Abstract
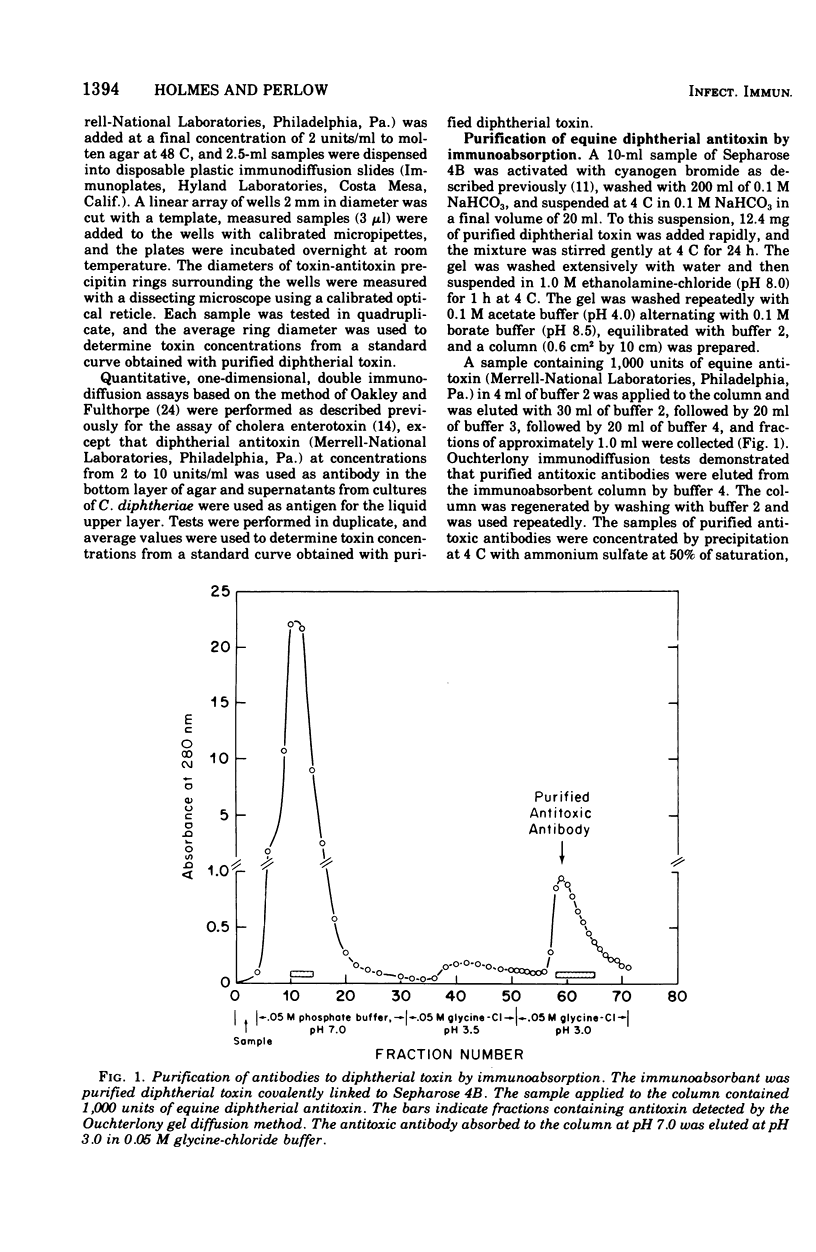
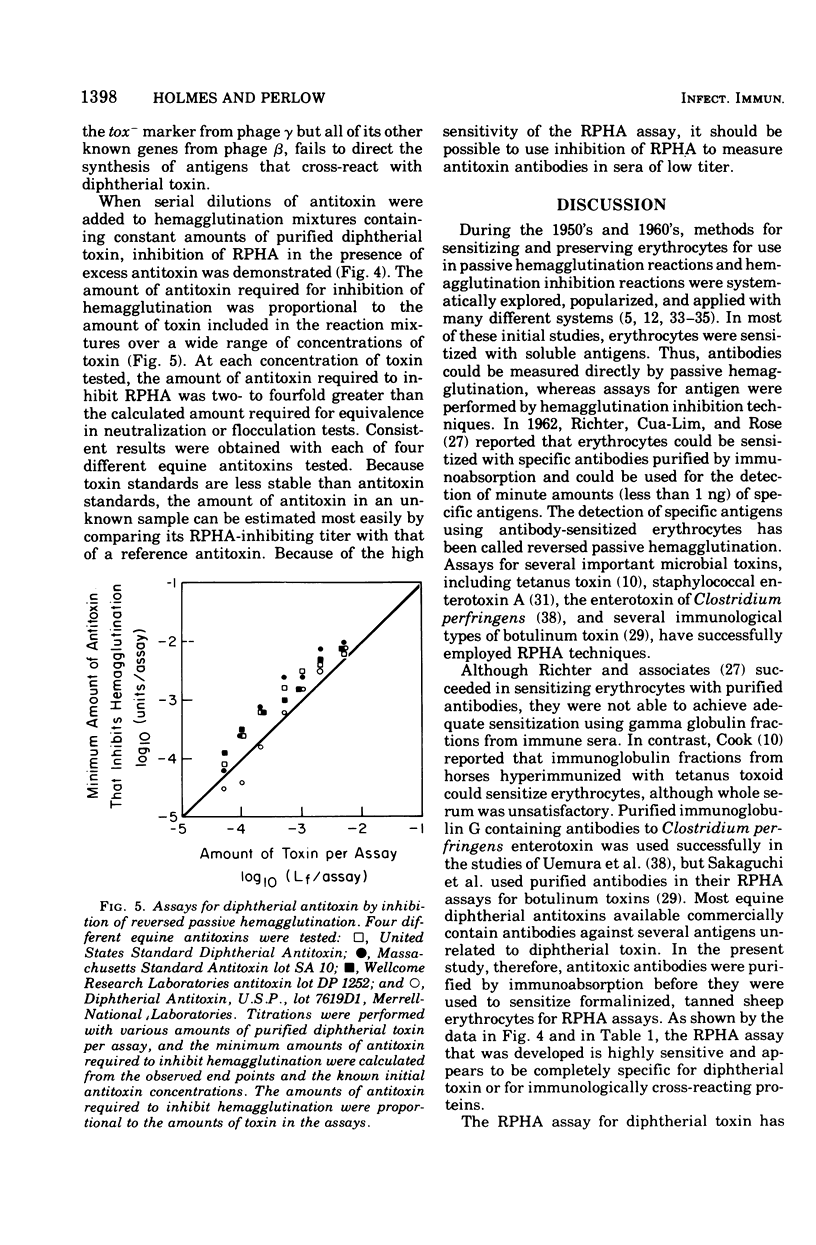
A reversed passive hemagglutination (RPHA) assay for diptherial toxin has been developed. Antitoxic antibodies were isolated from commercially available equine diptherial antitoxin by immunoabsorption using highly purified diphtherial toxin covalently linked to Sepharose 4B. Formalinized, tanned sheep erythrocytes sensitized with the purified antitoxic antibodies are specifically agglutinated by diphtherial toxin but are not agglutinated by extracellular antigens of Corynebacterium diptheriae that are unrelated to toxin. The RPHA assay described can detect less than 20 pg of diphtherial toxin and is comparable in sensitivity to intracutaneous tests for toxin. The RPHA assay was shown to be at least 1,000 times more sensitive than quantitative immunological assays for diptherial toxin performed by single radial immunodiffusion or by one-dimensional double diffusion in agar gels. Fragment A prepared from purified diphtherial toxin and nontoxic mutant proteins that cross-react immunologically with toxin can be assayed directly by RPHA, but the sensitivity of the assay for these proteins is less than for native diphtherial toxin. Inhibition of RPHA was also shown to be a sensitive quantitative method for measuring diptherial antitoxin in vitro.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUTLER W. T. HEMAGGLUTINATION STUDIES WITH FORMALINIZED ERYTHROCYTES. EFFECT OF BIS-DIAZO-BENZIDINE AND TANNIC ACID TREATMENT ON SENSITIZATION BY SOLUBLE ANTIGEN. J Immunol. 1963 May;90:663–671. [PubMed] [Google Scholar]
- Barile M. F., Kolb R. W., Pittman M. United States standard diphtheria toxin for the Schick text and the erythema potency assay for the Schick text dose. Infect Immun. 1971 Sep;4(3):295–306. doi: 10.1128/iai.4.3.295-306.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barksdale L., Arden S. B. Persisting bacteriophage infections, lysogeny, and phage conversions. Annu Rev Microbiol. 1974;28(0):265–299. doi: 10.1146/annurev.mi.28.100174.001405. [DOI] [PubMed] [Google Scholar]
