 mice display a trend toward an increase in β-cell mass by D7 (n = 3–5 for D2, n = 7–11 for D7; P < .05). C–J, The percentages of β-cells expressing HA-FoxM1ΔNRD at D2 and D7 after STZ treatment positively correlate with β-cell mass. C, HA-FoxM1ΔNRD expression in insulin+ cells (n = 3–4). D, HA-FoxM1ΔNRD expression in insulin− cells. E and F, Dox-treated β-FoxM1* mice with low β-cell mass 7 days after STZ treatment (E) express HA-FoxM1ΔNRD in fewer insulin (Ins)+ cells than β-FoxM1* mice with high β-cell mass (F). The arrow in E denotes an insulin− cell expressing HA-FoxM1ΔNRD. This correlation is quantified in both β-FoxM1*10 (G and I) and β-FoxM1*14 (H and J) mice at both D2 (G and H) and D7 (I and J).
Figure 5.
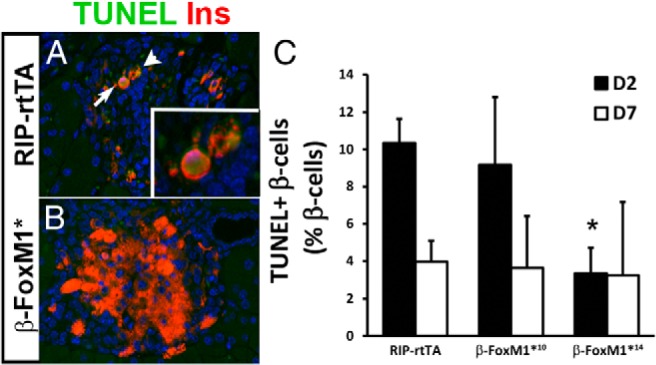
Decreased cell death is responsible for the increase in β-cell mass in β-FoxM1* mice. A and B, TUNEL labeling in RIP-rtTA (A) and β-FoxM1* (B) mice at D2. C, Quantification of TUNEL-labeled β-cells at D2 reveals a decrease in cell death in β-FoxM1*14 mice (n = 3;*, P < .05 vs RIP-rtTA D2).
Figure 7.
Increased immune cell infiltration in β-FoxM1* mice. A–C, No macrophages are observed near the islets of RIP-rtTA (A) or β-FoxM1*14 (B) mice, but spleen serves as an internal control for F4/80 immunolabeling (C). D and E, Immunolabeling for the common leukocyte marker CD45 indicates more leukocytes near islets of β-FoxM1*14 mice (E) than of RIP-rtTA mice (D). F and G, B220 immunolabeling indicates more B cells (arrows) near β-FoxM1*14 islets (G) than control islets (F). H and I, Increased T-cell infiltration (arrow) near β-FoxM1*14 islets than control islets, as indicated by CD3 immunolabeling. J and L, Isolated islets were treated with TNFα, IFNγ, and IL-1β for 48 hours, after which they were dispersed, and a TUNEL assay was performed (n = 2–8).
Results
Characterization of activated FoxM1 construct and transgenic lines
To derive a mouse model with inducible Foxm1 expression, an HA-tagged, activated Foxm1 cDNA was placed in a plasmid downstream of the tetracycline operator and a minimal CMV promoter (TetO; Figure 1A). Before deriving mouse lines, this construct was tested for activity in the immortalized rat β-cell line INS-1 using a FoxM1-responsive luciferase reporter (35). FoxM1 activity was up-regulated approximately 2-fold after the addition of TetO-HA-Foxm1ΔNRD and rtTA plasmids and 1 μg/mL Dox (Figure 1B). This low magnitude of HA-FoxM1ΔNRD–mediated luciferase induction was not unexpected, because FoxM1 is already highly expressed in all immortalized cell lines; however, higher activation of the luciferase construct has been reported in other cell lines using plasmids expressing wild-type FoxM1 (35). Therefore, to ensure that the HA-tagged FoxM1ΔNRD is not less functional than full-length FoxM1, wild-type FoxM1 activity in INS-1 cells was also examined. Compared with an empty vector, a vector containing wild-type FoxM1 up-regulated luciferase activity by approximately 1.6-fold (Figure 1C), indicating that the activities of HA-FoxM1ΔNRD and wild-type FoxM1 are similar in a setting of high endogenous FoxM1 levels and in the presence of other factors in cell culture systems that promote proliferation. Cyclin-dependent kinase 2 (Cdk2), which relieves the inhibition of the NRD, is already higher in cell culture lines than in their in vivo counterparts (36, 37); thus, in cell culture, removal of the NRD is less likely to have additional effects than in vivo.
