Abstract
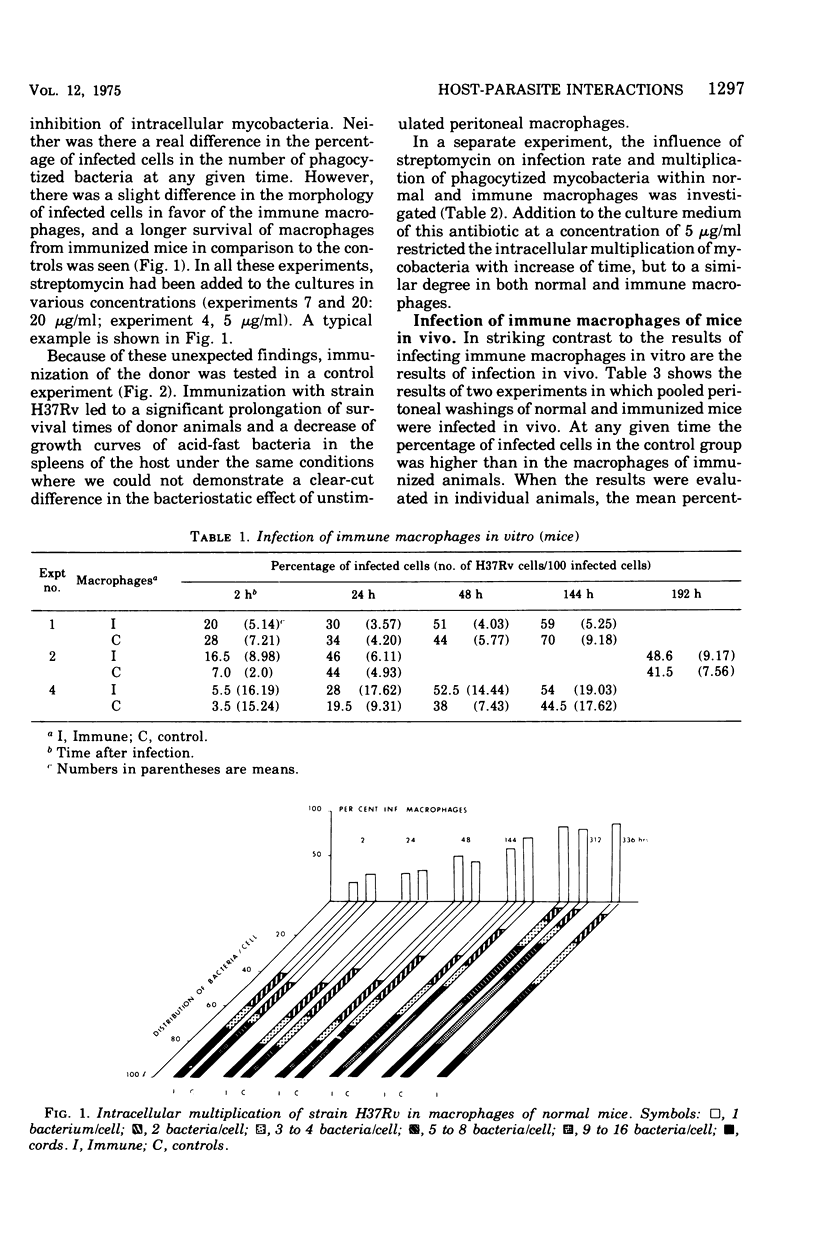
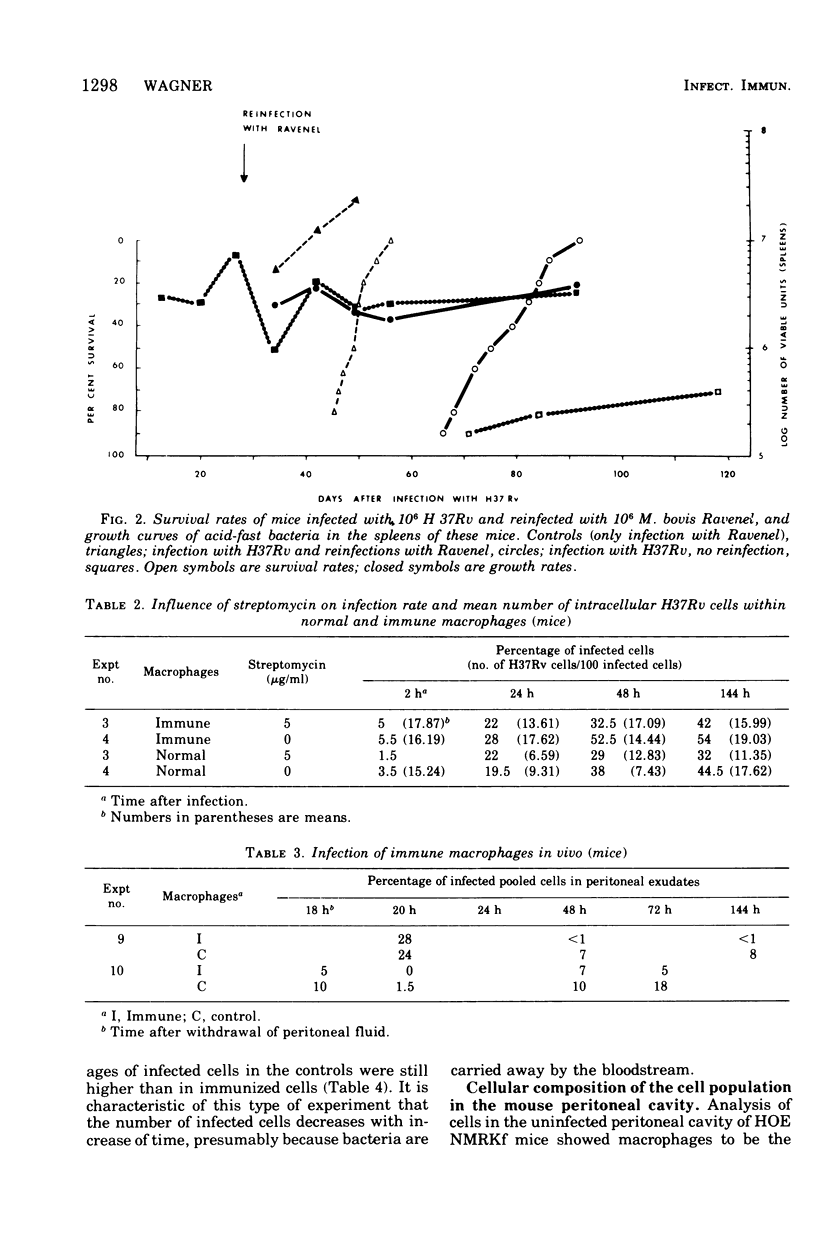
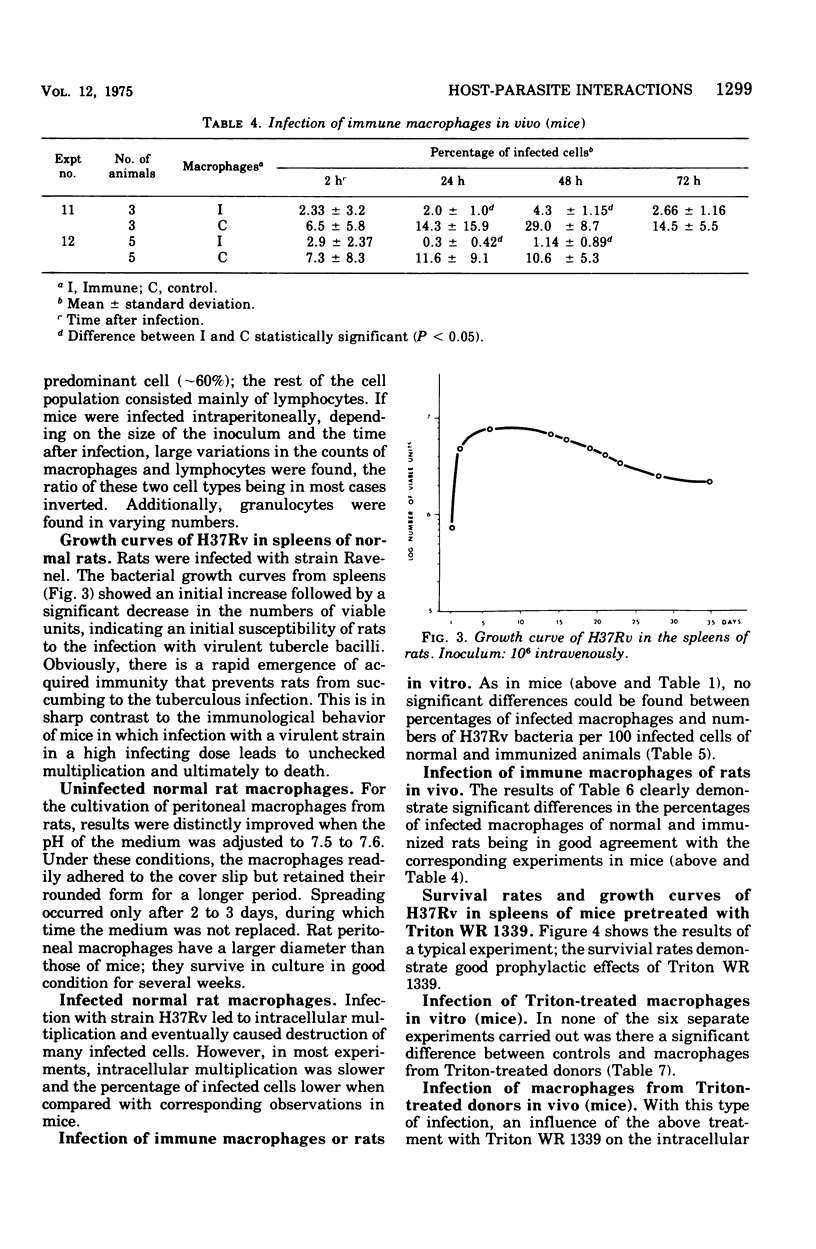
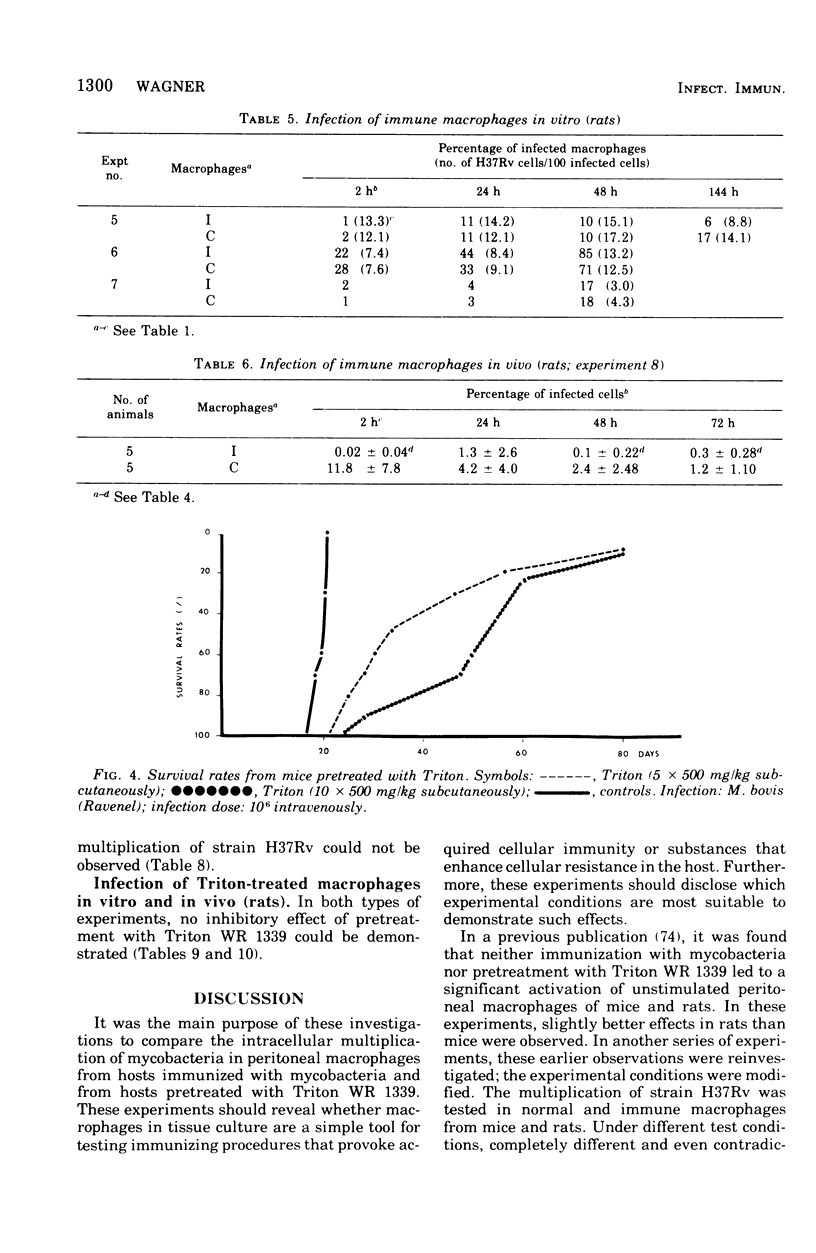
This paper deals with the intracellular multiplication of mycobacteria in peritoneal macrophages from mice and rats immunized with tubercle bacilli or pretreated with Triton WR 1339. If unstimulated macrophages were used, almost unrestricted multiplication of mycobacteria was observed in macrophages from both vaccinated and pretreated hosts after infection of the cells in vitro. Only when the infection of the cells was perfored in the peritoneal cavity of vaccinated hosts did the macrophages display a high degree of inhibition. This striking difference in the behavior of macrophages infected in vitro and in vivo is explained by the local inflammation caused by the intraperitoneal infection, which leads to an influx of T-cell mediators. When macrophages from hosts pretreated with Triton WR 1339 were used, inhibition of the multiplication of mycobacteria within cells infected in vitro or in vivo was very slight, though this compound displayed a marked protective effect in the host. Addition of streptomycin to the culture medium caused a strong inhibition of intracellular mycobacteria even in small concentrations; there was no difference between normal and "immune" macrophages. When rats were infected with virulent tubercle bacilli, they were initially fully susceptible to the infection but showed rapid onset of a strong immune response.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ando M., Dannenberg A. M., Jr, Shima K. Macrophage accumulation, division, maturation and digestive and microbicidal capacities in tuberculous lesions. II. Rate at which mononuclear cells enter and divide in primary BCG lesions and those of reinfection. J Immunol. 1972 Jul;109(1):8–19. [PubMed] [Google Scholar]