- Barksdale L. Corynebacterium diphtheriae and its relatives. Bacteriol Rev. 1970 Dec;34(4):378–422. doi: 10.1128/br.34.4.378-422.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazaral M., Goscienski P. J., Hamburger R. N. Characteristics of human antibody to diphtheria toxin. Infect Immun. 1973 Feb;7(2):130–136. doi: 10.1128/iai.7.2.130-136.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHATTERJEE S. C. A COMPARATIVE STUDY OF THE HAEMAGGLUTINATION AND BIOASSAY PROCEDURES FOR THE ASSAY OF GUINEA-PIG ANTI-DIPHTHERIA AND ANTITETANUS SERA. Indian J Med Res. 1964 Dec;52:1241–1249. [PubMed] [Google Scholar]
- COOK R. J. REVERSED PASSIVE HAEMAGGLUTINATION SYSTEMS FOR THE ESTIMATION OF TETANUS TOXINS AND ANTITOXINS. Immunology. 1965 Jan;8:74–80. [PMC free article] [PubMed] [Google Scholar]
- Cabau N., Levy F. M. Sur les résultats du titrage de l'antitoxine diphtérique par réaction d'hémagglutination passive. Rev Immunol Ther Antimicrob. 1968 Jan-Mar;32(1):31–36. [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Cuatrecasas P., Wilchek M., Anfinsen C. B. Selective enzyme purification by affinity chromatography. Proc Natl Acad Sci U S A. 1968 Oct;61(2):636–643. doi: 10.1073/pnas.61.2.636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DANIEL T. M., WEYAND J. G., Jr, STAVITSKY A. B. MICROMETHODS FOR THE STUDY OF PROTEINS AND ANTIBODIES. IV. FACTORS INVOLVED IN THE PREPARATION AND USE OF A STABLE PREPARATION OF FORMALINIZED, TANNIC ACID-TREATED, PROTEIN-SENSITIZED ERYTHROCYTES FOR DETECTION OF ANTIGEN AND ANTIBODY. J Immunol. 1963 May;90:741–750. [PubMed] [Google Scholar]
- Drazin R., Kandel J., Collier R. J. Structure and activity of diphtheria toxin. II. Attack by trypsin at a specific site within the intact toxin molecule. J Biol Chem. 1971 Mar 10;246(5):1504–1510. [PubMed] [Google Scholar]