Two transgenic mouse lines (nos. 10 and 14) carrying the TetO-HA-Foxm1ΔNRD construct were derived and mated to RIP-rtTA mice. Subsequently, RIP-rtTA;TetO-HA-Foxm1ΔNRD mice treated with Dox will be referred to as β-FoxM1* mice. Sixteen and 8 copies of the transgene were integrated into the genomes of lines 10 and 14, respectively, as measured by real-time qPCR (data not shown). To determine the degree of Foxm1 mRNA up-regulation in β-FoxM1* mice, we performed qPCR analysis on islets isolated from 8-week-old male mice treated with Dox for 2 weeks. Foxm1 mRNA was increased by approximately 150-fold in β-FoxM1*10 mice and 2200-fold in β-FoxM1*14 mice (Figure 2A). This increase in Foxm1 mRNA is entirely due to expression of the transgene because no change was observed in endogenous Foxm1 mRNA levels (Figure 2B). Immunohistochemistry against both insulin and the HA-tag of the transgenic protein demonstrated nuclear expression of HA-FoxM1ΔNRD in ∼10% of β-cells in β-FoxM1*10 mice and in ∼22% of β-cells in β-FoxM1*14 mice in the presence of Dox (Figures 2, C–E). The greater increase in mRNA compared with the number of cells expressing HA-FoxM1ΔNRD probably reflects regulation of FoxM1 translation or degradation. No cytoplasmic localization was observed, consistent with a previous report of endogenous FoxM1 in murine islets (11). In the absence of Dox, RIP-rtTA;HA-Foxm1ΔNRD10 mice express HA-FoxM1ΔNRD in ∼0.5% of β-cells, whereas RIP-rtTA;HA-Foxm1ΔNRD14 mice express HA-FoxM1ΔNRD in ∼17% of β-cells. Expression of HA-FoxM1ΔNRD in RIP-rtTA;HA-Foxm1ΔNRD14 mice before and after Dox administration was not significantly different (Figure 2E). This Dox-independent expression is probably due to integration in a transcriptionally active site in the genome but is fully dependent on the presence of rtTA. Mice carrying only an HA-Foxm1ΔNRD14 allele display no HA+ cells within the pancreas (data not shown). Very few insulin-negative cells (0.48% ± 0.12% in line 14 and 0.30% ± 0.30% in line 10; n = 3) were HA+ in either transgenic line (Figure 2, C and D, and Figure 3D).
After Dox treatment, both lines of transgenic mice expressed a protein of the predicted size of approximately 60 kDa within islets (Figure 2F), whereas RIP-rtTA mice do not express a protein recognized by the HA antibody, as expected. Quantification revealed an HA/β-tubulin ratio of 9.64 ± 4.06 for β-FoxM1*10 islets and 3.14 ± 1.05 in β-FoxM1*14 islets. Considering that β-FoxM1*10 mice express the transgene in fewer β-cells than β-FoxM1*14 mice, β-FoxM1*10 mice probably have higher expression of HA-FoxM1ΔNRD per HA+-β-cell. An antibody directed against the carboxyl terminus of FoxM1 that recognizes both the endogenous and transgenic forms of the protein, detected only transgenic FoxM1 expression, probably because endogenous islet FoxM1 expression is below the level of detection at this age (Figure 2G). Both antibodies revealed high variability in transgenic protein expression per animal. Despite expression of HA-FoxM1ΔNRD within β-FoxM1*14 islets in the absence of Dox, no transgene expression was observed in other metabolically responsive tissues such as the brain or liver (Supplemental Figure 1).
Activated FoxM1 promotes β-cell survival after STZ
To determine whether activated FoxM1 can stimulate β-cell proliferation and regeneration, β-cells were partially ablated using a single high dose of STZ. After STZ injections, mice were immediately treated with Dox to induce HA-FoxM1ΔNRD expression in the remaining β-cells. Blood glucose was monitored daily, and β-FoxM1*14 mice, which express HA-FoxM1ΔNRD before STZ treatment, displayed lowered ad libitum–fed blood glucose during the week after STZ treatment (Figure 3A). β-Cell mass was examined on D2 and D7 after STZ treatment. On D2, β-cell mass in STZ-treated RIP-rtTA mice was reduced by 80% compared with that in untreated RIP-rtTA mice. β-FoxM1*10 and β-FoxM1*14 mice displayed β-cell mass reductions of approximately 75% and 60%, respectively; however, the attenuation in β-cell mass loss was not statistically significant at this time point. On D7, β-cell mass in STZ-treated RIP-rtTA mice was further reduced to approximately 10% of that in untreated RIP-rtTA mice, and β-cell mass in STZ-treated β-FoxM1*10 mice remained at 20% of untreated β-cell mass levels. In contrast, β-FoxM1*14 mice displayed an increase in β-cell mass to approximately 75% of that observed in untreated β-FoxM1*14 mice and 10-fold higher than that in RIP-rtTA mice at D7 (Figure 3B).
Correlation of β-cell mass and HA-FoxM1ΔNRD expression after STZ treatment
To examine whether HA-FoxM1ΔNRD protein expression was maintained after STZ-treatment, colabeling with HA and insulin antibodies was performed. HA-FoxM1ΔNRD protein was still expressed in β-cells of STZ-treated β-FoxM1* mice. β-FoxM1*10 mice expressed the transgene in ∼9% of insulin-positive cells at both D2 and D7. β-FoxM1*14 mice expressed the transgene in ∼22% of β-cells at D2 and ∼8% of β-cells at D7 (Figure 3C). In contrast to untreated mice, STZ-treated β-FoxM1* mice expressed HA-FoxM1ΔNRD in many insulin− cells (Figure 3, D and E). These insulin−, HA+ cells are probably degranulated β-cells, resulting in a perceived reduction in the percentage of HA+ β-cells after STZ. Strikingly, mice with higher β-cell mass tended to have a higher percentage of β-cells expressing transgenic FoxM1 (Figure 3, E–J). This correlation was significant in β-FoxM1*14 mice on both D2 and D7. The correlation was weaker in β-FoxM1*10 mice at D2 and disappeared at D7.
