Abstract
During the past decade the zebrafish has emerged as a leading model for mechanistic cancer research due to its sophisticated genetic and genomic resources, its tractability for tissue targeting of transgene expression, its efficiency for forward genetic approaches to cancer model development, and its cost-effectiveness for enhancer and suppressor screens once a cancer model is established. However, in contrast to other laboratory animal species widely used as cancer models, much basic cancer biology information is lacking in zebrafish. As yet data are not published regarding dietary influences on neoplasm incidences in zebrafish. Little information is available regarding spontaneous tumor incidences or histologic types in wild-type (wt) lines of zebrafish. So far a comprehensive database documenting the full spectrum of neoplasia in various organ systems and tissues in not available for zebrafish as it is for other intensely studied laboratory animal species. This manuscript confirms that as in other species diet and husbandry can profoundly influence tumor incidences and histologic spectra in zebrafish. We show that in many laboratory colonies wt lines of zebrafish exhibit elevated neoplasm incidences and neoplasm associated lesions such as heptocyte megalocytosis. We present experimental evidence showing that certain diet and water management regimens can result in high incidences of neoplasia and neoplasm associated lesions. We document the wide array of benign and malignant neoplasms affecting nearly every organ, tissue and cell type in zebrafish, in some cases as a spontaneous aging change, and in other cases due to carcinogen treatment or genetic manipulation.
Keywords: Danio rerio, diet, hepatocyte megalocytosis, husbandry, neoplasia, naturally occurring carcinogen, non-protocol induced variation, zebrafish
Introduction
Zebrafish have emerged as a premier vertebrate model system for understanding genes and signaling pathways controlling development and mechanisms of disease affecting nearly every organ system (Dahme et al. 2009; Ingham 2009; Zhu and Zon 2002). Mutant lines of zebrafish produced worldwide have helped to clarify normal and abnormal development and have provided models for understanding human diseases from polycystic kidney disease to hereditary anemias to cancer. Optimization of tools for inducible tissue-specific expression of transgenes and recently developed techniques for efficient targeted mutagenesis such as zinc finger nucleases and transcription activator-like effector nucleases (TALENS) now allow production of “custom made” zebrafish models for precise histologic types of cancer affecting specific organs (Amatruda and Patton 2008, Foley et al., 2009; Koh et al. 2010; Mione and Trede 2010; Moore et al., 2012). Xenografts of human tumors into zebrafish embryos and zebrafish cancer models allowing tumor induction very early in life provide a cost-effective, high-throughput system for drug discovery (Lally et al. 2007; Mandrekar and Thakur 2009; Marques et al. 2009; Taylor et al. 2010; Yeh et al. 2008). Despite the high level of sophistication of genetic and genomic tools available for the zebrafish model, basic pathology data for this species still lag far behind the data available for most mammalian laboratory and domestic animal species. While we understand the genetics controlling induction of specific cancer types in zebrafish, little basic information is published regarding spontaneous tumor incidences or histologic types in commonly used wt or mutant lines (Smolowitz et al. 2002 ; Spitsbergen et al. 2009; Spitsbergen and Kent 2003). Because of a strong primary focus on cancer genetics, many of the recent reports of neoplasia in transgenic or mutant lines of zebrafish do not provide data regarding the spontaneous tumor incidences or histologic spectrum of neoplasia in the genetic lines of fish used in the research and do not report the incidences or morphologic diagnoses of all tumor types in mutant or carcinogen-treated fish. Additionally, data regarding dietary influences on neoplasia in zebrafish are not yet published.
As early as 1940 scientists recognized that dietary restriction could influence cancer incidence in animals (Tannenbaum 1940). Extensive data over the past 3 decades from carcinogenesis studies in rodents as well as human epidemiology clearly document strong influences of dietary components such as lipid and protein as well as caloric intake on cancer incidence (Abo and Kari 1996; Campbell 2007; Fontana et al. 2006; Hursting and Kari 1999; Kari et al. 1999; Li et al. 1999; Prentice et al. 2009; Wei et al. 2008). A wide variety of natural carcinogens and anticarcinogens in plants and other dietary factors are now well characterized in research focused on optimization of healthy aging (Aiyer et al. 2008; Akhtar et al. 2009). Recently dietary factors such as trace contamination by arsenic and other metals (Kozul et al. 2008) and estrogenic plant components in practical laboratory animal diets (Adlercreutz 2007; Adlercreutz et al. 2004; Cross et al. 2004; Green and Kelly 2008; Ziegler et al. 2004) have been recognized as confounding factors in toxicology and carcinogenesis studies.
To minimize such confounding effects of variable dietary components in practical diets in carcinogenesis studies using rainbow trout and aquarium fish, scientists at Oregon State University (OSU) developed a semi-purified diet, Oregon Test Diet (OTD), with gelatin and casein serving as the protein sources (Lee et al. 1991). This diet has ensured consistency and reproducibility in studies utilizing fish as cancer research models conducted over the past 30 years (Bailey et al. 1996, 2009; Spitsbergen et al. 2000a,b; Reddy et al. 1999).
As fish pathologists from OSU began providing diagnostic pathology expertise to zebrafish research laboratories from around the world as a service through the Zebrafish International Resource Center (ZIRC) we observed very different patterns of incidences and histologic types of neoplasia in untreated control fish from many laboratory colonies compared to our colony at OSU in which fish were fed OTD in a flow-through system receiving well water. Therefore we undertook a two-pronged approach to clarify the factors that might be contributing to these perplexing patterns of spontaneous tumors in many well-managed colonies. We conducted prospective studies of neoplasm incidences in replicate groups of AB wt strain that were fed either commercial flake diet or OTD and raised in either a flow-through or a recirculating water system. We also examined retired broodstock from various flow-through and recirculating water systems over a period of two years. This manuscript will discuss the variety of neoplasia observed in zebrafish submitted to the ZIRC diagnostic service, neoplasia and related lesions in sentinel fish from selected colonies, results of our prospective tumors studies with wt and mutant lines, our studies of neoplasia in retired broodstock from various colonies, and the diversity of neoplasia documented in carcinogen and genetic research using zebrafish. Wt lines of zebrafish fed semi-purified diets and raised in flow-through water systems had low incidences of neoplasia at one or two years of age and showed a limited variety of neoplasm types. Zebrafish fed commercial diets containing fish meal and reared in certain recirculating water systems showed far higher tumor incidences and a much wider variety of histologic types of neoplasia. Both diet and water system had strong influences on tumor incidences and a significant interaction occurred between diet and water system in determining tumor incidences. These studies highlight the need for careful consideration of diet and husbandry in order to ensure valid and reproducible data in research using the zebrafish model. Carcinogenesis studies with various lines as well as genetic studies to create zebrafish models for specific types of neoplasia have demonstrated convincingly that zebrafish can develop similar histologic types of neoplasia as those affecting humans as long as zebrafish have a similar tissue analog.
Experimental Methods and Results
Experimental methods and data from experimental studies investigating causes of neoplasia and carcinogen-induced neoplasia are reported as supplementary information (Supplemental Methods and Data). We present here in print illustrations highlighting the dramatic influence of diet and water systems on total neoplasm incidence (Fig. 1) and examples of key neoplasm types and neoplasm associated lesions occurring in zebrafish from these studies (Figs. 2 and 3). Detailed data on tumor incidence in specific treatment groups and tissue-specific tumor incidence are provided for the diet and water system studies (Tables S1 and S2). Table S3 summarizes relative incidence data for neoplasms of specific organ systems and tissues from studies of diagnostic cases, sentinels, retired broodstock, carcinogenesis studies and mutant tumor models. Table S4 reports tumor incidence and morphologic diagnoses for wt lines fed OTD in flow-through systems. Table S5 outlines target organs or tissues in wt and mutant lines from carcinogenesis studies. Figure S1, S2 and S3 illustrate the diversity of neoplasia occurring in studies of carcinogenesis and mutant tumor models. Figure S4 illustrates the typical location of small cell carcinoma of intestine near the ampulla of Vater in the anterior intestine and shows the paradoxical lack of high cell proliferation in this common site of tumor formation. S4 also shows that certain cytochrome P450 enzymes critical to carcinogen metabolism are highly expressed in the segment of intestine most prone to neoplasia.
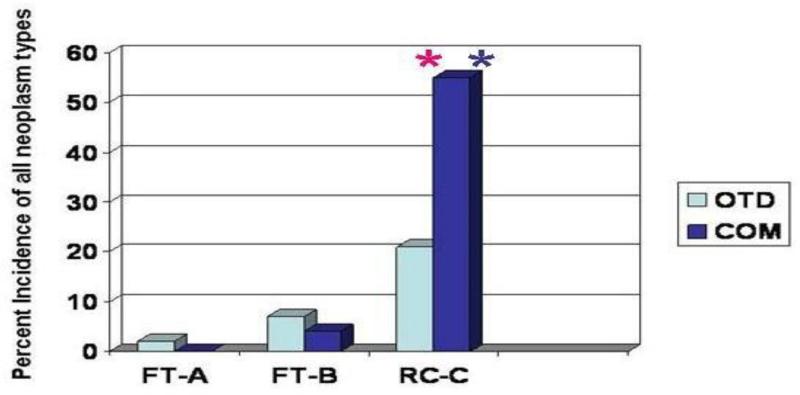
Figure 1.
Influences of diet and husbandry regimen on total neoplasia incidences in AB wild-type fish at 22 months of age. Replicate tanks of fish fed each of 2 diets, OTD (semi-purified Oregon Test Diet) or COM (mixture of commercial diets containing fish meal) and reared at each of 3 sites, FT-A (flow-through system design, location A), FT-B (flow-through system design, location B), or RC-C (recirculating system with fluidized sand biofiler). Tumor incidences were significantly higher in the recirculating system compared to flow through systems, regardless of diet (P=0.0000, chi-square with Yates’ correction; red asterisk). Also, diet did not significantly influence tumor incidences at the flow-through sites (P>0.24 for FT-A and P>0.7 for FT-B), but did significantly influence tumor incidences in the recirculating system (P<0.0001; blue asterisk).