- Finkelstein R. A., Vasil M. L., Holmes R. K. Studies on toxinogenesis in Vibrio cholerae. I. Isolation of mutants with altered toxinogenicity. J Infect Dis. 1974 Feb;129(2):117–123. doi: 10.1093/infdis/129.2.117. [DOI] [PubMed] [Google Scholar]
- Gill D. M., Dinius L. L. Observations on the structure of diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1485–1491. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Gill D. M., Uchida T., Singer R. A. Expression of diphtheria toxin genes carried by integrated and nonintegrated phage beta. Virology. 1972 Dec;50(3):664–668. doi: 10.1016/0042-6822(72)90420-5. [DOI] [PubMed] [Google Scholar]
- Holmes R. K., Barksdale L. Genetic analysis of tox+ and tox- bacteriophages of Corynebacterium diphtheriae. J Virol. 1969 Jun;3(6):586–598. doi: 10.1128/jvi.3.6.586-598.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampidis T., Barksdale L. Park-Williams number 8 strain of Corynebacterium diphtheriae. J Bacteriol. 1971 Jan;105(1):77–85. doi: 10.1128/jb.105.1.77-85.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- OAKLEY C. L., FULTHORPE A. J. Antigenic analysis by diffusion. J Pathol Bacteriol. 1953 Jan;65(1):49–60. doi: 10.1002/path.1700650105. [DOI] [PubMed] [Google Scholar]
- Pappenheimer A. M., Jr, Gill D. M. Diphtheria. Science. 1973 Oct 26;182(4110):353–358. doi: 10.1126/science.182.4110.353. [DOI] [PubMed] [Google Scholar]
- RICHTER M., CUA-LIM F., ROSE B. The agglutination of antibody coated red blood cells by antigen. Biochem Biophys Res Commun. 1962 Apr 20;7:241–244. doi: 10.1016/0006-291x(62)90182-1. [DOI] [PubMed] [Google Scholar]
- SCHEIBEL I., BENTZON M. W., TULINIUS S., BOJLEN K. Duration of immunity to diphtheria and tetanus after active immunization, with mention of non-conformity between haemagglutinating and neutralizing diphtheria antitoxin titers in some human sera. Acta Pathol Microbiol Scand. 1962;55:483–495. [PubMed] [Google Scholar]