β-Cell size, proliferation, and cell death after STZ treatment
We next examined the mechanism behind β-cell mass recovery in β-FoxM1*14 mice. No difference in β-cell size was observed between RIP-rtTA mice and β-FoxM1* mice on D7, indicating that hypertrophy was not contributing to recovery (Figure 4A).
Because FoxM1 is required for β-cell proliferation in other circumstances, we examined whether activated FoxM1 promoted β-cell mass recovery via β-cell proliferation after STZ treatment. No difference was observed in the percentage of Ki67-positive β-cells on D2, but on D7, a trend toward decreased proliferation in β-FoxM1*10 mice and a significant decrease in β-FoxM1*14 mice was apparent (Figure 4, B–D). Because β-FoxM1*14 mice had significantly increased β-cell mass on D7 and because increased β-cell mass could result in a decreased need to expand further, the correlation between proliferation and β-cell mass was determined (Figure 4, E and F). β-Cell proliferation negatively correlated with β-cell mass on D2 and D7, indicating that the likely reason for decreased proliferation in β-FoxM1*14 mice at D7 is the increased β-cell mass recovery observed at this time point.
Alternative explanations for the perceived reduction in β-cell proliferation are that the β-cells degranulate or partially dedifferentiate before undergoing replication (38) or that endocrine cells other than β-cells are proliferating and converting into β-cells. If either of these scenarios occurred, counting only Ki67+ and insulin+ cells would underestimate the total number of replicating cells contributing to the final β-cell population. All proliferating cells within the islet perimeter were therefore quantified to account for these 2 alternative possibilities. As with proliferating insulin-positive cells, β-FoxM1*14 mice displayed significantly less islet cell proliferation on D7 than RIP-rtTA mice (Figure 4G); therefore, the increased β-cell mass observed in β-FoxM1*14 mice after STZ treatment is not due to enhanced proliferation of mature β-cells, dedifferentiated or degranulated β-cells, or other endocrine cell types that subsequently undergo a conversion to β-cells.
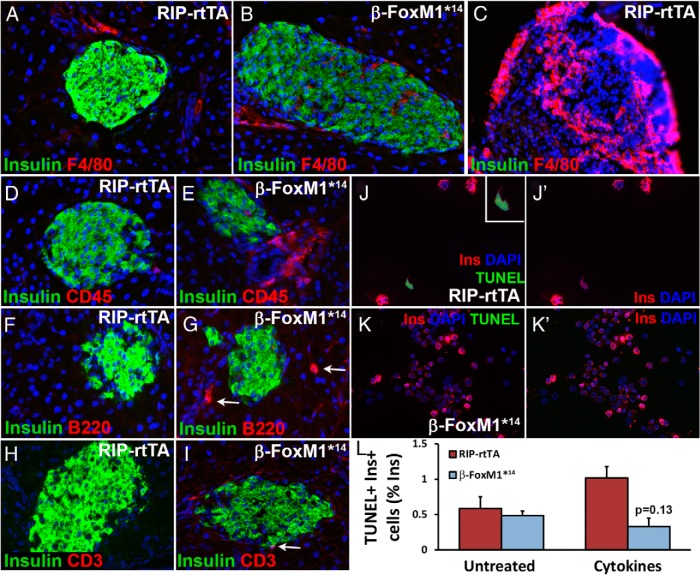
In addition to increased proliferation or individual cell size, reduced cell death can increase β-cell mass. On D2, β-cells in β-FoxM1*14 mice demonstrated a significant decrease in TUNEL labeling compared with that of RIP-rtTA mice; this difference was no longer apparent on D7 (Figure 5). These data suggest that expression of activated FoxM1 in β-cells protects against STZ-mediated cell death.
Altered immune response in β-FoxM1* mice
To investigate the possibility that FoxM1 mediates expression of genes involved in cell survival, we performed RNA-Seq analysis on islets from 8-week-old β-FoxM1*10 mice to identify genes differentially expressed after 2 weeks of Dox-induced HA-FoxM1ΔNRD expression. This transgenic line was chosen because very little activated FoxM1 expression was observed before Dox administration, and gene expression differences after only 2 weeks of HA-FoxM1ΔNRD activity are less likely to be due to compensatory changes that may be caused by continuous overexpression from the earliest stages of pancreas development, as occurs in the β-FoxM1*14 line. Approximately 250 genes exhibited significant differential expression between Dox-treated RIP-rtTA and β-FoxM1*10 mice (Supplemental Table 1). Because only 10% of β-cells in the β-FoxM1*10 line express the transgenic protein and RNA-Seq was performed on RNA from total islets, this analysis probably missed genes that are changed by a small magnitude.
To understand the biological relevance of genes altered in islets of β-FoxM1* mice, GO functions of significantly changed genes were examined using GOFFA software (Table 1) (34). Most of the GO function groups that were altered had some role in cell-cell adhesion and migration, such as changes in collagen and extracellular matrix (ECM). These GO functions are not unexpected because up-regulation of FoxM1 in cancer can increase metastasis (39). The ECM has a protective effect against β-cell death (40); therefore, changes in collagen and other ECM components could be partially responsible for the resistance to STZ displayed by β-FoxM1*14 mice.