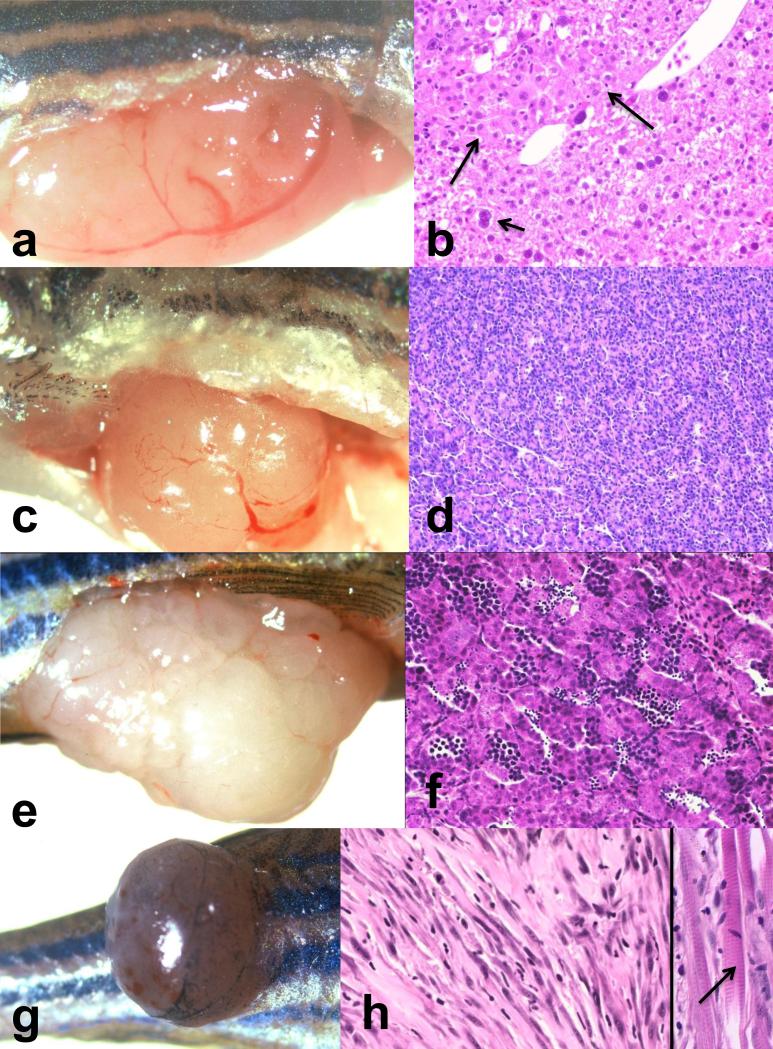
Figure 2.
Gross and microscopic lesions observed in zebrafish (Danio rerio) from the prospective study of diet and water system effects on tumors in AB wt fish. (a) and (b) Hepatocellular carcinoma and hepatocyte megalocytosis. Gross and histologic appearance of soft tan mass of hepatocellular carcinoma in liver of fish from system RC-C fed COM diet. Long arrows point to hepatocellular carcinoma. Short arrows point to enlarged nuclei characteristic of hepatocyte megalocytosis present in nonneoplastic liver adjacent to carcinoma. Inset illustrates cellular pleomorphism and nuclear atypia with prominent nucleoli characteristic of hepatocellular carcinoma. (c) and (d) Acinar cell carcinoma of exocrine pancreas. Gross and histologic appearance of soft tan mass of acinar cell carcinoma occurring in fish from Lot 17 system FT-A fed OTD. (e) and (f) Spermatocytic seminoma. Gross and microscopic appearance of soft white lobulated mass of spermatocytic seminoma in testis of fish from system RC-C fed COM diet. (g) and (h) Rhabdomyosarcoma in skeletal muscle of trunk. Gross and microscopic appearance of firm mass protruding from skeletal muscle of caudal trunk in fish from system RC-C fed COM diet. Inset in h shows striations (arrow) in some of the neoplastic myocytes viewed under Nomarsky differential interference microscopy.
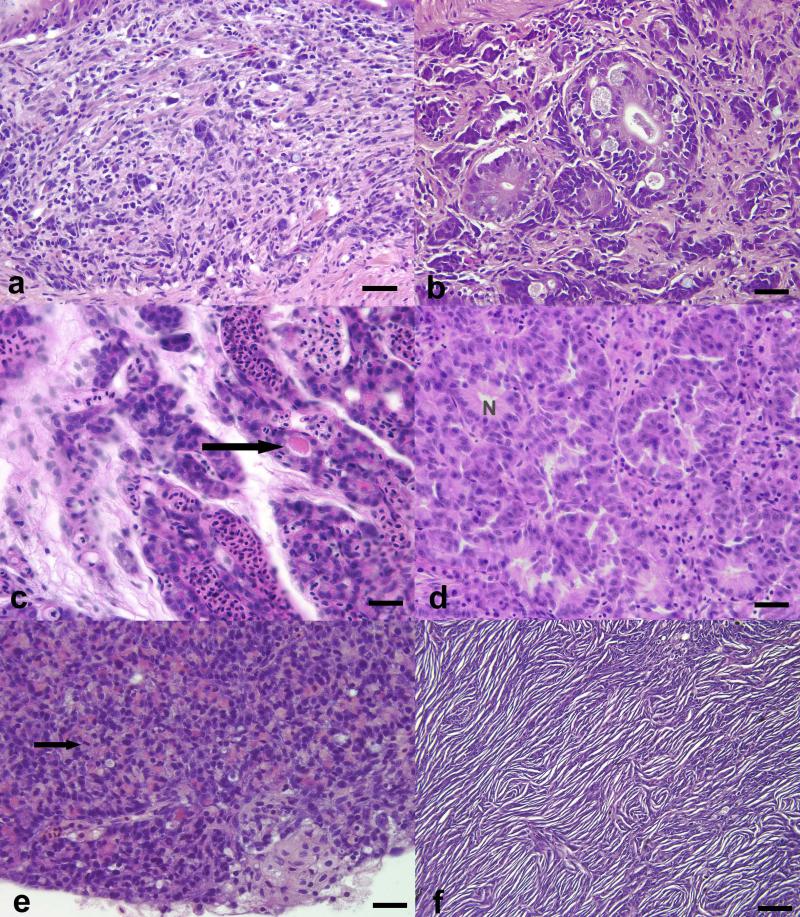
Figure 3.
Histomorphologic patterns and features of relatively common types of neoplasia in adult zebrafish. Images are from AB wt (a-e) and tp53zdf1 null (f) zebrafish. (a) Small cell carcinoma of the anterior intestine. Small clusters and packets of 3-8 basophilic polygonal cells infiltrating the lamina propria, embedded within a dense fibrous stroma and interspersed chronic inflammatory cells. It is common in zebrafish for small cell carcinoma to invade into the coelomic cavity and line the serosal surfaces of adjacent organs (carcinomatosis). (b) Adenocarcinoma of the anterior to mid-intestine. Irregularly shaped, disorganized acinar structures lined by hyper- and dysplastic epithelial cells and nests of neoplastic cells within the lamina propria, surrounded by dense schirrous matrix intermingled with chronic inflammatory cells. (c) Thyroid gland carcinoma. Cords and nests of basophilic neoplastic cells within an edematous fibrovascular matrix; rare follicular structures contain intraluminal colloid (arrow). (d) Ultimobranchial gland carcinoma. Nests, cords and ribbons of amphophilic polygonal cells surrounded by fibrovascular tissue; occasional “normal” acinar structures (N) can be observed. (e) Pancreatic carcinoma. Sheets of densely packed neoplastic acinar cells completely efface normal pancreas architecture; mitotic figures (arrow) are common and some of the neoplastic cells retain eosinophilic zymogen granules. (f) Malignant peripheral nerve sheath tumor. Dense, streaming and interlacing fascicles of basophilic spindle cells with interfascicular clefts and prominent whorls; there was extensive local invasion and extension of this tumor. (a-e); bar = 25 microns; (f); bar = 50 microns
Role of Infectious Agents in Neoplasia
Pseudocapillaria tomentosa is a nematode parasite infecting the gut of zebrafish. This parasite causes moderate to severe multifocal to diffuse hyperplasia and dysplasia in intestine. Elevated incidences of intestinal neoplasia occur in colonies infected with this parasite, and gut neoplasms often occur in close proximity to profiles of nematodes (Kent et al. 2002). In dietary studies with DMBA at OSU, zebrafish were more likely to develop intestinal neoplasia if infected with the nematode parasite (Spitsbergen et al. 2000b). We have recently shown that the specific protocol for infection of zebrafish with P. tomentosa is critical for optimal tumor induction and promotion in carcinogen studies. Bath treatment of 3 wk old fry of the sensitive uma s2068 mutant line with DMBA followed by infection with nematodes induced no more than a 10% incidence of intestinal neoplasia. However, natural early life infection in a colony endemically infected with P. tomentosa acted as a potent tumor promoter when infected fry were given bath treatments with DMBA at 3 and 5 wk. Incidences of intestinal neoplasia in this study were greater than 50% by 1 yr following carcinogen treatment. Higher incidences of myelodysplastic syndrome also occurred in the uma s2068 line infected with nematodes compared to uninfected fish (Spitsbergen et al. 2008; Figure S1).
Certain other infectious agents that often cause profound hyperplasia in zebrafish tissues, such as Piscinoodinium pillulare in the gill have not acted as a tumor promoter in any carcinogen experiments that we have conducted. The strain of mycobacterium seems critical in determining whether this infectious agent will act as a tumor promoter in carcinogen experiments with zebrafish. The mycobacterial strain which most often infects zebrafish colonies in Oregon is Mycobacterium chelonae, a relatively nonpathogenic agent which typically causes mild focal lesions in and around the gas bladder (Kent et al. 2004; Murray 2012; Whipps et al 2008). This strain does not appear to increase the incidence of neoplasia in zebrafish colonies with or without carcinogen treatment. In contrast the more pathogenic strain Mycobacterium haemophilum occurring in zebrafish colonies in Singapore causes severe diffuse inflammation throughout most visceral organs (Whipps et al. 2007). This greater inflammation acts as a tumor promoter in carcinogen studies and neoplasms often arise in the center of inflammatory lesions in tissues such as liver or intestine. We have not yet investigated any possible role of enteric bacterial flora such as Helicobacter in spontaneous intestinal neoplasia in zebrafish.
