Abstract
Addition of iron componds to inocula of the relatively avirulent T3 or T4 colony types of gonococci increased their lethality for chicken embryos after intravenous inoculation but had little or no effect on the highly virulent T1 or T2 types. The toxicity of nonviable inocula, killed cells or sonicates, was not significantly affected by ecogenous iron. Addition of the iron-binding protein conalbumin reduced or delayed the lethal effect of T1, but not T3, gonococci although growth of both colony types in the allantoic cavity of the embryo was inhibited by this protwin. This effect can be attributed specifically to deprivation of iron since the iron-complexed form of conalbumin had no apparent influence on growth or virulence. The results indicate that the ability to acquire iron in vivo is a significant factor in gonococcal virulence. The virulent colony types appear to have enhanced ability to compete with the host for iron and this may be related to the presence of pili, other surface components, or the synthesis of iron-chelating compounds.
Full text
PDF

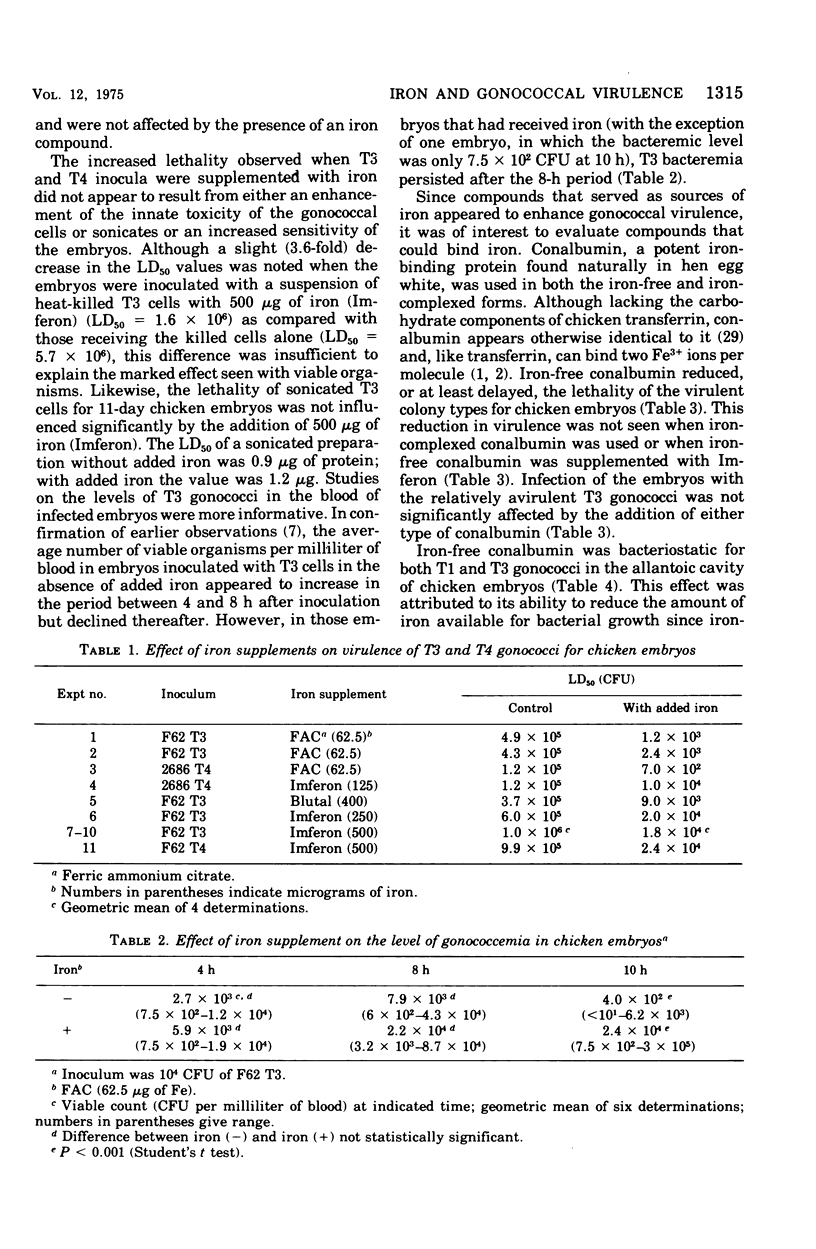


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AASA R., MALMSTROEM B. G., SALTMAN P. THE SPECIFIC BINDING OF IRON(III) AND COPPER(II) TO TRANSFERRIN AND CONALBUMIN. Biochim Biophys Acta. 1963 Sep 24;75:203–222. doi: 10.1016/0006-3002(63)90599-7. [DOI] [PubMed] [Google Scholar]
- Aisen P., Leibman A. The stability constants of the Fe3+ conalbumin complexes. Biochem Biophys Res Commun. 1968 Feb 26;30(4):407–413. doi: 10.1016/0006-291x(68)90759-6. [DOI] [PubMed] [Google Scholar]