- Armstrong J. A., Hart P. D. Response of cultured macrophages to Mycobacterium tuberculosis, with observations on fusion of lysosomes with phagosomes. J Exp Med. 1971 Sep 1;134(3 Pt 1):713–740. doi: 10.1084/jem.134.3.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERTHRONG M., HAMILTON M. A. Tissue culture studies on resistance in tuberculosis. II. Monocytes from normal and immunized guinea pigs infected with virulent human tubercle bacilli. Am Rev Tuberc. 1959 Feb;79(2):221–231. doi: 10.1164/artpd.1959.79.2.221. [DOI] [PubMed] [Google Scholar]
- Berthrong M. Biology of the mycobacterioses. The macrophage-tubercle bacillus relationship and resistance to tuberculosis. Ann N Y Acad Sci. 1968 Sep 5;154(1):157–166. doi: 10.1111/j.1749-6632.1968.tb16706.x. [DOI] [PubMed] [Google Scholar]
- Bonventre P. F., Imhoff J. G. Uptake of h-dihydrostreptomycin by macrophages in culture. Infect Immun. 1970 Jul;2(1):89–95. doi: 10.1128/iai.2.1.89-95.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANG Y. T. LONG-TERM CULTIVATION OF MOUSE PERITONEAL MACROPHAGES. J Natl Cancer Inst. 1964 Jan;32:19–35. [PubMed] [Google Scholar]
- CORNFORTH J. W., HART P. D., NICHOLLS G. A., REES R. J., STOCK J. A. Antituberculous effects of certain surface-active polyoxyethylene ethers. Br J Pharmacol Chemother. 1955 Mar;10(1):73–88. doi: 10.1111/j.1476-5381.1955.tb00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CORNFORTH J. W., HART P. D., REES R. J. W., STOCK J. A. Antituberculous effect of certain surface-active polyoxyethylene ethers in mice. Nature. 1951 Jul 28;168(4265):150–153. doi: 10.1038/168150a0. [DOI] [PubMed] [Google Scholar]
- Catanzaro P. J., Graham R. C., Jr, Burns C. P. Mouse peritoneal lymphocytes: general properties of normal peritoneal lymphocytes. J Reticuloendothel Soc. 1974 Sep;16(3):150–160. [PubMed] [Google Scholar]
- Catanzaro P. J., Graham R. C., Jr, Hogrefe W. R. Mouse peritoneal lymphocytes: a morphologic comparison of normal and exudate peritoneal lymphocytes. J Reticuloendothel Soc. 1974 Sep;16(3):161–174. [PubMed] [Google Scholar]