To date, no pathogenic viruses have been isolated from zebrafish. Ultrastructural studies of a variety of histologic types of neoplasia from zebrafish including seminomas, neuroblastoma of brain, esthesioneuroblastoma of nose, spindle cell sarcoma of skeletal muscle, malignant peripheral nerve sheath neoplasia, benign and malignant vascular tumors, and enlarged spleens with myelodysplastic syndrome have not revealed viral agents in the tissues. Likewise tumor transmission trials in which whole live cells from neoplasms were injected intraperitoneally into zebrafish fry have failed to yield evidence of any transmissible neoplasms. Since the wt Nadia (NA) line was recently introduced to the laboratory from field conditions in India, we considered this line most likely to be harboring possible latent pathogenic viruses. We used large seminomas from 2–year-old NA zebrafish to inject zebrafish fry of the TL and AB lines. A year following injection, the fish were free of neoplasia grossly and histologically. We are anxious to obtain live zebrafish with skin or fin papillomas as we believe that these papillomas are the best neoplasm candidates for harboring a tumorigenic virus of zebrafish.
The Need for an Immunohistochemistry Panel for Better Identification of Specific Types of Neoplasia in Diagnostic Pathology and Research
Investigators worldwide have extensively used immunohistochemical analysis of teleost fish tissues as a research technique for the past 35 years. Varied antibodies, chromogens, antigen retrieval and blocking procedures have been used to answer specific questions regarding cellular and tissue composition as well as gene and protein expression. However to date little effort has focused on creation and utilization of specific antibodies for general application in fish diagnostic and toxicologic pathology We currently lack standardized, validated immunohistochemical protocols for formalin-fixed and paraffin-embedded tissues for a comprehensive panel of antibodies to be used to characterize cell and tissue types in fish neoplasms. Custom made antibodies directed against zebrafish-specific target antigens are available if the antigen amino acid sequence is known. Current application of common basic antibody panels, familiar to human and veterinary pathologists, can be generated using the amino acid sequence of known zebrafish protein antigens (National Center for Biotechnology Information GenBank; www.ncbi.nlm.nih.gov) for epithelial, mesenchymal, neural and endocrine neoplasia that include cytokeratin, vimentin, desmin, smooth muscle actin, myoglobin, glial fibrillary acidic protein, S-100, thyroglobulin and insulin (Ramos-Vara 2005).
One of the best examples of the utility of immunohistochemistry in pathology is cytokeratin filament expression profiling of suspected or questionable carcinoma, which frequently allows definitive determination of the neoplastic cell's epithelial origin. Cytokeratins are thought to be highly conserved across vertebrate species and in teleost fish they have been previously characterized, demonstrating similar molecular weights and isoelectric points among different genera (Garcia et al. 2005). Attempts to examine cytokeratin expression profiles in the medaka and common carp, a close relative of zebrafish, met with limited success as a wide spectrum of tissues showed non-specific immunopositive reactivity. Although many of the epithelial tissues, such as epidermis, branchial, biliary, intestinal and renal epithelium stained cytokeratin positive using mammalian AE1/AE3 antibody, several tissues other than those of ectodermal origin stained positive including fibroblasts, chondrocytes, testicular myoid cells, vascular adventitia, skeletal muscle and glial cells (Bunton 1993, 1994; Groff et al. 1997). Similar to cytokeratins, other mammalian antibodies have been used to identify or confirm the histotype and mitotic activity of certain teleostean fish tumors such as peripheral nerve sheath tumors, intestinal adenocarcinoma, gonadal tumors such as seminoma and perineoplastic stromal cells that includes calretinin, S-100, PCNA (proliferating cell nuclear antigen), vimentin, placental alkaline phosphatase, alpha fetal protein, neuron-specific enolase, c-KIT, estrogen receptor, actin and desmin with variable success (Bunton, 1994; Bunton 1995; Faro et al. 2009, Marino et al. 2007, Sirri et al. 2010). The issue of what constitutes appropriate antigen and control tissues as a means of validating the immunohistochemical reactivity remains problematic as long as mammalian antibodies are used.
Discussion and Conclusions
Neoplasia in Liver of Zebrafsh and Other Species
Considering spontaneous tumors in diagnostic cases and retired broodstock as well as carcinogenesis bioassays and mutant tumor models, we have observed neoplasia of a wide variety of histologic types affecting nearly every organ and most cell types. Liver is the most common target organ for nearly all of the carcinogens studied in all wt and mutant lines of zebrafish (Table S4). This targeting of liver by most carcinogens is similar to the data regarding rainbow trout (Bailey et al. 1996) and other small aquarium fish such as medaka and guppy (Bunton 1996). Liver is more often targeted in neoplasia in fish than in mammals, most likely because fish liver grows throughout life, whereas, adult mammal liver is quiescent unless damaged. Compared to mammals, zebrafish and trout more often show mixed hepatic neoplasms comprised of biliary and hepatic components (Bailey et al. 1996; Hendricks 1996; Spitsbergen et al. 2000b; Tsai 1996).
Species Differences in Target Tissues and Histologic Types of Neoplasia
The range of tumor types and affected tissues that we have observed in zebrafish, differ from those seen in mammals and in other well-studied fish species such as rainbow trout. Regardless of the class of carcinogen, rainbow trout show 3 primary target organs: liver, stomach, and gas bladder, developing almost exclusively epithelial neoplasia (Bailey et al. 1996). In contrast zebrafish and other small aquarium fish such as medaka and guppy show a much wider range of target organs, and a broader range of histologic types of neoplasia, including epithelial, mesenchymal, neural and neural crest tumors. Zebrafish are agastric, so no stomach neoplasia occurs. Neoplasia of gas bladder is quite rare in zebrafish, with or without carcinogen treatment. However, as we have examined large numbers of fish from a variety of fish strains and treatment regimens, we have now observed several benign as well as malignant neoplasms of gas bladder in zebrafish (Spitsbergen et al. 2000a; Zhan et al. 2010).
Neural Neoplasia in Zebrafish and Other Species
Neural neoplasms affecting brain, eye and spinal cord are relatively common spontaneous tumors in diagnostic cases and retired broodstock from systems with recirculating biofilters in which fish are fed commercial diets. Neural tissues are also common targets for several carcinogens including DMBA, MNNG and MAMA (Table 5). In adult humans, dogs and cats, gliomas are the most frequent primary brain tumor (Behin et al. 2003; Koestner et al. 1999). In contrast, in wt zebrafish most spontaneous or induced primary neurogenic neoplasms of the central nervous system (CNS) are poorly differentiated, highly embryonal neuroblastomas or primitive neuroectodermal tumors (PNETS). Brain of adult zebrafish is histologically quite distinct from that of mammals, with a much greater component of highly cellular areas comprised of deeply basophilic embryonal cells surrounding the ventricular system of forebrain, diencephalon and myelencephalon (Kizil et al. 2012; Kroehne et al. 2011). These abundant embryonal periventricular cells may predispose zebrafish to develop more embryonal neoplasms of CNS resembling those seen in pediatric cases in humans. Until recent collaborations with investigators creating transgenic tumor models (Ju et al. 2010) we had not documented a glioma of brain or spinal cord in our studies of spontaneous or carcinogen induced neoplasia in zebrafish. Now we have observed low grade astrocytomas as well as glioblastomas in zebrafish CNS. In comparison to mammals, zebrafish are unusually predisposed to develop neoplasia of nerve sheath of peripheral and cranial nerves. Many of the zebrafish models with inactivating mutation in tumor suppressor genes including tp53, mlh1, msh2, msh6, and ribosomal genes show high incidences of malignant peripheral nerve sheath tumors when the human or rodent cancer spectrum from inactivating mutations in the orthologous tumor suppressor genes cause a much wider range of neoplasms in mesenchymal, epithelial or lymphomyeloid tissues (Amsterdam et al. 2004; Berghmans et al. 2005; Feitsma et al. 2008; Parant et al. 2010).
Enteric Neoplasia in Zebrafish and Other Species
Like liver tumors occurring in zebrafish, gastrointestinal tumors (GI) of zebrafish are more likely to be pluripotential neoplasms comprised of multiple cell lineages than the spontaneous or carcinogen-induced GI tumors of mammals. Mixed malignant intestinal neoplasia comprised of malignant smooth muscle cells and malignant mucosal epithelial cells is a relatively common lesion induced by DMBA in zebrafish (Spitsbergen et al. 2000b). Such carcinosarcomas of the GI tract are rare in mammals (Riddell et al 2003; Whiteley 1996). Interestingly, most of the spontaneous GI neoplasia occurring in zebrafish diagnostic cases and retired broodstock is strictly epithelial, principally small cell carcinomas or mucosal adenocarcinomas.
Renal Neoplasia in Zebrafish and Other Species
Kidney is a common target of carcinogens including DMBA, MNNG and MAMA in rainbow trout. When treatment with MNNG occurs early in life, up to 50% of rainbow trout develop nephroblastomas (Bailey et al. 1996). In contrast to rainbow trout, kidney is rarely a target for carcinogens in zebrafish. We have seen a single renal adenoma and one renal carcinoma following early life stage exposure to MAMA and MNNG, respectively, in the 5-D Florida wt line. We have observed nephroblastoma primarily in the TL line, and then only in diagnostic cases or retired broodstock from systems with recirculating biofilters and/or feeding commercial diets (Figure S3). We have not yet observed nephroblastoma in TL or other lines of zebrafish intentionally treated with carcinogens.
