Abstract
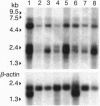
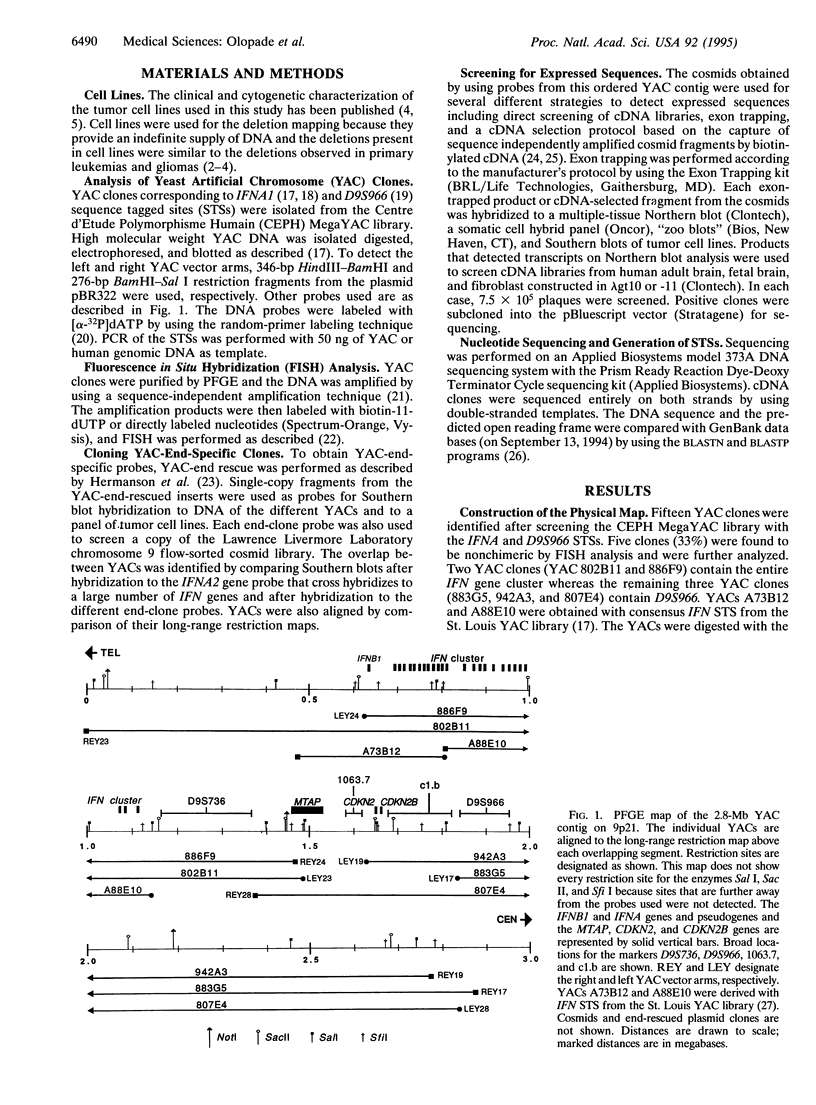
Many human malignant cells lack methylthioadenosine phosphorylase (MTAP) enzyme activity. The gene (MTAP) encoding this enzyme was previously mapped to the short arm of chromosome 9, band p21-22, a region that is frequently deleted in multiple tumor types. To clone candidate tumor suppressor genes from the deleted region on 9p21-22, we have constructed a long-range physical map of 2.8 megabases for 9p21 by using overlapping yeast artificial chromosome and cosmid clones. This map includes the type IIFN gene cluster, the recently identified candidate tumor suppressor genes CDKN2 (p16INK4A) and CDKN2B (p15INK4B), and several CpG islands. In addition, we have identified other transcription units within the yeast artificial chromosome contig. Sequence analysis of a 2.5-kb cDNA clone isolated from a CpG island that maps between the IFN genes and CDKN2 reveals a predicted open reading frame of 283 amino acids followed by 1302 nucleotides of 3' untranslated sequence. This gene is evolutionarily conserved and shows significant amino acid homologies to mouse and human purine nucleoside phosphorylases and to a hypothetical 25.8-kDa protein in the pet gene (coding for cytochrome bc1 complex) region of Rhodospirillum rubrum. The location, expression pattern, and nucleotide sequence of this gene suggest that it codes for the MTAP enzyme.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bohlander S. K., Dreyling M. H., Hagos F., Sveen L., Olopade O. I., Díaz M. O. Mapping a putative tumor suppressor gene on chromosome 9 bands p21-p22 with microdissection probes. Genomics. 1994 Nov 15;24(2):211–217. doi: 10.1006/geno.1994.1608. [DOI] [PubMed] [Google Scholar]
- Bohlander S. K., Espinosa R., 3rd, Fernald A. A., Rowley J. D., Le Beau M. M., Díaz M. O. Sequence-independent amplification and labeling of yeast artificial chromosomes for fluorescence in situ hybridization. Cytogenet Cell Genet. 1994;65(1-2):108–110. doi: 10.1159/000133612. [DOI] [PubMed] [Google Scholar]
- Buckler A. J., Chang D. D., Graw S. L., Brook J. D., Haber D. A., Sharp P. A., Housman D. E. Exon amplification: a strategy to isolate mammalian genes based on RNA splicing. Proc Natl Acad Sci U S A. 1991 May 1;88(9):4005–4009. doi: 10.1073/pnas.88.9.4005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Carrera C. J., Eddy R. L., Shows T. B., Carson D. A. Assignment of the gene for methylthioadenosine phosphorylase to human chromosome 9 by mouse-human somatic cell hybridization. Proc Natl Acad Sci U S A. 1984 May;81(9):2665–2668. doi: 10.1073/pnas.81.9.2665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng J. Q., Jhanwar S. C., Klein W. M., Bell D. W., Lee W. C., Altomare D. A., Nobori T., Olopade O. I., Buckler A. J., Testa J. R. p16 alterations and deletion mapping of 9p21-p22 in malignant mesothelioma. Cancer Res. 1994 Nov 1;54(21):5547–5551. [PubMed] [Google Scholar]