- Chang Y. T. Suppressive activity of streptomycin on the growth of Mycobacterium lepraemurium in macrophage cultures. Appl Microbiol. 1969 May;17(5):750–754. doi: 10.1128/am.17.5.750-754.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANNENBERG A. M., Jr, BURSTONE M. S., WALTER P. C., KINSLEY J. W. A histochemical study of phagocytic and enzymatic functions of rabbit mononuclear and polymorphonuclear exudate cells and alveolar macrophages. I. Survey and quantitation of enzymes, and states of cellular activation. J Cell Biol. 1963 Jun;17:465–486. doi: 10.1083/jcb.17.3.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Ando M., Shima K. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. 3. The turnover of macrophages and its relation to their activation and antimicrobial immunity in primary BCG lesions and those of reinfection. J Immunol. 1972 Nov;109(5):1109–1121. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr Cellular hypersensitivity and cellular immunity in the pathogensis of tuberculosis: specificity, systemic and local nature, and associated macrophage enzymes. Bacteriol Rev. 1968 Jun;32(2):85–102. doi: 10.1128/br.32.2.85-102.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Meyer O. T., Esterly J. R., Kambara T. The local nature of immunity in tuberculosis, illustrated histochemically in dermal BCG lesions. J Immunol. 1968 May;100(5):931–941. [PubMed] [Google Scholar]
- Dannenberg A. M., Jr, Roessler W. G., Meyer O. T., Chandrasekhar S., Kambara T. Radiation, infection, and macrophage function. 3. Recovery from the effects of radiation illustrated by dermal BCG lesions; resistance of pulmonary alveolar macrophages to radiation illustrated by tuberculosis produced by the airborne route. J Reticuloendothel Soc. 1970 Jan;7(1):91–108. [PubMed] [Google Scholar]
- Hahn H. Zelluläre antibakterielle Immunität. Dtsch Med Wochenschr. 1974 Mar 29;99(13):651–656. doi: 10.1055/s-0028-1107820. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Armstrong J. A., Brown C. A., Draper P. Ultrastructural study of the behavior of macrophages toward parasitic mycobacteria. Infect Immun. 1972 May;5(5):803–807. doi: 10.1128/iai.5.5.803-807.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart P. D., Gordon A. H. Suggested role of lysosomal lipid in the contrasting effects of 'triton WR-1339' and dextran on tuberculous infection. Nature. 1969 May 17;222(5194):672–673. doi: 10.1038/222672a0. [DOI] [PubMed] [Google Scholar]