- STAVITSKY A. B., ARQUILLA E. R. Micromethods for the study of proteins and antibodies. III. Procedures and applications of hemagglutination and hemagglutination-inhibition reactions with bis-diazotized benzidine and protein-conjugated red blood cells. J Immunol. 1955 Apr;74(4):306–312. [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. I. Procedure and general applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):360–367. [PubMed] [Google Scholar]
- STAVITSKY A. B. Micromethods for the study of proteins and antibodies. II. Specific applications of hemagglutination and hemagglutination-inhibition reactions with tannic acid and protein-treated red blood cells. J Immunol. 1954 May;72(5):368–375. [PubMed] [Google Scholar]
- SURJAN M., NYERGES G. Diphtheria antitoxin titration of human sera by haemagglutination. Z Immun exp ther. 1962 Dec;124:401–410. [PubMed] [Google Scholar]
- Sakaguchi G., Sakaguchi S., Kozaki S., Sugii S., Oishi I. Cross reaction in reversed passive hemagglutination between Clostridium botulinum type A and B toxins and its avoidance by the sue of anti-toxic component immunoglobulin isolated by affinity chromatography. Jpn J Med Sci Biol. 1974 Jun;27(3):161–172. doi: 10.7883/yoken1952.27.161. [DOI] [PubMed] [Google Scholar]
- Silverman S. J., Knott A. R., Howard M. Rapid, sensitive assay for staphylococcal enterotoxin and a comparison of serological methods. Appl Microbiol. 1968 Jul;16(7):1019–1023. doi: 10.21236/ad0838753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchida T., Pappenheimer A. M., Jr, Greany R. Diphtheria toxin and related proteins. I. Isolation and properties of mutant proteins serologically related to diphtheria toxin. J Biol Chem. 1973 Jun 10;248(11):3838–3844. [PubMed] [Google Scholar]
- Uemura T., Sakaguchi G., Riemann H. P. In vitro production of Clostridium perfringens enterotoxin and its detection by reversed passive hemagglutination. Appl Microbiol. 1973 Sep;26(3):381–385. doi: 10.1128/am.26.3.381-385.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ramshorst J. D. Titration of diphtheria and tetanus antitoxins in sera of low titre. Bull World Health Organ. 1971;45(2):213–218. [PMC free article] [PubMed] [Google Scholar]