Table 1.
Top altered GO functions between β-FoxM1*10 and RIP-rtTA islets
| GO Function | P | E |
|---|---|---|
| Molecular function | ||
| Platelet-derived growth factor binding | <.000001 | 42.74 |
| Extracellular matrix structural constituent | <.000001 | 11.47 |
| Integrin binding | .000014 | 8.89 |
| Identical protein binding | .000019 | 2.73 |
| Protein homodimerization activity | .000023 | 3.38 |
| Protein dimerization activity | .000028 | 2.84 |
| Structural molecule activity | .000033 | 2.9 |
| Amine binding | .000085 | 5.7 |
| Vitamin binding | .000099 | 5.57 |
| Growth factor binding | .000229 | 5.72 |
| Biological processes | ||
| Cell adhesion | <.000001 | 2.96 |
| Biological adhesion | <.000001 | 2.96 |
| Collagen fibril organization | <.000001 | 22.7 |
| Digestion | .000002 | 7.98 |
| Extracellular matrix organization | .000003 | 7.62 |
| Protein heterotrimerization | .000023 | 47.02 |
| Skin development | .000037 | 13.06 |
| Regulation of triglyceride catabolic process | .00004 | 40.3 |
| Cell-cell adhesion | .00005 | 3.74 |
| Amine transport | .00008 | 5.04 |
| Cellular component | ||
| Fibrillar collagen | <.000001 | 50.63 |
| Platelet-derived growth factor binding | <.000001 | 42.74 |
| Collagen fibril organization | <.000001 | 22.7 |
| Extracellular matrix structural constituent | <.000001 | 11.47 |
| Collagen | <.000001 | 10.69 |
| Extracellular matrix part | <.000001 | 7.88 |
| Proteinaceous extracellular matrix | <.000001 | 5.64 |
| Extracellular matrix | <.000001 | 5.06 |
| Extracellular region part | <.000001 | 3.75 |
| Extracellular space | <.000001 | 3.44 |
Pathways were considered significant if E = >2 and P < .005.
To find other pathways possibly mediating the decreased β-cell death observed in β-FoxM1*14 mice, we used Ingenuity Pathway Analysis. Three of the top 5 altered pathways contained cytokines and genes expressed in immune cells. Most of the observed changes were either down-regulation of markers for inflammatory cells such as macrophages or up-regulation of markers for anti-inflammatory T cells. In addition, genes with known protective effects against STZ treatment were up-regulated in β-FoxM1*10 mice, including Vgf (41) and p21 (42) (Supplemental Table 1).
To confirm that the gene expression changes observed in the β-FoxM1*10 line were also present in the β-FoxM1*14 line, we designed a TLDA, a 384-well plate preloaded with primers and probes for qPCR. We examined a subset of genes of interest using the TLDA. Islets from β-FoxM1*14 mice before and 2 weeks after Dox treatment displayed down-regulation of the cytokines CCL19 and IFNγ, the interleukin IL10, and the chemokine Xcl1, confirming gene expression changes observed in β-FoxM1*10 islets by RNA-Seq (Figure 6A and data not shown). Vgf expression was examined by immunohistochemistry. More cells expressing Vgf were observed in the islets of β-FoxM1*10 and β-FoxM1*14 mice after Dox treatment than in RIP-rtTA islets (Figure 6, B and C).
Figure 6.
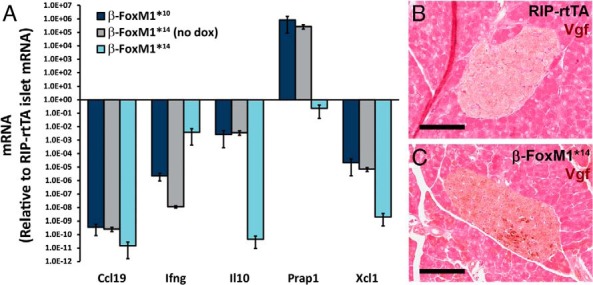
Confirmation of gene expression changes observed in β-FoxM1*10 mice by RNA-Seq. A, TLDA performed on islets from β-FoxM1*10 mice, as well as β-FoxM1*14 mice before and after Dox treatment. Gene expression is normalized to a control mouse. B and C, Vgf immunolabeling on Dox-treated β-FoxM1*14 mice (scale bar corresponds to 100 μm).
The presence of immune cells in and near islets in Dox-treated control and β-FoxM1*14 mice was assessed using immunohistochemistry. Very few macrophages, as detected by immunolabeling against F4/80, were observed within the pancreatic parenchyma of either control or β-FoxM1*14 mice, and almost none were observed close to islets (Figure 7, A–C). Lymph nodes within the pancreas served as a control for F4/80 immunolabeling of macrophages (Figure 7C). More leukocytes (CD45+ cells) were detected in and near β-FoxM1*14 mice islets than control islets (Figure 7, D and E). To determine which subsets of leukocytes were adjacent to β-FoxM1*14 islets, immunolabeling against B220 (B cells) and CD3 (T cells) was performed, which indicated that the leukocyte population consisted of both B and T cells (Figure 7, F–I).