- Hart P. D. Mycobacterium tuberculosis in macrophages: effect of certain surfactants and other membrane-active compounds. Science. 1968 Nov 8;162(3854):686–689. doi: 10.1126/science.162.3854.686. [DOI] [PubMed] [Google Scholar]
- Hart P. D., Payne S. N. Effects of non-ionic surfactants that modify experimental tuberculosis on lipase activity of macrophages. Br J Pharmacol. 1971 Sep;43(1):190–196. doi: 10.1111/j.1476-5381.1971.tb07168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambara T., Chandrasekhar S., Dannenberg A. M., Jr, Meyer O. T. Radiation, infection, and macrophage function. I. Effects of whole body radiation on dermal tuberculous lesions in rabbits: development, histology, and histochemistry. J Reticuloendothel Soc. 1970 Jan;7(1):53–78. [PubMed] [Google Scholar]
- Koster F. T., McGregor D. D., Mackaness G. B. The mediator of cellular immunity. II. Migration of immunologically committed lymphocytes into inflammatory exudates. J Exp Med. 1971 Feb 1;133(2):400–409. doi: 10.1084/jem.133.2.400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster F., McGregor D. D. Rat thoracic duct lymphocytes: types that participate in inflammation. Science. 1970 Feb 20;167(3921):1137–1139. doi: 10.1126/science.167.3921.1137. [DOI] [PubMed] [Google Scholar]
- LOVELOCK J. E., REES R. J. Possible site and mode of action of certain lipotropic macromolecules in tuberculosis. Nature. 1955 Jan 22;175(4447):161–163. doi: 10.1038/175161a0. [DOI] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Immune response to Mycobacterium tuberculosis in rats. Infect Immun. 1973 Aug;8(2):182–189. doi: 10.1128/iai.8.2.182-189.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J., McGregor D. D., Mackaness G. B. Properties of lymphocytes which confer adoptive immunity to tuberculosis in rats. Immunology. 1973 Oct;25(4):703–715. [PMC free article] [PubMed] [Google Scholar]