- BURROWS T. W., JACKSON S. The virulence-enhancing effect of iron on nonpigmented mutants of virulent strains of Pasteurella pestis. Br J Exp Pathol. 1956 Dec;37(6):577–583. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Leigh L. C., Rogers H. J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology. 1968 Oct;15(4):581–588. [PMC free article] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J. Bacterial iron metabolism and immunity to Pasteurella septica and Escherichia coli. Nature. 1969 Oct 25;224(5217):380–382. doi: 10.1038/224380a0. [DOI] [PubMed] [Google Scholar]
- Bullen J. J., Rogers H. J., Lewin J. E. The bacteriostatic effect of serum on Pasteurella septica and its abolition by iron compounds. Immunology. 1971 Mar;20(3):391–406. [PMC free article] [PubMed] [Google Scholar]
- Bumgarner L. R., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: virulence of colony types of Neisseria gonorrhoeae for chicken embryos. Infect Immun. 1973 Dec;8(6):919–924. doi: 10.1128/iai.8.6.919-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilworth J. A., Hendley J. O., Mandell G. L. Attachment and ingestion of gonococci human neutrophils. Infect Immun. 1975 Mar;11(3):512–516. doi: 10.1128/iai.11.3.512-516.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gladstone G. P., Walton E. The effect of iron and haematin on the killing of staphylococci by rabbit polymorphs. Br J Exp Pathol. 1971 Oct;52(5):452–464. [PMC free article] [PubMed] [Google Scholar]
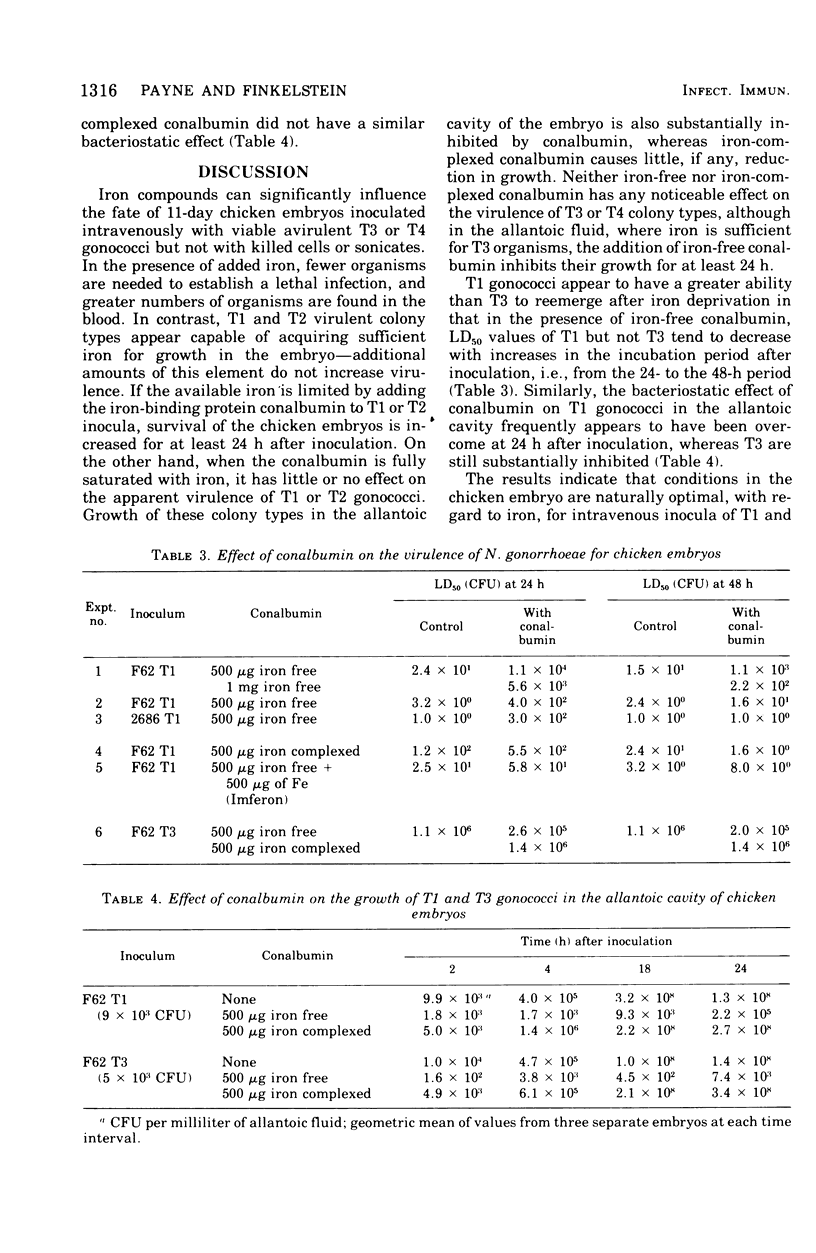
- James-Holmquest A. N., Swanson J., Buchanan T. M., Wende R. D., Williams R. P. Differential attachment by piliated and nonpiliated Neisseria gonorrhoeae to human sperm. Infect Immun. 1974 May;9(5):897–902. doi: 10.1128/iai.9.5.897-902.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochan I., Golden C. A., Bukovic J. A. Mechanism of tuberculostasis in mammalian serum. II. Induction of serum tuberculostasis in guinea pigs. J Bacteriol. 