To determine whether activated FoxM1 directly protects β-cells from cytokine-induced cell death, we treated islets isolated from RIP-rtTA and β-FoxM1*14 mice with a combination of TNFα, IFNγ, and IL-1β for 2 days. Islets were then dispersed and assessed for β-cell death. After cytokine treatment, β-cells from Dox-treated β-FoxM1*14 mice displayed a trend toward reduced β-cell death compared with those from RIP-rtTA mice (Figure 7, J–L).
Discussion
We derived 2 lines of transgenic mice expressing a truncated, activated form of FoxM1 under the control of the tetracycline operator. When bred to RIP-rtTA mice, the β-FoxM1*10 line was appropriately responsive to Dox, expressing detectable HA-FoxM1ΔNRD in ∼10% of β-cells after Dox administration and in only ∼0.5% of β-cells in the absence of Dox. In contrast, β-FoxM1*14 mice expressed HA-FoxM1ΔNRD in ∼17% of β-cells before and in ∼22% after Dox.
Neither line of β-FoxM1* mice displayed increased β-cell mass in the absence of a proliferative stimulus at the age studied here. The reason could be that FoxM1 is not limiting at younger ages, because the capacity for β-cell proliferation is not as limited in younger mice as in older mice (43). In addition, the repressive effect of the NRD is not the only mechanism by which FoxM1 activity is controlled, and FoxM1 could require a second stimulus, such as high blood glucose, to be activated fully.
Mice expressing activated FoxM1 both before and after STZ administration (β-FoxM1*14) but not after STZ treatment alone (β-FoxM1*10) displayed reduced proliferation and β-cell death but increased β-cell mass compared with those of RIP-rtTA mice, indicating that FoxM1 is acting to prevent β-cell death. Compared with untreated mice, both lines of β-FoxM1* mice displayed increased β-cell proliferation 2 days after STZ, which is typically between 0.1 and 0.5% in young, unstimulated adult mice (44, 45). However, because proliferation was reduced compared with STZ-treated RIP-rtTA mice, the increased proliferation alone cannot explain the increased β-cell mass in β-FoxM1*14 mice.
FoxM1 is typically thought of as a regulator of the cell cycle. Because the increase in β-cell mass in β-FoxM1*14 mice is due at least in part to decreased β-cell death, RNA-Seq was used to identify genes altered upon FoxM1 overexpression that could prevent β-cell death. β-FoxM1* mice displayed altered gene regulation of ECM components, chemokines and cytokines, p21, and the neurosecretory protein precursor Vgf. In addition, FoxM1 is also a transcriptional regulator of survivin (46), which has been shown to enhance survival of transplanted islets due to decreased apoptosis (47). Islets from β-FoxM1*10 mice did not display differences in survivin, but a change in gene expression may have been too small to detect because activated FoxM1 was only expressed in ∼10% of β-cells. β-FoxM1* mice also displayed increased numbers of leukocytes near islets compared with those in control islets. Although immune cells are typically thought of as proapoptotic when associated with islets, certain types of immune cells can protect against type 1 diabetes (48, 49), and immune cells have been reported to have a prosurvival or recovery effect for β-cells and other cell types (50–52). In light of our findings, it is noteworthy that other groups have recently reported defects in immune cell migration in Foxm1 loss-of-function models (53, 54). These data indicate that activated FoxM1 is acting as a prosurvival factor. This effect is probably mediated by Vgf, p21, changes in ECM components, and immune cell infiltration, all of which are known to protect against β-cell death (41). Although we cannot rule out the possibility that chronic exposure of β-cells to activated FoxM1 in line 14 results in gene expression changes that alter influx of STZ, it is clear that STZ is causing significant (although reduced) β-cell death in this line because β-cell mass is reduced by D2.
On D2 after STZ treatment, β-FoxM1*14 mice displayed decreased blood glucose levels before an increase in β-cell mass was observed. The discrepancy in blood glucose and β-cell mass could indicate that β-FoxM1*14 mice are either more insulin sensitive than control mice, which seems unlikely given the fact that we did not alter FoxM1 expression in insulin-responsive tissues, or that β-FoxM1* β-cells have enhanced insulin secretion. This latter conclusion is supported by the discrepancy in β-cell mass and glucose tolerance in Foxm1Δpanc male mice. These mice display glucose intolerance or diabetes by 9 weeks of age, although their β-cell mass is only reduced by 60% (11). Many other mouse models of genetic or physical β-cell ablation display normal glucose tolerance with a reduction in β-cell mass up to 70% (29, 55, 56). Maintenance of euglycemia despite a severe reduction in β-cell mass echoes what is observed in type 1 diabetic patients: they display the most severe symptoms only after a loss in β-cell mass of >90% (57).
Although β-cell mass in STZ-treated β-FoxM1*14 mice is increased to approximately 75% of that observed in untreated β-FoxM1*14 mice, blood glucose is elevated over pre-STZ levels. This may be due either to the immaturity of recently replicated β-cells or altered islet morphology; islets from β-FoxM1*14 mice tend to have gaps in insulin immunoreactivity on D7 after STZ treatment (Figures 3, C and D, and 4D), which could disrupt intraislet communication and thus coordinated insulin secretion (58).