Pigment Neoplasia in Zebrafish and Other Species
Melanomas are common skin tumors in mammals (Goldschmidt et al. 1998), and occur at high incidences spontaneously or after carcinogen exposure in certain genetic lines of Xiphophorus (Kazianis and Walter 2002; Walter and Kazianis 2001). We have observed a single case of malignant melanoma in a diagnostic case (Table S3) and a single benign melanocytoma of optic nerve in retired broodstock. We have not yet found a carcinogen treatment regimen that yields increased numbers of melanomas. However in recent years several laboratories have developed genetic protocols to induce high incidences of melanoma in zebrafish (Patton et al. 2005; Santoriello et al. 2010).
Vascular Neoplasia in Zebrafish
Vascular neoplasia has occurred in many tissues throughout the body of zebrafish treated with carcinogens. However, most vascular neoplasms occur in the rete of the choroid gland of the eye or in the gill (Figures S1 and S3). Perhaps this tissue tropism reflects the high density of small blood vessels in these two sites.
Epithelial Skin Neoplasia in Zebrafish and Other Species
In our studies epithelial skin neoplasia was exceedingly rare in zebrafish spontaneously or following carcinogen treatment. We were unable to induce papillomas of skin or fin by bath treatment of fry of the TL, TU or KOLN lines with the maximum tolerated dose of ENU. Various factors might explain our findings compared to the 100% incidence of cutaneous papillomas occurring in Florida wt zebrafish treated as adults with ENU (Beckwith et al. 2000). Only Florida wt skin may respond to ENU. Although it seems unlikely to us, adult exposure to ENU may be required to induce papillomas. Typically early life stages of fish are more responsive to all carcinogens than adults, so long as the fish have reached a stage of development at which they metabolize carcinogens requiring metabolic activation. ENU is a direct-acting carcinogen and does not require metabolic activation for effect. Epithelial skin neoplasms in a variety of fish species are associated with oncogenic viruses (McAllister et al. 1985; Yoshimizu et al. 1995). Pennsylvania State College of Medicine zebrafish may have carried a virus, which was activated following treatment with ENU (see Supplemental Methods and Data, Section on Carcinogen-Induced Neoplasia). This hypothesis that the colony at Pennsylvania State College of Medicine has some unique factor predisposing it to epithelial neoplasia is supported by the finding that papillomas have not been observed on the skin or fins in several large zebrafish colonies including those at the University of Oregon, Cornell University (Paul Bowser, personal communication) and Harvard University (Leonard Zon, personal communication) in which adult males have been mutagenized using ENU by protocols similar that used by Beckwith et al.
Soft Tissue Sarcoma in Zebrafish and Other Species
Liposarcoma is the most common soft tissue sarcoma in humans (Dei Tos, 2000). We have not yet documented a single liposarcoma or lipoma in zebrafish from diagnostic cases, retired broodstock, or carcinogen studies. We find no reports of liposarcoma in zebrafish in the literature. The reasons that zebrafish adipocytes do not act as targets in tumorigenesis are unclear. Lipomas do occur rarely in other fish species (Bruno et al., 1991; Chen et al., 1996).
Neoplasia of Gonad in Zebrafish and Other Species
One factor influencing tissue tropism of carcinogens is the rate of cell proliferation in the tissue. Cell division is required for activity of most mutagens (Winn et al. 2000). Yet cell proliferation rates alone do not adequately explain the disparity between spontaneous and carcinogen-induced neoplasia in zebrafish testis in comparison to ovary. We have observed just 2 spontaneous, no carcinogen-induced, and a few genetically influenced (morphant or mutant fish) ovarian neoplasms in zebrafish. In contrast seminomas in males are the most common spontaneous and one of the common carcinogen-induced neoplasms in zebrafish. Most of the ovarian neoplasms in zebrafish were carcinomas, fewer adenomas, and one dysgerminoma. In contrast to zebrafish, in medaka, spontaneous seminomas occur in females as well as in males (Hawkins et al. 1996) and dysgerminomas are a common finding in control fish in some studies (Reddy et al, 1999b). In female zebrafish with normal functional adult ovaries, we have seen sperm producing testicular tissue in pancreas of wt lines. In wt fish treated with DMBA we have also seen occasional spermatocytic seminomas in pancreas of female fish. Ectopic germ cells which develop outside of the gonad in zebrafish and medaka differentiate into testis in female fish.
Lymphomyeloid Neoplasia in Zebrafish and Other Species
Hemopoietic tissues of fish have a relatively high proliferation rate, but except for lymphoma, spontaneous or carcinogen-induced hemopoietic neoplasia is extremely rare in wt lines of zebrafish. Acute or chronic myeloblastic leukemia are important cancers in humans and other mammals (Iovino and Camacho 2003; Wertheim et al. 2002), yet we had not documented granulocytic leukemia until we examined mutant lines of zebrafish. Now that we have extensively studied some of the mutant lines of zebrafish predisposed to lymphomyeloid neoplasia, we have documented numerous cases of myelodysplastic syndrome and neoplasia in erythroid and granulocytic lineages.
Ultimobranchial Neoplasia in Zebrafish and the Role of Diet in this Neoplasm
Ultimobranchial neoplasia is among the most common histologic tumor types in diagnostic cases, retired broodstock, and carcinogenesis studies, regardless of diet and husbandry. In recent years we have evaluated in our carcinogenesis bioassays the usefulness of Aquatox (Ziegler), a commercial diet formulated to have low nitrosamine levels, and have typically found low tumor incidences in most tissues in control fish, however in some experiments hyperplasia of ultimobranchial glands occurred that was associated with elevated carcinogen-induced neoplasia in these lines of fish. In our assays of tissue-specific cell proliferation rates, we have found PCNA expression in ultimobranchial to be among the highest in any tissue (Figure S4). Diet analyses indicated that the batch of Aquatox used in these experiments contained 2% calcium on a dry weight basis compared to the 1% calcium present in OTD. More controlled experiments are needed in order to define the optimal calcium levels in zebrafish diets (Watts et al, 2012), but we speculate that as with bulls showing medullary thyroid neoplasia when fed diets high in calcium designed for lactating dairy cows (Geelhoed 1996), elevated dietary calcium may cause hyperplasia of the ultimobranchial gland and predispose zebrafish to elevated neoplasm levels. In contrast to zebrafish, in medaka, ultimobranchial neoplasia does not occur with or without carcinogen treatment (Bunton et al. 1996; Masahito et al 1989). One might speculate that medaka evolved in an environment with high calcium levels, so that high dietary calcium does not cause ultimobranchial hyperplasia as in zebrafish. Much more information is needed regarding tissue-specific carcinogen metabolism, DNA repair and cell turnover rates to begin to understand tissue tropism of carcinogens in zebrafish (Law 2001).
A single diet is not likely to be optimal for all research applications with zebrafish (Watts et al, 2012). We did not rigorously compare OTD with Aquatox in carcinogenesis or spontaneous tumor studies, however, we conducted selected carcinogenesis and aging experiments using Aquatox to determine its influence on tumor incidences. We observed rather profound senescence of aging zebrafish over 2 years old of most genetic lines when fed OTD. Although these fish were free of infectious diseases, they showed reduced appetite and became cachexic. We found that these same lines reared under similar conditions in our flow-through systems but fed Aquatox maintained good appetites and showed healthier aging, in some cases living over 4 years while maintaining normal weight. We have not carefully compared the composition of Aquatox versus OTD, but visually Aquatox contains much more carotenoids which may act as antioxidants that promote healthy aging. OTD was optimized for tumor bioassays, not for healthy aging.
Critical Role of Genetic Background as an Influence on Neoplasia in Zebrafish and Other Species
A factor that has received little attention in zebrafish research until the past 5 years is the critical role of genetic background in determining tumor incidences as well as other physiological parameters such as disease resistance, immune responses and other endpoints in response to toxicant exposure. Extensive data from rodents and other laboratory animal species confirm that genetic background as well as specific mutations and transgenes are critical in determining both spontaneous and carcinogen induced neoplasm incidences, target organs and the histologic spectrum of tumors which occur (Ward and Devor-Henneman 2004). Observations regarding hyperplasia of bile ducts in the TL line of zebrafish illustrate the importance of genetic background in determining tumor phenotype in fish. The TL line from most laboratories shows moderate to severe hyperplasia of bile ducts in liver as well as about 10% incidences of biliary neoplasia by 1-1.5 years of age. This trait is not linked to the long findt2 (lof dt2) gene because siblings of lof dt2 fish with wt fin length show similar biliary hyperplasia and neoplasia. This biliary trait acts as a dominant genetic factor in crosses to other wt strains. Surprisingly older adult fish of the TL line from certain laboratories do not show bile duct hyperplasia, biliary neoplasia or myelodysplastic syndrome seen in the TL line from most laboratories, so these traits seem likely to be determined by the genetic background of the commonly occurring TL lines.