- Coleman A., Fountain J. W., Nobori T., Olopade O. I., Robertson G., Housman D. E., Lugo T. G. Distinct deletions of chromosome 9p associated with melanoma versus glioma, lung cancer, and leukemia. Cancer Res. 1994 Jan 15;54(2):344–348. [PubMed] [Google Scholar]
- Della Ragione F., Oliva A., Palumbo R., Russo G. L., Gragnaniello V., Zappia V. Deficiency of 5'-deoxy-5'-methylthioadenosine phosphorylase activity in malignancy. Absence of the protein in human enzyme-deficient cell lines. Biochem J. 1992 Jan 15;281(Pt 2):533–538. doi: 10.1042/bj2810533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz M. O., Rubin C. M., Harden A., Ziemin S., Larson R. A., Le Beau M. M., Rowley J. D. Deletions of interferon genes in acute lymphoblastic leukemia. N Engl J Med. 1990 Jan 11;322(2):77–82. doi: 10.1056/NEJM199001113220202. [DOI] [PubMed] [Google Scholar]
- Diaz M. O., Ziemin S., Le Beau M. M., Pitha P., Smith S. D., Chilcote R. R., Rowley J. D. Homozygous deletion of the alpha- and beta 1-interferon genes in human leukemia and derived cell lines. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5259–5263. doi: 10.1073/pnas.85.14.5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyling M. H., Bohlander S. K., Adeyanju M. O., Olopade O. I. Detection of CDKN2 deletions in tumor cell lines and primary glioma by interphase fluorescence in situ hybridization. Cancer Res. 1995 Mar 1;55(5):984–988. [PubMed] [Google Scholar]
- Díaz M. O., Pomykala H. M., Bohlander S. K., Maltepe E., Malik K., Brownstein B., Olopade O. I. Structure of the human type-I interferon gene cluster determined from a YAC clone contig. Genomics. 1994 Aug;22(3):540–552. doi: 10.1006/geno.1994.1427. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Taruscio D., Graw S. L., Buckler A. J., Ward D. C., Dracopoli N. C., Housman D. E. Genetic and physical map of the interferon region on chromosome 9p. Genomics. 1992 Sep;14(1):105–112. doi: 10.1016/s0888-7543(05)80290-3. [DOI] [PubMed] [Google Scholar]
- Hannon G. J., Beach D. p15INK4B is a potential effector of TGF-beta-induced cell cycle arrest. Nature. 1994 Sep 15;371(6494):257–261. doi: 10.1038/371257a0. [DOI] [PubMed] [Google Scholar]
- Hermanson G. G., Hoekstra M. F., McElligott D. L., Evans G. A. Rescue of end fragments of yeast artificial chromosomes by homologous recombination in yeast. Nucleic Acids Res. 1991 Sep 25;19(18):4943–4948. doi: 10.1093/nar/19.18.4943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Jen J., Harper J. W., Bigner S. H., Bigner D. D., Papadopoulos N., Markowitz S., Willson J. K., Kinzler K. W., Vogelstein B. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994 Dec 15;54(24):6353–6358. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kamb A., Shattuck-Eidens D., Eeles R., Liu Q., Gruis N. A., Ding W., Hussey C., Tran T., Miki Y., Weaver-Feldhaus J. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994 Sep;8(1):23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Kwiatkowski D. J., Diaz M. O. Dinucleotide repeat polymorphism at the IFNA locus (9p22). Hum Mol Genet. 1992 Nov;1(8):658–658. doi: 10.1093/hmg/1.8.658-a. [DOI] [PubMed] [Google Scholar]
- Lovett M., Kere J., Hinton L. M. Direct selection: a method for the isolation of cDNAs encoded by large genomic regions. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9628–9632. doi: 10.1073/pnas.88.21.9628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Nobori T., Szinai I., Amox D., Parker B., Olopade O. I., Buchhagen D. L., Carson D. A. Methylthioadenosine phosphorylase deficiency in human non-small cell lung cancers. Cancer Res. 1993 Mar 1;53(5):1098–1101. [PubMed] [Google Scholar]
- Olopade O. I., Bohlander S. K., Pomykala H., Maltepe E., Van Melle E., Le Beau M. M., Diaz M. O. Mapping of the shortest region of overlap of deletions of the short arm of chromosome 9 associated with human neoplasia. Genomics. 1992 Oct;14(2):437–443. doi: 10.1016/s0888-7543(05)80238-1. [DOI] [PubMed] [Google Scholar]
- Olopade O. I., Buchhagen D. L., Malik K., Sherman J., Nobori T., Bader S., Nau M. M., Gazdar A. F., Minna J. D., Diaz M. O. Homozygous loss of the interferon genes defines the critical region on 9p that is deleted in lung cancers. Cancer Res. 1993 May 15;53(10 Suppl):2410–2415. [PubMed] [Google Scholar]
- Olopade O. I., Jenkins R. B., Ransom D. T., Malik K., Pomykala H., Nobori T., Cowan J. M., Rowley J. D., Diaz M. O. Molecular analysis of deletions of the short arm of chromosome 9 in human gliomas. Cancer Res. 1992 May 1;52(9):2523–2529. [PubMed] [Google Scholar]
- Rowley J. D., Diaz M. O., Espinosa R., 3rd, Patel Y. D., van Melle E., Ziemin S., Taillon-Miller P., Lichter P., Evans G. A., Kersey J. H. Mapping chromosome band 11q23 in human acute leukemia with biotinylated probes: identification of 11q23 translocation breakpoints with a yeast artificial chromosome. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9358–9362. doi: 10.1073/pnas.87.23.9358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano M., Hannon G. J., Beach D. A new regulatory motif in cell-cycle control causing specific inhibition of cyclin D/CDK4. Nature. 1993 Dec 16;366(6456):704–707. doi: 10.1038/366704a0. [DOI] [PubMed] [Google Scholar]
- Weaver-Feldhaus J., Gruis N. A., Neuhausen S., Le Paslier D., Stockert E., Skolnick M. H., Kamb A. Localization of a putative tumor suppressor gene by using homozygous deletions in melanomas. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7563–7567. doi: 10.1073/pnas.91.16.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]