Compared with control STZ-treated mice, mice expressing an activated form of FoxM1 exhibited higher β-cell mass without increased β-cell proliferation than RIP-rtTA mice. This augmented β-cell mass was achieved by a reduction in β-cell death. This is the first evidence that FoxM1 plays a protective role and enhances β-cell survival. Recently, interest in identifying therapeutic targets capable of inhibiting β-cell death in addition to increasing β-cell proliferation has been rekindled. We propose that FoxM1 is a good experimental target for achieving both of these goals simultaneously.
Additional material
Supplementary data supplied by authors.
Acknowledgments
We thank Dr Timothy Blackwell for the gift of the pTetO vector, Dr Vladamir Kalinichenko for the gift of the FoxM1-responsive Cdx2-luciferase plasmid, Dr Travis Clark in Vanderbilt Technology for Advanced Genomics, and Drs Yu Shyr and Yan Guo in Vanderbilt Technology for Advanced Genomics and Research Design for help with RNA-Seq and pathway analysis, and Dr Danielle Dean and Bethany Carboneau for technical assistance.
This work was supported by the VA (Grant 1BX000990–01A1 to M.A.G.) and the Juvenile Diabetes Research Foundation (Grant 3–2010-563 to M.L.G.). M.L.G. was also supported by the National Cancer Institute (Integrated Biological Systems Training in Oncology Training Grant 5T32 CA119925). The Islet Procurement and Analysis Core of the Vanderbilt Diabetes Research and Training Center is supported by the National Institutes of Health (Grant DK20593). The Transgenic Mouse/ESC Shared Resource is supported by the Cancer Center (Support Grant P30 CA68485), the Vanderbilt Diabetes Research and Training Center (Grant DK20593), the Center for Molecular Neuroscience, and the Center for Stem Cells. VANTAGE is supported by the Vanderbilt Ingram Cancer Center (Grant P30 CA58645), the Vanderbilt Vision Center (Grant P30 EY08126), and the National Institutes of Health/National Center for Research Resources (Grant G20 RR030956).
M.L.G. designed and performed experiments and wrote and edited the manuscript. M.F.M. and J.C.D. performed experiments. J.S. analyzed RNA sequencing data. K.H.K. oversaw RNA sequencing analysis and edited the manuscript. M.A.G. designed experiments and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CMV
- cytomegalovirus
- D
- day
- DAB
- 3,3′-diaminobenzidine
- Dox
- doxycycline
- ECM
- extracellular matrix
- FBS
- fetal bovine serum
- Fox
- forkhead box
- GFP
- green fluorescent protein
- GO
- gene ontology
- HA
- hemagglutinin
- IFN
- interferon
- NRD
- N-terminal repressor domain
- qPCR
- quantitative PCR
- RNA-Seq
- RNA sequencing
- STZ
- streptozotocin
- TLDA
- TaqMan low-density array
- TUNEL
- terminal deoxynucleotidyl transferase dUTP nick-end labeling
- VANTAGE
- Vanderbilt Technology for Advanced Genomics.
References
- 1. Like AA, Rossini AA. Streptozotocin-induced pancreatic insulitis: new model of diabetes mellitus. Science. 1976;193:415–417. [DOI] [PubMed] [Google Scholar]
- 2. Junod A, Lambert AE, Orci L, Pictet R, Gonet AE, Renold AE. Studies of the diabetogenic action of streptozotocin. Proc Soc Exp Biol Med. 1967;126:201–205. [DOI] [PubMed] [Google Scholar]
- 3. Saini KS, Thompson C, Winterford CM, Walker NI, Cameron DP. Streptozotocin at low doses induces apoptosis and at high doses causes necrosis in a murine pancreatic β cell line, INS-1. Biochem Mol Biol Int. 1996;39:1229–1236. [DOI] [PubMed] [Google Scholar]
- 4. Takamura T, Ando H, Nagai Y, Yamashita H, Nohara E, Kobayashi K. Pioglitazone prevents mice from multiple low-dose streptozotocin-induced insulitis and diabetes. Diabetes Res Clin Pract. 1999;44:107–114. [DOI] [PubMed] [Google Scholar]
- 5. Kodama S, Toyonaga T, Kondo T, et al. Enhanced expression of PDX-1 and Ngn3 by exendin-4 during β cell regeneration in STZ-treated mice. Biochem Biophys Res Commun. 2005;327:1170–1178. [DOI] [PubMed] [Google Scholar]
- 6. Li L, Seno M, Yamada H, Kojima I. Betacellulin improves glucose metabolism by promoting conversion of intraislet precursor cells to β-cells in streptozotocin-treated mice. Am J Physiol Endocrinol Metab. 2003;285:E577–E583. [DOI] [PubMed] [Google Scholar]
- 7. Ye H, Holterman AX, Yoo KW, Franks RR, Costa RH. Premature expression of the winged helix transcription factor HFH-11B in regenerating mouse liver accelerates hepatocyte entry into S phase. Mol Cell Biol. 1999;19:8570–8580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Korver W, Schilham MW, Moerer P, et al. Uncoupling of S phase and mitosis in cardiomyocytes and hepatocytes lacking the winged-helix transcription factor Trident. Curr Biol. 1998;8:1327–1330. [DOI] [PubMed] [Google Scholar]
- 9. Wang IC, Chen YJ, Hughes DE, et al. FoxM1 regulates transcription of JNK1 to promote the G1/S transition and tumor cell invasiveness. J Biol Chem. 2008;283:20770–20778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Petrovic V, Costa RH, Lau LF, Raychaudhuri P, Tyner AL. FoxM1 regulates growth factor-induced expression of kinase-interacting stathmin (KIS) to promote cell cycle progression. J Biol Chem. 2008;283:453–460. [DOI] [PubMed] [Google Scholar]