Role of Diet and Water Systems in Neoplasia in Zebrafish
A great advantage of small aquarium fish for cancer bioassays has been their low background tumor incidences in comparison to mammals (Hawkins et al. 1985, 2003; Spitsbergen et al. 2000a,b). Recently we have found that water system design and diet exert profound effects on spontaneous tumor incidences in zebrafish. One of the most urgent issues in the rapidly growing field of cancer research using the zebrafish model is the need to optimize aquaculture systems and diets to eliminate or minimize the natural carcinogens and possible tumor promoters that currently confound research in many recirculating systems feeding commercial diets. Many gastrointestinal, pancreas, ultimobranchial, thyroid, nerve sheath, brain and eye tumors seen in diagnostic cases and retired broodstock probably are caused by natural carcinogens and/or tumor promoters in water systems or diets. These neoplasms are rare, even in 2 year old fish of most wt and certain mutant lines when born and raised in flow-through systems and fed a semi-purified diet. When spontaneous seminomas and other neoplasms occur in older fish in these flow-through systems, these tumors are typically quite small (1-4 mm) rather than 10-14 mm as are many seminomas from recirculating systems feeding commercial diets. The finding of elevated age-specific tumor incidences, remarkably large spontaneous tumors, and hepatocyte megalocytosis in a high percentage of intensive zebrafish aquaculture facilities from around the world has profound implications for many institutes at the National Institutes of Health, not just those funding cancer research. Now that the zebrafish genome is sequenced (Bowen et al. 2012; Leshchiner et al. 2012), and our knowledge of genetics, genomics, and molecular and cellular development mechanisms has become very sophisticated, interest has grown regarding use of zebrafish as models for understanding the genetic mechanisms underlying a wide variety of human diseases. Episodic exposure of zebrafish colonies to potent naturally occurring mutagens and carcinogens from recirculating systems, and continuous exposure to possible carcinogens and/or tumor promoters in commercial diets, jeopardizes the integrity of many types of research using fish more than a few weeks old. Physiology and histology of these fish will not be normal, in addition to problems with elevated liver lesions and neoplasia in various tissues. Perhaps some of the genetic polymorphism seen in certain colonies is more a reflection of husbandry protocols than actual baseline polymorphism. We suspect that there are multiple carcinogens and/or tumor promoters in recirculating aquaculture systems, which vary in predominance over time, because some cohorts of a given line of fish at a particular facility show just hepatic megalocytosis, without elevated age-specific tumor incidences, some lots show liver tumors only, and other groups show predominately gastrointestinal neoplasia, with or without hepatic megalocytosis. There are many possible sources of natural carcinogens in aquaculture systems including biofilters, microbial and algal biofilms in water distribution systems and fish tanks, Paramecium cultures, and leechates from system components. Nitrosamines and nitrosamides are the most plausible natural carcinogens, which might form in recirculating aquaculture systems. These carcinogens are known to form in natural waters and sediments in microenvironments in which organic matter is high and pH is low. Such naturally formed carcinogens in sediments in waterways can occur at concentrations that induce cancer in fish (Alexander and Tate 1975; Ayanaba and Alexander 1974; Mills and Alexander 1976; Yordy and Alexander 1981; Spitsbergen and Wolfe 1995). Clearly some recirculating aquaculture systems house zebrafish colonies that are free from hepatocyte megalocytosis and elevated age-specific tumor incidences. Recirculating aquaculture systems have been used for decades and we have studied a variety of species of fish housed in conventional recirculating systems in which the biofilter material consists of polyurethane foam, plastic cylinders or beads, gravel, crushed oyster shell, or activated carbon. So far we have observed hepatocyte megalocytosis and elevated tumor incidences only in certain systems with fluidized sand biofilters. However some systems with fluidized sand biofilters used for small aquarium fish are free of these problems (Dr. Gary Marty, personal communication).
Many commercial fish foods contain detectable levels of nitrosamines principally from formation of these agents during the manufacture of fish meals used in the diets. To minimize the risk of elevated spontaneous tumor incidences in their carcinogenesis bioassays, scientists at the Gulf Coast Research Laboratory, Ocean Springs, MS, arrange for pretesting of fish meal to ensure acceptable levels of nitrosamines. They have found that a tolerance level of 100 ppb for the most commonly occurring nitrosamine in fish meal, N-nitrosodimethylamine (DMN), ensures low background tumor incidences in medaka and guppy carcinogen bioassays (Dr. William Hawkins, personal communication; Hawkins et al. 2003). Aquatox Flake, a diet manufactured by Ziegler Brothers (Gardners, PA) and also supplied in small batches through Aquatic Ecosystems, Inc. (Apopka, FL) is available pretested for nitrosamine levels in fish meal.
Summary
Over the past 15 years our knowledge of zebrafish carcinogenesis and tumor biology has advanced greatly and the number of laboratories studying transgenic and mutant zebrafish models for cancer has grown rapidly. Yet much potential remains for applying this highly sophisticated and facile model to clarify mechanisms occurring at each stage in the carcinogenesis process and to better understand interactions of the complex array of oncogenes and tumor suppressor genes in oncogenesis. Models for several tumor types of humans are now available, yet many neoplasm types remain to be studied in detail using this model system. The immune system of zebrafish is fundamentally similar to that of humans, however the roles of innate and adaptive immunity in all stages of the tumorigenesis process have not yet been addressed in zebrafish. The zebrafish model can play a unique role in discovery of novel contrast agents for tumor imaging (Canaple et al, 2008; Ullmann et al, 2011; Spitsbergen et al, 2007; Zheng et al, 2011) as well as in development of innovative anticancer drugs and more effective delivery methods such as use of nanoparticles to deliver drug combinations in a tissue targeted fashion (Harfouche et al, 2009).
Supplementary Material
Acknowledgments
Research was funded by U.S. Public Health Service grants R01ES011587, R21ES013124, P30ESO3850, and P30ES00210 from the National Institutes of Environmental Health Sciences, by grant 3P40RR12546 and its supplement 03S1 from the National Center for Research Resources, and by U.S. Army contract DAMD 17-91Z1043. Additional assistance was provided by the Center for Fish Disease Research, the John Fryer Salmon Disease Laboratory, the Department of Microbiology and the Agricultural Research Foundation at Oregon State University. We thank Tom Miller, Renee Norred, Amber Taylor, Keri St. Clair, Sireesha Vemula, Faunya Campbell, Lauren Brown, Janelle Bishop-Stewart, Sheila Cleveland, Chance MacDonald, Don Stevens, Karen Larison, April Mazanac, Bill Trevarrow, and Dan Arbogast for technical support. We thank Cliff Pereira and George Weaver for assistance with statistical analyses.
References
- Abdo KM, Kari FW. The sensitivity of the NTP bioassay for carcinogen hazard evaluation can be modulated by dietary restriction. Exp Toxic Pathol. 1996:48. doi: 10.1016/S0940-2993(96)80033-9. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H. Lignans and human health. Crit Rev Clin Lab Sci. 2007;44:483–525. doi: 10.1080/10408360701612942. [DOI] [PubMed] [Google Scholar]
- Adlercreutz H, Heinonen SM, Penalvo-Garcia J. Phytoestrogens, cancer and coronary heart disease. Biofactors. 2004;22:229–236. doi: 10.1002/biof.5520220146. [DOI] [PubMed] [Google Scholar]
- Aiyer HS, Srinivasan C, Gupta RC. Dietary berries and ellagic acid diminish estrogen-mediated mammary tumorigenesis in ACI rats. Nutr Cancer. 2008;60:227–234. doi: 10.1080/01635580701624712. [DOI] [PubMed] [Google Scholar]
- Akhtar S, Meeran SM, Katiyar N, Katiyar SK. Grape seed proanthocyanidins inhibit the growth of human non-small cell lung cancer xenografts by targeting insulin-like growth factor binding protein-3, tumor cell proliferation, and angiogenic factors. Clin Cancer Res. 2009;15:821–831. doi: 10.1158/1078-0432.CCR-08-1901. [DOI] [PubMed] [Google Scholar]
- Albores-Saavedra J, Henson DE, Klimstra DS. Atlas of Tumor Pathology. Armed Forces Institute of Pathology; Washington: 2000. Tumors of the gallbladder, extrahepatic bile ducts, and ampulla of Vater. [Google Scholar]
- Alexander M, Tate RLI. Stability of nitrosamines in samples of lake water, soil, and sewage. Journal of The National Cancer Institute. 1975;54:327–330. [PubMed] [Google Scholar]