- Lefford M. J. Transfer of adoptive immunity to tuberculosis in mice. Infect Immun. 1975 Jun;11(6):1174–1181. doi: 10.1128/iai.11.6.1174-1181.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. Artificial cellular immunity against tubercle bacilli; an effect of polyoxyethylene ethers (Triton). Am Rev Tuberc. 1954 May;69(5):690–704. doi: 10.1164/art.1954.69.5.690. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N. The action of isoniazid (isonicotinic acid hydrazide) on intracellular tubercle bacilli. Am Rev Tuberc. 1952 Aug;66(2):125–133. doi: 10.1164/art.1952.66.2.125. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N. The bactericidal action of isoniazid, streptomycin and terramycin on extracellular and intracellular tubercle bacilli. Am Rev Tuberc. 1953 Mar;67(3):322–340. doi: 10.1164/art.1953.67.3.322. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B., SMITH N., WELLS A. Q. The growth of intracellular tubercle bacilli in relation to their virulence. Am Rev Tuberc. 1954 Apr;69(4):479–494. doi: 10.1164/art.1954.69.4.479. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. THE IMMUNOLOGICAL BASIS OF ACQUIRED CELLULAR RESISTANCE. J Exp Med. 1964 Jul 1;120:105–120. doi: 10.1084/jem.120.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKANESS G. B. The action of drugs on intracellular tubercle bacilli. J Pathol Bacteriol. 1952 Jul;64(3):429–446. doi: 10.1002/path.1700640302. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The growth of tubercle bacilli in monocytes from normal and vaccinated rabbits. Am Rev Tuberc. 1954 Apr;69(4):495–504. doi: 10.1164/art.1954.69.4.495. [DOI] [PubMed] [Google Scholar]
- MACKANESS G. B. The intracellular activation of pyrazinamide and nicotinamide. Am Rev Tuberc. 1956 Nov;74(5):718–728. doi: 10.1164/artpd.1956.74.5.718. [DOI] [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., FARISS B. Lysozyme content of alveolar and peritoneal macrophages from the rabbit. J Immunol. 1961 Feb;86:133–136. [PubMed] [Google Scholar]
- MYRVIK Q. N., LEAKE E. S., OSHIMA S. A study of macrophages and epitheloid-like cells from granulomatous (BCG-induced) lungs of rabbits. J Immunol. 1962 Nov;89:745–751. [PubMed] [Google Scholar]