- 11. Zhang H, Ackermann AM, Gusarova GA, et al. The FoxM1 transcription factor is required to maintain pancreatic beta-cell mass. Mol Endocrinol. 2006;20:1853–1866. [DOI] [PubMed] [Google Scholar]
- 12. Zhang H, Zhang J, Pope CF, et al. Gestational diabetes mellitus resulting from impaired β-cell compensation in the absence of FoxM1, a novel downstream effector of placental lactogen. Diabetes. 2010;59:143–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ackermann Misfeldt A, Costa RH, Gannon M. β-Cell proliferation, but not neogenesis, following 60% partial pancreatectomy is impaired in the absence of FoxM1. Diabetes. 2008;57:3069–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Golson ML, Ackermann AM, Kopsumbut UG, Petersen CP, Gannon MA. High fat diet regulation of β-cell proliferation and β-cell mass. Open Endocrinol. 2010;4:66–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu Y, Sadikot RT, Adami GR, et al. FoxM1 mediates the progenitor function of type II epithelial cells in repairing alveolar injury induced by Pseudomonas aeruginosa. J Exp Med. 2011;208:1473–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang X, Krupczak-Hollis K, Tan Y, Dennewitz MB, Adami GR, Costa RH. Increased hepatic forkhead box M1B (FoxM1B) levels in old-aged mice stimulated liver regeneration through diminished p27Kip1 protein levels and increased Cdc25B expression. J Biol Chem. 2002;277:44310–44316. [DOI] [PubMed] [Google Scholar]
- 17. Huang X, Zhao YY. Transgenic expression of FoxM1 promotes endothelial repair following lung injury induced by polymicrobial sepsis in mice. PLoS One. 2012;7:e50094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao YY, Gao XP, Zhao YD, et al. Endothelial cell-restricted disruption of FoxM1 impairs endothelial repair following LPS-induced vascular injury. J Clin Invest. 2006;116:2333–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The forkhead box M1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci USA. 2002;99:16881–16886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang X, Bhattacharyya D, Dennewitz MB, et al. Rapid hepatocyte nuclear translocation of the forkhead box M1B (FoxM1B) transcription factor caused a transient increase in size of regenerating transgenic hepatocytes. Gene Expr. 2003;11:149–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Park HJ, Wang Z, Costa RH, Tyner A, Lau LF, Raychaudhuri P. An N-terminal inhibitory domain modulates activity of FoxM1 during cell cycle. Oncogene. 2008;27:1696–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wierstra I, Alves J. Despite its strong transactivation domain, transcription factor FOXM1c is kept almost inactive by two different inhibitory domains. Biol Chem. 2006;387:963–976. [DOI] [PubMed] [Google Scholar]
- 23. Laoukili J, Alvarez M, Meijer LA, et al. Activation of FoxM1 during G2 requires cyclin A/Cdk-dependent relief of autorepression by the FoxM1 N-terminal domain. Mol Cell Biol. 2008;28:3076–3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ma RY, Tong TH, Cheung AM, Tsang AC, Leung WY, Yao KM. Raf/MEK/MAPK signaling stimulates the nuclear translocation and transactivating activity of FOXM1c. J Cell Sci. 2005;118:795–806. [DOI] [PubMed] [Google Scholar]
- 25. Park HJ, Costa RH, Lau LF, Tyner AL, Raychaudhuri P. Anaphase-promoting complex/cyclosome-CDH1-mediated proteolysis of the forkhead box M1 transcription factor is critical for regulated entry into S phase. Mol Cell Biol. 2008;28:5162–5171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fu Z, Malureanu L, Huang J, Wang W, et al. Plk1-dependent phosphorylation of FoxM1 regulates a transcriptional programme required for mitotic progression. Nat Cell Biol. 2008;10:1076–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang IC, Zhang Y, Snyder J, et al. Increased expression of FoxM1 transcription factor in respiratory epithelium inhibits lung sacculation and causes Clara cell hyperplasia. Dev Biol. 2010;347:301–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Milo-Landesman D, Surana M, Berkovich I, et al. Correction of hyperglycemia in diabetic mice transplanted with reversibly immortalized pancreatic β cells controlled by the tet-on regulatory system. Cell Transplant. 2001;10:645–650. [PubMed] [Google Scholar]
- 29. Golson ML, Loomes KM, Oakey R, Kaestner KH. Ductal malformation and pancreatitis in mice caused by conditional Jag1 deletion. Gastroenterology. 2009;136:1761–1771.e1. [DOI] [PubMed] [Google Scholar]
- 30. Golson ML, Bush WS, Brissova M. Automated quantification of pancreatic β-cell mass. Am J Physiol Endocrinol Metab. 2014;306:E1460–E1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chandler KJ, Chandler RL, Broeckelmann EM, Hou Y, Southard-Smith EM, Mortlock DP. Relevance of BAC transgene copy number in mice: transgene copy number variation across multiple transgenic lines and correlations with transgene integrity and expression. Mamm Genome. 2007;18:693–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Grant GR, Farkas MH, Pizarro AD, et al. Comparative analysis of RNA-Seq alignment algorithms and the RNA-Seq unified mapper (RUM). Bioinformatics. 2011;27:2518–2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sun H, Fang H, Chen T, Perkins R, Tong W. GOFFA: gene ontology for functional analysis—a FDA gene ontology tool for analysis of genomic and proteomic data. BMC Bioinformatics. 