- Amatruda JF, Patton EE. Genetic models of cancer in zebrafish. Int Rev Cell Mol Biol. 2008;271:1–34. doi: 10.1016/S1937-6448(08)01201-X. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Sadler KC, Lai K, Farrington S, Bronson RT, Lees JA, Hopkins N. Many ribosomal protein genes are cancer genes in zebrafish. PLoS Biol. 2004;2:E139. doi: 10.1371/journal.pbio.0020139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayanaba A, Alexander M. Transformations of methylamines and formation of a hazardous product, dimethylnitrosamine, in samples of treated sewage and lake water. J. Environ. Qual. 1974;3:83–89. [Google Scholar]
- Bailey GS, Reddy A, Pereira C, Harttig U, Baird W, Spitsbergen JM, Hendricks JD, Orner G, Williams DE, Swenberg J. Non-linear cancer response at ultra-low dose: A 40,800-animal ED001 tumor and biomarker study. Chemical Research in Toxicology. 2009a doi: 10.1021/tx9000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GS, Reddy AP, Pereira CB, Harttig U, Baird W, Spitsbergen JM, Hendricks JD, Orner GA, Williams DE, Swenberg JA. Nonlinear cancer response at ultralow dose: a 40,800-animal ED(001) tumor and biomarker study. Chemical research in toxicology. 2009b Jul;22:1264–1276. doi: 10.1021/tx9000754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey GS, Williams DE, Hendricks JD. Fish models for environmental carcinogenesis: the rainbow trout. Environ Health Perspect. 1996;104(Suppl 1):5–21. doi: 10.1289/ehp.96104s15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckwith LG, Moore JL, Tsao-Wu GS, Harshbarger JC, Cheng KC. Ethylnitrosourea induces neoplasia in zebrafish (Danio rerio). Lab Invest. 2000;80:379–385. doi: 10.1038/labinvest.3780042. [DOI] [PubMed] [Google Scholar]
- Behin A, Hoang-Xuan K, Carpentier AF, Delattre JY. Primary brain tumours in adults. Lancet. 2003;361:323–331. doi: 10.1016/S0140-6736(03)12328-8. [DOI] [PubMed] [Google Scholar]
- Berghmans S, Murphey RD, Wienholds E, Neuberg D, Kutok JL, Fletcher CD, Morris JP, Liu TX, Schulte-Merker S, Kanki JP, Plasterk R, Zon LI, Look AT. tp53 mutant zebrafish develop malignant peripheral nerve sheath tumors. Proc Natl Acad Sci U S A. 2005;102:407–412. doi: 10.1073/pnas.0406252102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen ME, Henke K, Siegfried KR, Warman ML, Harris MP. Efficient mapping and cloning of mutations in zebrafish by low-coverage whole-genome sequencing. Genetics. 2012;190:1017–1024. doi: 10.1534/genetics.111.136069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno DW, NcVicar AH, Fraser CO. Multiple lipoma in the common dab, Limanda limanda L. Journal of applied ichthyology/Zeitschrift fur angewandte Ichthyologie , Berlin. 1991;7:238–243. [Google Scholar]
- Bunton TE. The immunocytochemistry of cytokeratin in fish tissues. Vet Pathol. 1993;30:418–425. doi: 10.1177/030098589303000503. [DOI] [PubMed] [Google Scholar]
- Bunton TE. Intermediate filament reactivity in hyperplastic and neoplastic lesions from medaka (Oryzias latipes). Exp Toxicol Pathol. 1994;46:389–396. doi: 10.1016/S0940-2993(11)80122-3. [DOI] [PubMed] [Google Scholar]
- Bunton TE. Expression of actin and desmin in experimentally induced hepatic lesions and neoplasms from medaka (Oryzias latipes). Carcinogenesis. 1995;16:1059–1063. doi: 10.1093/carcin/16.5.1059. [DOI] [PubMed] [Google Scholar]
- Bunton TE. Experimental chemical carcinogenesis in fish. Toxicol Pathol. 1996;24:603–618. doi: 10.1177/019262339602400511. [DOI] [PubMed] [Google Scholar]
- Campbell TC. Dietary protein, growth factors, and cancer. Am J Clin Nutr. 2007;85:1667. doi: 10.1093/ajcn/85.6.1667. [DOI] [PubMed] [Google Scholar]
- Canaple L, Beuf O, Armenean M, Hasserodt J, Samarut J, Janier M. Fast screening of paramagnetic molecules in zebrafish embryos by MRI. NMR Biomed. 2008;21:129–137. doi: 10.1002/nbm.1169. [DOI] [PubMed] [Google Scholar]
- Chen HC, Pan IJ, Tu WJ, Lin WH, Hong CC, Brittelli MR. Neoplastic response in Japanese medaka and channel catfish exposed to N-methyl-N′-nitro-N-nitrosoguanidine. Toxicol Pathol. 1996;24:696–706. doi: 10.1177/019262339602400604. [DOI] [PubMed] [Google Scholar]
- Corley-Smith GE, Su HT, Wang-Buhler JL, Tseng HP, Hu CH, Hoang T, Chung WG, Buhler DR. CYP3C1, the first member of a new cytochrome P450 subfamily found in zebrafish (Danio rerio). Biochem Biophys Res Commun. 2006;340:1039–1046. doi: 10.1016/j.bbrc.2005.12.110. [DOI] [PubMed] [Google Scholar]
- Cross HS, Kallay E, Lechner D, Gerdenitsch W, Adlercreutz H, Armbrecht HJ. Phytoestrogens and vitamin D metabolism: a new concept for the prevention and therapy of colorectal, prostate, and mammary carcinomas. J Nutr. 2004;134:1207S–1212S. doi: 10.1093/jn/134.5.1207S. [DOI] [PubMed] [Google Scholar]
- Dahme T, Katus HA, Rottbauer W. Fishing for the genetic basis of cardiovascular disease. Dis Model Mech. 2009;2:18–22. doi: 10.1242/dmm.000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dei Tos AP. Liposarcoma: new entities and evolving concepts. Ann Diagn Pathol. 2000;4:252–266. doi: 10.1053/adpa.2000.8133. [DOI] [PubMed] [Google Scholar]
- Faro A, Boj SF, Clevers H. Fishing for intestinal cancer models: unraveling gastrointestinal homeostasis and tumorigenesis in zebrafish. Zebrafish. 2009;6:361–376. doi: 10.1089/zeb.2009.0617. [DOI] [PubMed] [Google Scholar]
- Feitsma H, Kuiper RV, Korving J, Nijman IJ, Cuppen E. Zebrafish with mutations in mismatch repair genes develop neurofibromas and other tumors. Cancer Res. 2008;68:5059–5066. doi: 10.1158/0008-5472.CAN-08-0019. [DOI] [PubMed] [Google Scholar]
- Fleiss JL, Levin B, Paik MC. Statistical Methods for Rates & Proportions. 3rd ed. Wiley-Interscience; Hoboken, NJ: 2003. [Google Scholar]
- Foley JE, Yeh JR, Maeder ML, Reyon D, Sander JD, Peterson RT, Joung JK. Rapid mutation of endogenous zebrafish genes using zinc finger nucleases made by Oligomerized Pool ENgineering (OPEN). PLoS One. 2009;4:e4348. doi: 10.1371/journal.pone.0004348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L, Klein S, Holloszy JO. Long-term low-protein, low-calorie diet and endurance exercise modulate metabolic factors associated with cancer risk. Am J Clin Nutr. 2006;84:1456–1462. doi: 10.1093/ajcn/84.6.1456. [DOI] [PubMed] [Google Scholar]
- Garcia DM, Bauer H, Dietz T, Schubert T, Markl J, Schaffeld M. Identification of keratins and analysis of their expression in carp and goldfish: comparison with the zebrafish and trout keratin catalog. Cell Tissue Res. 2005;322:245–256. doi: 10.1007/s00441-005-0031-1. [DOI] [PubMed] [Google Scholar]
- Geelhoed GW. “Aging bull’. Med Hypotheses. 1996;47:471–479. doi: 10.1016/s0306-9877(96)90160-7. [DOI] [PubMed] [Google Scholar]
- Goldschmidt MH, Dunstan RW, Stannard AA, von Tscharner C, Walder EJ, Yager JA. International Histological Classification of Tumors of Domestic Animals. Armed Forces Institute of Pathology and World Health Organization; Washington, D.C.: 1998. Histological classification of epithelial and melanocytic tumors of the skin of domestic animals. [Google Scholar]
- Green CC, Kelly AM. Effects of the estrogen mimic genistein as a dietary component on sex differentiation and ethoxyresorufin-O-deethylase (EROD) activity in channel catfish (Ictalurus punctatus). Fish Physiol Biochem. 2008 doi: 10.1007/s10695-008-9260-z. [DOI] [PubMed] [Google Scholar]
- Groff JM, Naydan DK, Higgins RJ, Zinkl JG. Cytokeratin-filament expression in epithelial and non-epithelial tissues of the common carp (Cyprinus carpio). Cell Tissue Res. 1997;287:375–384. doi: 10.1007/s004410050763. [DOI] [PubMed] [Google Scholar]
- Harfouche R, Basu S, Soni S, Hentschel DM, Mashelkar RA, Sengupta S. Nanoparticle-mediated targeting of phosphatidylinositol-3-kinase signaling inhibits angiogenesis. Angiogenesis. 2009;12:325–338. doi: 10.1007/s10456-009-9154-4. [DOI] [PubMed] [Google Scholar]
- Haschek WM, Rousseaux CG. Fundamentals of Toxicologic Pathology. Academic Press; San Diego: 1998. [Google Scholar]
- Hawkins WE, Fournie JW, Ishikawa T, Walker WW. Germ cell neoplasms in Japanese medaka. Journal of Aquatic Animal Health. 1996;8:120–129. [Google Scholar]
- Hawkins WE, Overstreet RM, Fournie JW, Walker WW. Development of aquarium fish models for environmental carcinogenesis: tumor induction in seven species. J Appl Toxicol. 1985;5:261–264. doi: 10.1002/jat.2550050408. [DOI] [PubMed] [Google Scholar]
- Hawkins WE, Walker WW, Fournie JW, Manning CS, Krol RM. Use of the Japanese medaka (Oryzias latipes) and guppy (Poecilia reticulata) in carcinogenesis testing under national toxicology program protocols. Toxicol Pathol. 2003;31(Suppl):88–91. doi: 10.1080/01926230390174968. [DOI] [PubMed] [Google Scholar]
- Hendricks JD. Development of the Zebra Danio Model: Carcinogenesis and Gene Transfer Studies. Re. NTIS/AD-A328 886/7; DAMD17-91-Z-1043. U.S. Army; Springfield VA: 1996. p. 337. [Google Scholar]
- Higginbotham S, RamaKrishna NV, Johansson SL, Rogan EG, Cavalieri EL. Tumor-initiating activity and carcinogenicity of dibenzo[a,l]pyrene versus 7,12-dimethylbenz[a]anthracene and benzo[a]pyrene at low doses in mouse skin. Carcinogenesis. 1993;14:875–878. doi: 10.1093/carcin/14.5.875. [DOI] [PubMed] [Google Scholar]
- Hursting SD, Kari FW. The anti-carcinogenic effects of dietary restriction: mechanisms and future directions. Mutat Res. 1999;443:235–249. doi: 10.1016/s1383-5742(99)00021-6. [DOI] [PubMed] [Google Scholar]