1969 Oct;100(1):64–70. doi: 10.1128/jb.100.1.64-70.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J. Iron-Binding Catechols and Virulence in Escherichia coli. Infect Immun. 1973 Mar;7(3):445–456. doi: 10.1128/iai.7.3.445-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. An Iron-binding Component in Human Blood Plasma. Science. 1946 Oct 11;104(2702):340–341. doi: 10.1126/science.104.2702.340. [DOI] [PubMed] [Google Scholar]
- Schade A. L., Caroline L. RAW HEN EGG WHITE AND THE ROLE OF IRON IN GROWTH INHIBITION OF SHIGELLA DYSENTERIAE, STAPHYLOCOCCUS AUREUS, ESCHERICHIA COLI AND SACCHAROMYCES CEREVISIAE. Science. 1944 Jul 7;100(2584):14–15. doi: 10.1126/science.100.2584.14. [DOI] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILLIAMS J. A comparison of conalbumin and transferrin in the domestic fowl. Biochem J. 1962 May;83:355–364. doi: 10.1042/bj0830355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wake A., Morita H., Yamamoto M. The effect of an iron drug on host response to experimental plague infection. Jpn J Med Sci Biol. 1972 Apr;25(2):75–84. doi: 10.7883/yoken1952.25.75. [DOI] [PubMed] [Google Scholar]
- Ward M. E., Watt P. J., Robertson J. N. The human fallopian tube: a laboratory model for gonococcal infection. J Infect Dis. 1974 Jun;129(6):650–659. doi: 10.1093/infdis/129.6.650. [DOI] [PubMed] [Google Scholar]
- Watt P. J. The fate of gonococci in polymorphonuclear leucocytes. J Med Microbiol. 1970 Aug;3(3):501–509. doi: 10.1099/00222615-3-3-501. [DOI] [PubMed] [Google Scholar]
- Weinberg E. D. Iron and susceptibility to infectious disease. Science. 1974 May 31;184(4140):952–956. doi: 10.1126/science.184.4140.952. [DOI] [PubMed] [Google Scholar]