2006;7(suppl 2):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Major ML, Lepe R, Costa RH. Forkhead box M1B transcriptional activity requires binding of Cdk-cyclin complexes for phosphorylation-dependent recruitment of p300/CBP coactivators. Mol Cell Biol. 2004;24:2649–2661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Cozar-Castellano I, Harb G, Selk K, et al. Lessons from the first comprehensive molecular characterization of cell cycle control in rodent insulinoma cell lines. Diabetes. 2008;57:3056–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lüscher-Firzlaff JM, Lilischkis R, Lüscher B. Regulation of the transcription factor FOXM1c by cyclin E/CDK2. FEBS Lett. 2006;580:1716–1722. [DOI] [PubMed] [Google Scholar]
- 38. de la Tour D, Halvorsen T, Demeterco C, et al. β-Cell differentiation from a human pancreatic cell line in vitro and in vivo. Mol Endocrinol. 2001;15:476–483. [DOI] [PubMed] [Google Scholar]
- 39. Raychaudhuri P, Park HJ. FoxM1: a master regulator of tumor metastasis. Cancer Res. 2011;71:4329–4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hammar E, Parnaud G, Bosco D, et al. Extracellular matrix protects pancreatic beta-cells against apoptosis: role of short- and long-term signaling pathways. Diabetes. 2004;53:2034–2041. [DOI] [PubMed] [Google Scholar]
- 41. Stephens SB, Schisler JC, Hohmeier HE, et al. A VGF-derived peptide attenuates development of type 2 diabetes via enhancement of islet β-cell survival and function. Cell Metab. 2012;16:33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Yang J, Zhang W, Jiang W, et al. P21cip-overexpression in the mouse beta cells leads to the improved recovery from streptozotocin-induced diabetes. PLoS One. 2009;4:e8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krishnamurthy J, Ramsey MR, Ligon KL, et al. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. [DOI] [PubMed] [Google Scholar]
- 44. Rankin MM, Kushner JA. Adaptive β-cell proliferation is severely restricted with advanced age. Diabetes. 2009;58:1365–1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Tschen SI, Dhawan S, Gurlo T, Bhushan A. Age-dependent decline in β-cell proliferation restricts the capacity of β-cell regeneration in mice. Diabetes. 2009;58:1312–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wang IC, Chen YJ, Hughes D, Petrovic V, et al. Forkhead box M1 regulates the transcriptional network of genes essential for mitotic progression and genes encoding the SCF (Skp2-Cks1) ubiquitin ligase. Mol Cell Biol. 2005;25:10875–10894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Dohi T, Salz W, Costa M, Ariyan C, Basadonna GP, Altieri DC. Inhibition of apoptosis by survivin improves transplantation of pancreatic islets for treatment of diabetes in mice. EMBO Rep. 2006;7:438–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Richer MJ, Straka N, Fang D, Shanina I, Horwitz MS. Regulatory T-cells protect from type 1 diabetes after induction by coxsackievirus infection in the context of transforming growth factor-beta. Diabetes. 2008;57:1302–1311. [DOI] [PubMed] [Google Scholar]
- 49. Petzold C, Riewaldt J, Watts D, Sparwasser T, Schallenberg S, Kretschmer K. Foxp3+ regulatory T cells in mouse models of type 1 diabetes. J Diabetes Res. 2013;2013:940710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Brissova M, Aamodt K, Brahmachary P, et al. Islet microenvironment, modulated by vascular endothelial growth factor-A signaling, promotes β cell regeneration. Cell Metab. 2014;19:498–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Dirice E, Kahraman S, Jiang W, et al. Soluble factors secreted by T cells promote β-cell proliferation. Diabetes. 2014;63:188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J Physiol. 2007;578:327–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Balli D, Ren X, Chou FS, et al. Foxm1 transcription factor is required for macrophage migration during lung inflammation and tumor formation. Oncogene. 2012;31:3875–3888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Ren X, Shah TA, Ustiyan V, et al. FOXM1 promotes allergen-induced goblet cell metaplasia and pulmonary inflammation. Mol Cell Biol. 2013;33:371–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Peshavaria M, Larmie BL, Lausier J, et al. Regulation of pancreatic β-cell regeneration in the normoglycemic 60% partial-pancreatectomy mouse. Diabetes. 2006;55:3289–3298. [DOI] [PubMed] [Google Scholar]
- 56. Leahy JL, Bonner-Weir S, Weir GC. Minimal chronic hyperglycemia is a critical determinant of impaired insulin secretion after an incomplete pancreatectomy. J Clin Invest. 1988;81:1407–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Matveyenko AV, Butler PC. Relationship between beta-cell mass and diabetes onset. Diabetes Obes Metab. 2008;10(suppl 4):23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Benninger RK, Head WS, Zhang M, Satin LS, Piston DW. Gap junctions and other mechanisms of cell-cell communication regulate basal insulin secretion in the pancreatic islet. J Physiol. 2011;589:5453–5466. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.