- Ingham PW. The power of the zebrafish for disease analysis. Hum Mol Genet. 2009;18:R107–112. doi: 10.1093/hmg/ddp091. [DOI] [PubMed] [Google Scholar]
- Iovino CS, Camacho LH. Acute myeloid leukemia: a classification and treatment update. Clin J Oncol Nurs. 2003;7:535–540. doi: 10.1188/03.CJON.535-540. [DOI] [PubMed] [Google Scholar]
- Ju B, Spitsbergen J, Eden CJ, Taylor MR, Chen W. Co-activation of hedgehog and AKT pathways promote tumorigenesis in zebrafish. Mol Cancer. 2009;8:40. doi: 10.1186/1476-4598-8-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kari FW, Dunn SE, French JE, Barrett JC. Roles for insulin-like growth factor-1 in mediating the anti-carcinogenic effects of caloric restriction. J Nutr Health Aging. 1999;3:92–101. [PubMed] [Google Scholar]
- Kazianis S, Walter RB. Use of platyfishes and swordtails in biological research. Lab Anim. 2002;31:46–52. doi: 10.1038/5000142. [DOI] [PubMed] [Google Scholar]
- Kent ML, Bishop-Stewart JK, Matthews JL, Spitsbergen JM. Pseudocapillaria tomentosa, a nematode pathogen, and associated neoplasms of zebrafish (Danio rerio) kept in research colonies. Comp Med. 2002;52:354–358. [PubMed] [Google Scholar]
- Kent ML, Spitsbergen JM, Matthews JM, Fournie JW, Westerfield M. Diseases of zebrafish in research facilities. Zebrafish International Resource Center; 2007. Available online ( http://zebrafish.org/zirc/health/diseaseManual.php), accessed June 2012. [Google Scholar]
- Kent ML, Whipps CM, Matthews JL, Florio D, Watral V, Bishop-Stewart JK, Poort M, Bermudez L. Mycobacteriosis in zebrafish (Danio rerio) research facilities. Comp Biochem Physiol C Toxicol Pharmacol. 2004;138:383–390. doi: 10.1016/j.cca.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Khudoley VV. Use of aquarium fish, Danio rerio and Poecilia reticulata, as test species for evaluation of nitrosamine carcinogenicity. Natl Cancer Inst Monogr. 1984;65:65–70. [PubMed] [Google Scholar]
- Kizil C, Kaslin J, Kroehne V, Brand M. Adult neurogenesis and brain regeneration in zebrafish. Dev Neurobiol. 2012;72:429–461. doi: 10.1002/dneu.20918. [DOI] [PubMed] [Google Scholar]
- Koestner A, Bilzer T, Fatzer R, Schulman FY, Summers BA, Van Winkle TJ. International Histological Classification of Tumors of Domestic Animals. World Health Organization/Armed Forces Institute of Pathology/American Registry of Pathology; Washington, D.C.: 1999. Histological Classification of Tumors of the Nervous System of Domestic Animals. [Google Scholar]
- Koh VCH, Nguyen AT, Lam SH, Spitsbergen J, Emelyanov A, Parinov S, Gong Z. Molecular genetics of liver neoplasia: The zebrafish model of liver cancer. In: Wang XW, Grisham JW, Thorgeirsson SS, editors. Molecular Genetics of Liver Neoplasia. Springer; New York, NY: 2010. [Google Scholar]
- Kozul CD, Nomikos AP, Hampton TH, Warnke LA, Gosse JA, Davey JC, Thorpe JE, Jackson BP, Ihnat MA, Hamilton JW. Laboratory diet profoundly alters gene expression and confounds genomic analysis in mouse liver and lung. Chem Biol Interact. 2008;173:129–140. doi: 10.1016/j.cbi.2008.02.008. [DOI] [PubMed] [Google Scholar]
- Kroehne V, Freudenreich D, Hans S, Kaslin J, Brand M. Regeneration of the adult zebrafish brain from neurogenic radial glia-type progenitors. Development. 2011;138:4831–4841. doi: 10.1242/dev.072587. [DOI] [PubMed] [Google Scholar]
- Lally BE, Geiger GA, Kridel S, Arcury-Quandt AE, Robbins ME, Kock ND, Wheeler K, Peddi P, Georgakilas A, Kao GD, Koumenis C. Identification and biological evaluation of a novel and potent small molecule radiation sensitizer via an unbiased screen of a chemical library. Cancer Res. 2007;67:8791–8799. doi: 10.1158/0008-5472.CAN-07-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law JM. Mechanistic considerations in small fish carcinogenicity testing. ILAR J. 2001;42:274–284. doi: 10.1093/ilar.42.4.274. [DOI] [PubMed] [Google Scholar]
- Lee BC, Hendricks JD, Bailey GS. Toxicity of mycotoxins in the feed of fish. In: Smith JE, editor. Mycotoxins and Animal Feedstuff: Natural Occurrence, Toxicity and Control. CRC Press; Boca Raton, FL: 1991. pp. 607–626. [Google Scholar]
- Leshchiner I, Alexa K, Kelsey P, Adzhubei I, Austin C, Cooney J, Anderson H, King M, Stottmann RW, Ha S, Drummond I, Paw BH, North T, Beier D, Goessling W, Sunyaev S. Mutation mapping and identification by whole genome sequencing. Genome Res. 2012 Jun 14; doi: 10.1101/gr.135541.111. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Bai X, Wang S, Tomiyama-Miyaji C, Nagura T, Kawamura T, Abo T. Immunopotentiation of NKT cells by low-protein diet and the suppressive effect on tumor metastasis. Cell Immunol. 2004;231:96–102. doi: 10.1016/j.cellimm.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Mandrekar N, Thakur NL. Significance of the zebrafish model in the discovery of bioactive molecules from nature. Biotechnol Lett. 2009;31:171–179. doi: 10.1007/s10529-008-9868-1. [DOI] [PubMed] [Google Scholar]
- Marino F, Germana A, Bambir S, Helgason S, De Vico G, Macri B. Calretinin and S-100 expression in goldfish, Carassius auratus (L.), schwannoma. J Fish Dis. 2007;30:251–253. doi: 10.1111/j.1365-2761.2007.00776.x. [DOI] [PubMed] [Google Scholar]
- Marques IJ, Weiss FU, Vlecken DH, Nitsche C, Bakkers J, Lagendijk A, Partecke LI, Heidecke CD, Lerch MM, Bagowski CP. Metastatic behaviour of primary human tumours in a zebrafish xenotransplantation model. BMC Cancer. 2009;9:128. doi: 10.1186/1471-2407-9-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masahito P, Aoki K, Egami N, Ishikawa T, Sugano H. Life-span studies on spontaneous tumor development in the medaka (Oryzias latipes). Jpn J Cancer Res. 1989;80:1058–1065. doi: 10.1111/j.1349-7006.1989.tb02259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews DE, Farewell VT. Karger, AG; Basel, Switzerland: 1996. Using and Understanding Medical Statistics. [Google Scholar]
- McAllister PE, Lidgerding BC, Herman RL, Hoyer LC, Hankins J. Viral diseases of fish: first report of carp pox in golden ide (Leuciscus idus) in North America. J Wildl Dis. 1985;21:199–204. doi: 10.7589/0090-3558-21.3.199. [DOI] [PubMed] [Google Scholar]
- Mills AL, Alexander M. Factors affecting dimethylnitrosamine formation in samplers of soils and water. J. Environ. Qual. 1976;5:437–440. [Google Scholar]
- Mione MC, Trede NS. The zebrafish as a model for cancer. Dis Model Mech. 2010;3:517–523. doi: 10.1242/dmm.004747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray KN. Prevalence and presentation of pathologies diagnosed in laboratory zebrafish (Danio rerio) submitted to the Zebrafish International Resource Center Diagnostic Service. ILAR Journal. 2012 [Google Scholar]
- Pan MH, Lai CS, Wu JC, Ho CT. Molecular mechanisms for chemoprevention of colorectal cancer by natural dietary compounds. Mol Nutr Food Res. 2011;55:32–45. doi: 10.1002/mnfr.201000412. [DOI] [PubMed] [Google Scholar]
- Parant JM, George SA, Holden JA, Yost HJ. Genetic modeling of Li-Fraumeni syndrome in zebrafish. Dis Model Mech. 2010;3:45–56. doi: 10.1242/dmm.003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton EE, Widlund HR, Kutok JL, Kopani KR, Amatruda JF, Murphey RD, Berghmans S, Mayhall EA, Traver D, Fletcher CD, Aster JC, Granter SR, Look AT, Lee C, Fisher DE, Zon LI. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr Biol. 2005;15:249–254. doi: 10.1016/j.cub.2005.01.031. [DOI] [PubMed] [Google Scholar]
- Pliss GB, Khudoley VV. Tumor induction by carcinogenic agents in aquarium fish. J Natl Cancer Inst. 1975;55:129–136. doi: 10.1093/jnci/55.1.129. [DOI] [PubMed] [Google Scholar]
- Pliss GB, Zabezhinski MA, Petrov AS, Khudoley VV. Peculiarities of N-nitramines carcinogenic action. Arch Geschwulstforsch. 1982;52:629–634. [PubMed] [Google Scholar]
- Prentice RL, Shaw PA, Bingham SA, Beresford SA, Caan B, Neuhouser ML, Patterson RE, Stefanick ML, Satterfield S, Thomson CA, Snetselaar L, Thomas A, Tinker LF. Biomarker-calibrated energy and protein consumption and increased cancer risk among postmenopausal women. Am J Epidemiol. 2009;169:977–989. doi: 10.1093/aje/kwp008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Vara JA. Technical aspects of immunohistochemistry. Vet Pathol. 2005;42:405–426. doi: 10.1354/vp.42-4-405. [DOI] [PubMed] [Google Scholar]
- Reddy AP, Harttig U, Barth MC, Baird WM, Schimerlik M, Hendricks JD, Bailey GS. Inhibition of dibenzo[a,l]pyrene-induced multi-organ carcinogenesis by dietary chlorophyllin in rainbow trout. Carcinogenesis. 1999a;20:1919–1926. doi: 10.1093/carcin/20.10.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddy AP, Spitsbergen JM, Mathews C, Hendricks JD, Bailey GS. Experimental hepatic tumorigenicity by environmental hydrocarbon dibenzo[a,l]pyrene. J Environ Pathol Toxicol Oncol. 1999b;18:261–269. [PubMed] [Google Scholar]
- Riddell RH, Petras RE, Williams GT, Sobin LH. Tumors of the Intestines. AFIP; Washington DC: 2003. [Google Scholar]