- Mackaness G. B. The immunology of antituberculous immunity. Am Rev Respir Dis. 1968 Mar;97(3):337–344. doi: 10.1164/arrd.1968.97.3.337. [DOI] [PubMed] [Google Scholar]
- Mackaness G. B. The influence of immunologically committed lymphoid cells on macrophage activity in vivo. J Exp Med. 1969 May 1;129(5):973–992. doi: 10.1084/jem.129.5.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor D. D., Hahn H. H., Mackaness G. B. The mediator of cellular immunity. V. Development of cellular resistance to infection in thymectomized irradiated rats. Cell Immunol. 1973 Feb;6(2):186–199. doi: 10.1016/0008-8749(73)90021-x. [DOI] [PubMed] [Google Scholar]
- McGregor D. D., Koster F. T., Mackaness G. B. The mediator of cellular immunity. I. The life-span and circulation dynamics of the immunologically committed lymphocyte. J Exp Med. 1971 Feb 1;133(2):389–399. doi: 10.1084/jem.133.2.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O. T., Dannenberg A. M., Jr Radiation, infection, and macrophage function. II. Effect of whole body radiation on the number of pulmonary alveolar macrophages and their levels of hydrolytic enzymes. J Reticuloendothel Soc. 1970 Jan;7(1):79–90. [PubMed] [Google Scholar]
- North R. J., Spitalny G. Inflammatory lymphocyte in cell-mediated antibacterial immunity: factors governing the accumulation of mediator T cells in peritoneal exudates. Infect Immun. 1974 Sep;10(3):489–498. doi: 10.1128/iai.10.3.489-498.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Demonstration in tissue culture of lymphocyte-mediated immunity to tuberculosis. Infect Immun. 1970 Jun;1(6):600–603. doi: 10.1128/iai.1.6.600-603.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson R. J., Youmans G. P. Multiplication of Mycobacterium tuberculosis Within Normal and "Immune" Mouse Macrophages Cultivated With and Without Streptomycin. Infect Immun. 1970 Jan;1(1):30–40. doi: 10.1128/iai.1.1.30-40.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SUTER E. Multiplication of tubercle bacilli within mononuclear phagocytes in tissue cultures derived from normal animals and animals vaccinated with BCG. J Exp Med. 1953 Feb 1;97(2):235–245. doi: 10.1084/jem.97.