- Santoriello C, Gennaro E, Anelli V, Distel M, Kelly A, Koster RW, Hurlstone A, Mione M. Kita driven expression of oncogenic HRAS leads to early onset and highly penetrant melanoma in zebrafish. PLoS One. 2010;5:e15170. doi: 10.1371/journal.pone.0015170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sirri R, Mandrioli L, Grieco V, Bacci B, Brunetti B, Sarli G, Schmidt-Posthaus H. Seminoma in a koi carp Cyprinus carpio: histopathological and immunohistochemical findings. Dis Aquat Organ. 2010;92:83–88. doi: 10.3354/dao02273. [DOI] [PubMed] [Google Scholar]
- Smolowitz R, Hanley J, Richmond H. A three-year retrospective study of abdominal tumors in zebrafish maintained in an aquatic laboratory animal facility. Biol Bull. 2002;203:265–266. doi: 10.2307/1543433. [DOI] [PubMed] [Google Scholar]
- Spitsbergen J. Imaging neoplasia in zebrafish. Nat Methods. 2007;4:548–549. doi: 10.1038/nmeth0707-548. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Blazer VS, Bowser PR, Cheng KC, Cooper KR, Cooper TK, Frasca S, Groman DB, Harper CM, Law JMM, Marty GD, Smolowitz RM, St Leger J, Wolf DC, Wolf JC. Finfish and aquatic invertebrate pathology resources for now and the future. Comparative biochemistry and physiology. Toxicology & pharmacology : CBP. 2009 Mar;149:249–257. doi: 10.1016/j.cbpc.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Buhler DR, Miller T. 6th International Conference on Zebrafish Development and Genetics. University of Wisconsin; Madison, WI: 2004. another long fin and uma mutant lines are highly sensitive to polycyclic aromatic hydrocarbon-induced liver neoplasia. [Google Scholar]
- Spitsbergen JM, Kent ML. The state of the art of the zebrafish model for toxicology and toxicologic pathology research--advantages and current limitations. Toxicol Pathol. 2003;31(Suppl):62–87. doi: 10.1080/01926230390174959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spitsbergen JM, Norred ER, Zhan H, Gong Z, Lo LC, Johnson NJ, Banks HK. The Role of Inflammation in Intestinal and Blood Neoplasia in Zebrafish. 8th International Conference on Zebrafish Development and Genetics. University of Wisconsin; Madison, WI: 2008. [Google Scholar]
- Spitsbergen JM, Tsai H, Reddy A, Hendricks J. Response of zebrafish to a panel of structurally diverse carcinogens. Proc. Am. Assoc. Cancer Res. 1997;38:354. [Google Scholar]
- Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS. Neoplasia in zebrafish (Danio rerio) treated with 7,12-dimethylbenz[a]anthracene by two exposure routes at different developmental stages. Toxicol Pathol. 2000a;28:705–715. doi: 10.1177/019262330002800511. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Tsai HW, Reddy A, Miller T, Arbogast D, Hendricks JD, Bailey GS. Neoplasia in zebrafish (Danio rerio) treated with N-methyl-N′-nitro-N-nitrosoguanidine by three exposure routes at different developmental stages. Toxicol Pathol. 2000b;28:716–725. doi: 10.1177/019262330002800512. [DOI] [PubMed] [Google Scholar]
- Spitsbergen JM, Wolfe MJ. The riddle of hepatic neoplasia in brown bullheads from relatively unpolluted waters in New York State. Toxicol Pathol. 1995;23:716–725. doi: 10.1177/019262339502300610. [DOI] [PubMed] [Google Scholar]
- Stanton M. Hepatic neoplasms of aquarium fish exposed to Cycas cercinalis. Fed. Proc. 1966;26:661. [Google Scholar]
- Stanton MF. Diethylnitrosamine-induced hepatic degeneration and neoplasia in the aquarium fish , Brachydanio rerio. JNCI. 1965;34:117–130. doi: 10.1093/jnci/34.1.117. [DOI] [PubMed] [Google Scholar]
- Tannenbaum A. The initiation and growth of tumors. Introduction. 1. Effects of underfeeding. Am J Cancer. 1940;38:335. [Google Scholar]
- Taylor A. Senior B.S. Thesis. Department of Environmental and Molecular Toxicology. Oregon State University; Corvallis, OR: 2005. Immunohistochemical localization of cytochrome P450s 1A, 2K6, 2K7, 3A65 and 3C1 and expression of P4501A in tumor sensitive and resistant lines of juvenile zebrafish. p. 41. [Google Scholar]
- Taylor KL, Grant NJ, Temperley ND, Patton EE. Small molecule screening in zebrafish: an in vivo approach to identifying new chemical tools and drug leads. Cell Commun Signal. 2010;8:11. doi: 10.1186/1478-811X-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai H. Ph.D. Thesis. Dept. Food Science and Technology. Oregon State University; Corvallis, OR: 1996. Evaluation of zebrafish (Brachydanio rerio) as a model for carcinogenesis. p. 146. [Google Scholar]
- Ullmann JF, Cowin G, Kurniawan ND, Collin SP. Magnetic resonance histology of the adult zebrafish brain: optimization of fixation and gadolinium contrast enhancement. NMR Biomed. 2010;23:341–346. doi: 10.1002/nbm.1465. [DOI] [PubMed] [Google Scholar]
- Walter RB, Kazianis S. Xiphophorus interspecies hybrids as genetic models of induced neoplasia. ILAR Journal. 2001;42:299–321. doi: 10.1093/ilar.42.4.299. [DOI] [PubMed] [Google Scholar]
- Wang-Buhler J, Chung W, Tseng H, Miranda C, Hu C, Hseu T, Taylor A, Spitsbergen JM, Buhler DR. The Toxicologist. Society of Toxicology Conference Proceedings; New Orleans, LA: 2005a. Development of specific antipeptide antibodies against zebrafish xenobiotic metabolising forms of cytochrome P450. [Google Scholar]
- Wang-Buhler JL, Lee SJ, Chung WG, Stevens JF, Tseng HP, Hseu TH, Hu CH, Westerfield M, Yang YH, Miranda CL, Buhler DR. CYP2K6 from zebrafish (Danio rerio): cloning, mapping, developmental/tissue expression, and aflatoxin B1 activation by baculovirus expressed enzyme. Comp Biochem Physiol C Toxicol Pharmacol. 2005b;140:207–219. doi: 10.1016/j.cca.2005.02.002. [DOI] [PubMed] [Google Scholar]
- Ward JM, Devor-Henneman DE. Mouse models of human familial cancer syndromes. Toxicol Pathol. 2004;32(Suppl 1):90–98. doi: 10.1080/01926230490424680. [DOI] [PubMed] [Google Scholar]
- Watts SA, Powell M, D'Abramo LR. Fundamental approaches to the study of zebrafish nutrition. 2012. [DOI] [PMC free article] [PubMed]; ILAR J, Wei N, Wang B, Zhang QY, Mi MT, Zhu JD, Yu XP, Yuan JL, Chen K, Wang J, Chang H. Effects of different dietary fatty acids on the fatty acid compositions and the expression of lipid metabolic-related genes in mammary tumor tissues of rats. Nutr Cancer. 2008;60:810–825. doi: 10.1080/01635580802192858. [DOI] [PubMed] [Google Scholar]
- Wertheim JA, Miller JP, Xu L, He Y, Pear WS. The biology of chronic myelogenous leukemia:mouse models and cell adhesion. Oncogene. 2002;21:8612–8628. doi: 10.1038/sj.onc.1206089. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio) 5th ed. University of Oregon Press; Eugene, Oregon: 2007. [Google Scholar]
- Whipps CM, Dougan ST, Kent ML. Mycobacterium haemophilum infections of zebrafish (Danio rerio) in research facilities. FEMS Microbiol Lett. 2007;270:21–26. doi: 10.1111/j.1574-6968.2007.00671.x. [DOI] [PubMed] [Google Scholar]
- Whipps CM, Matthews JL, Kent ML. Distribution and genetic characterization of Mycobacterium chelonae in laboratory zebrafish Danio rerio. Dis Aquat Organ. 2008;82:45–54. doi: 10.3354/dao01967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteley LO, Anver MR, Botts S, Jokinen MP. Guides for Toxicologic Pathology. STP/ARP/AFIP; Washington, D.C.: 1996. Proliferative lesions of the intestine, salivary glands, oral cavity and esophagus in rats. p. 18. [Google Scholar]
- Williams DE, Bailey GS, Reddy A, Hendricks JD, Oganesian A, Orner GA, Pereira CB, Swenberg JA. The rainbow trout (Oncorhynchus mykiss) tumor model: recent applications in low-dose exposures to tumor initiators and promoters. Toxicol Pathol. 2003;31(Suppl):58–61. [PubMed] [Google Scholar]
- Winn RN, Norris MB, Brayer KJ, Torres C, Muller SL. Detection of mutations in transgenic fish carrying a bacteriophage lambda cII transgene target. Proc Natl Acad Sci U S A. 2000;97:12655–12660. doi: 10.1073/pnas.220428097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh JR, Munson KM, Chao YL, Peterson QP, Macrae CA, Peterson RT. AML1-ETO reprograms hematopoietic cell fate by downregulating scl expression. Development. 2008;135:401–410. doi: 10.1242/dev.008904. [DOI] [PubMed] [Google Scholar]
- Yordy JR, Alexander M. Formation of N-Nitrosodiethanolamine From diethanolamine in lake water and sewage. Journal of Environmental Quality. 1981;10:266–270. [Google Scholar]
- Yoshimizu M, Fukuda H, Sano T, Kimura T. Salmonid herpesvirus 2. Epizootiology and serological relationship. Vet Res. 1995;26:486–492. [PubMed] [Google Scholar]
- Zhan H, Spitsbergen JM, Qing W, Wu YL, Paul TA, Casey JW, Her GM, Gong Z. Transgenic expression of walleye dermal sarcoma virus rv-cyclin gene in zebrafish and its suppressive effect on liver tumor development after carcinogen treatment. Mar Biotechnol (NY) 2010;12:640–649. doi: 10.1007/s10126-009-9251-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng PP, Romme E, van der Spek PJ, Dirven CM, Willemsen R, Kros JM. HeNe laser (633 nm)-coupled confocal microscope allows simulating magnetic resonance imaging/computed tomography scan of the brain and eye: a noninvasive optical approach applicable to small laboratory animals. Zebrafish. 2011;8:83–85. doi: 10.1089/zeb.2011.0698. [DOI] [PubMed] [Google Scholar]
- Zhu H, Zon LI. Use of zebrafish models for the analysis of human disease. Curr Protoc Hum Genet. 2002 doi: 10.1002/0471142905.hg1503s34. Chapter 15:Unit 15 13. [DOI] [PubMed] [Google Scholar]
- Ziegler RG. Phytoestrogens and breast cancer. Am J Clin Nutr. 2004;79:183–184. doi: 10.1093/ajcn/79.2.183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.