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shima K., Dannenberg A. M., Jr, Ando M., Chandrasekhar S., Seluzicki J. A., Fabrikant J. I. Macrophage accumulation, division, maturation, and digestive and microbicidal capacities in tuberculous lesions. I. Studies involving their incorporation of tritiated thymidine and their content of lysosomal enzymes and bacilli. Am J Pathol. 1972 Apr;67(1):159–180. [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Cellular immunity in vitro. I. Immunologically mediated enhancement of macrophage bactericidal capacity. J Exp Med. 1971 Jun 1;133(6):1377–1389. doi: 10.1084/jem.133.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon H. B., Sheagren J. N. Enhancement of macrophage bactericidal capacity by antigenically stimulated immune lymphocytes. Cell Immunol. 1972 Jun;4(2):163–174. doi: 10.1016/0008-8749(72)90015-9. [DOI] [PubMed] [Google Scholar]
- Thompson J., van Furth R. The effect of glucocorticosteroids on the kinetics of mononuclear phagocytes. J Exp Med. 1970 Mar 1;131(3):429–442. doi: 10.1084/jem.131.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouet A. Immunisation de lapins par des lysosomes hépatiques de rats traités au Triton WR 1339. Arch Int Physiol Biochim. 1964 Sep;72(4):698–700. [PubMed] [Google Scholar]
- WAGNER W. H., SAAR G. Die quantitative Bestimmung von Tuberkelbakterien mittels Zählplatten. Arb Paul Ehrlich Inst Georg Speyer Haus Ferdinand Blum Inst Frankf A M. 1954;51:178–186. [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Diesselhoff-Den Dulk M. M. The kinetics of promonocytes and monocytes in the bone marrow. J Exp Med. 1970 Oct 1;132(4):813–828. doi: 10.1084/jem.132.4.813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Hirsch J. G., Fedorko M. E. Morphology and peroxidase cytochemistry of mouse promonocytes, monocytes, and macrophages. J Exp Med. 1970 Oct 1;132(4):794–812. doi: 10.1084/jem.132.4.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R. Origin and kinetics of monocytes and macrophages. Semin Hematol. 1970 Apr;7(2):125–141. [PubMed] [Google Scholar]


