Abstract
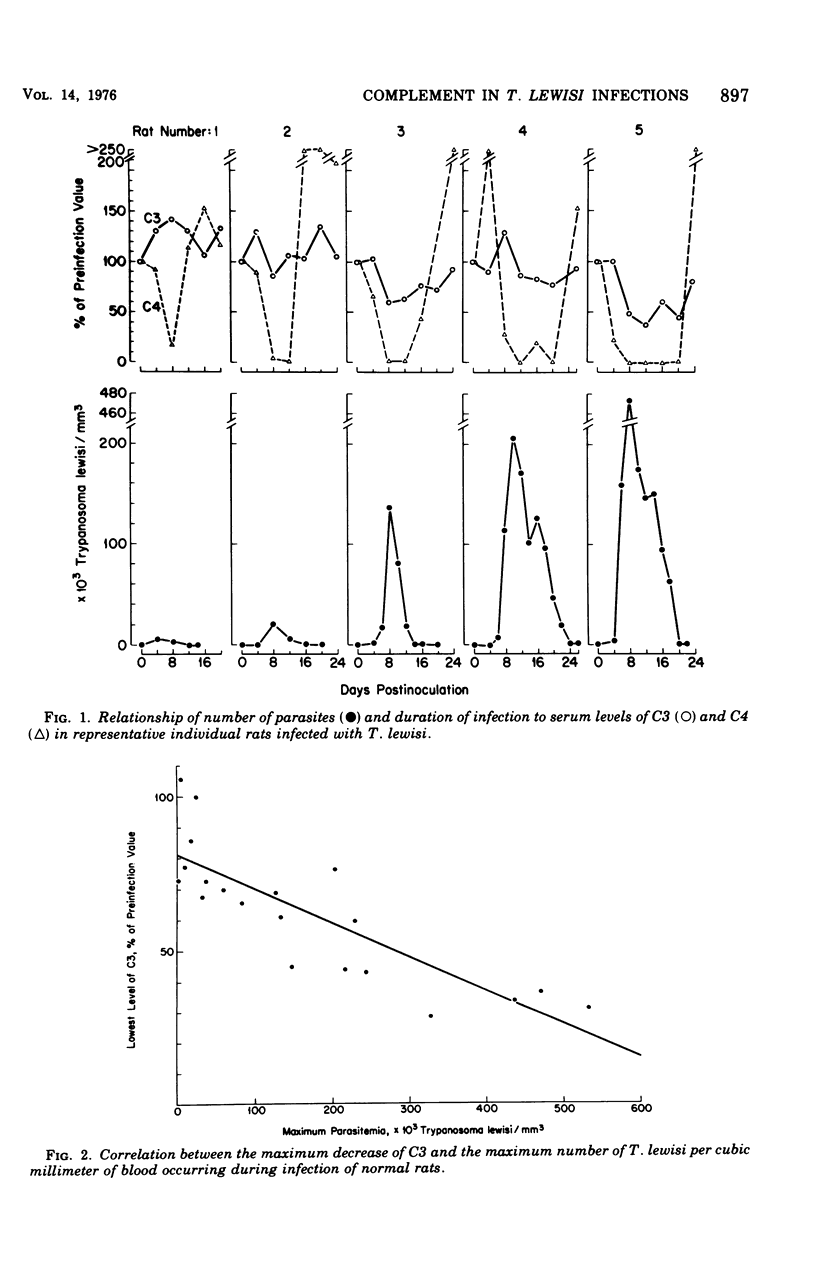
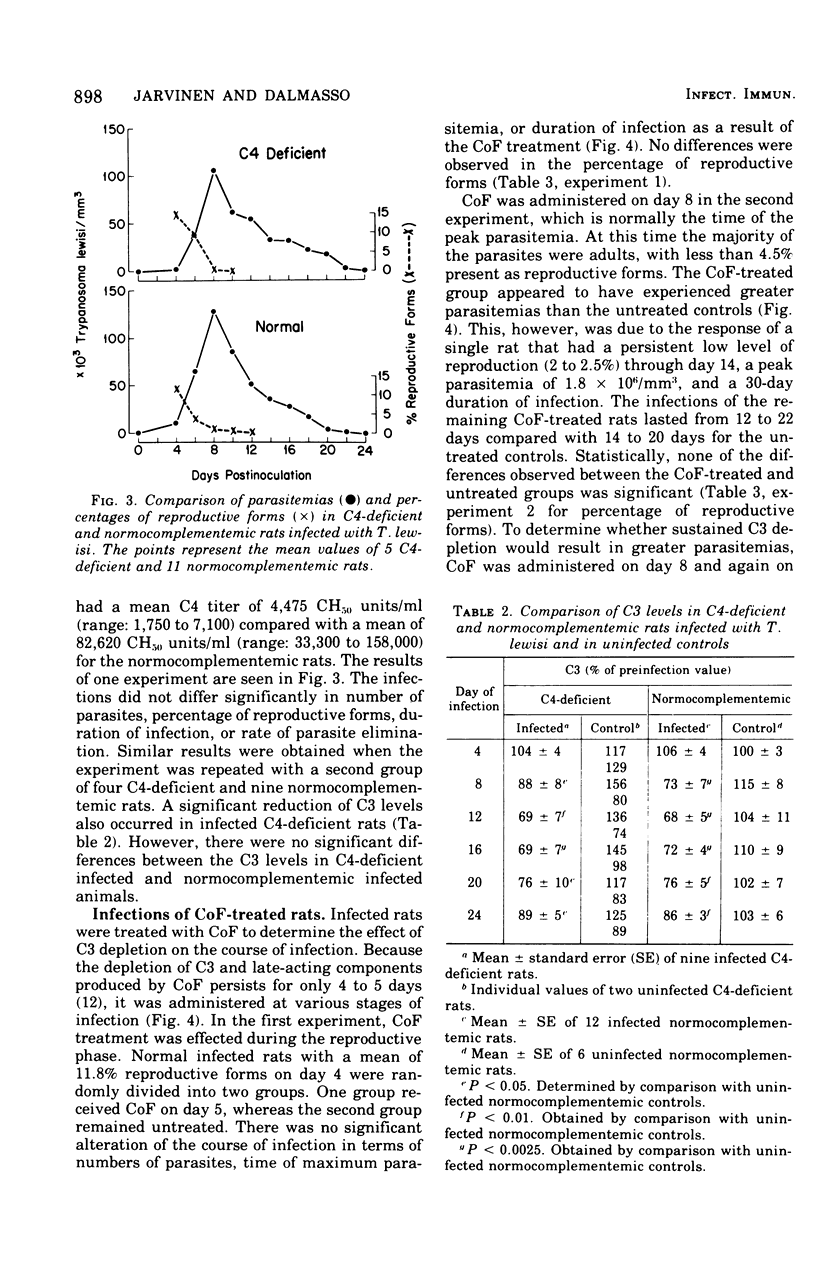
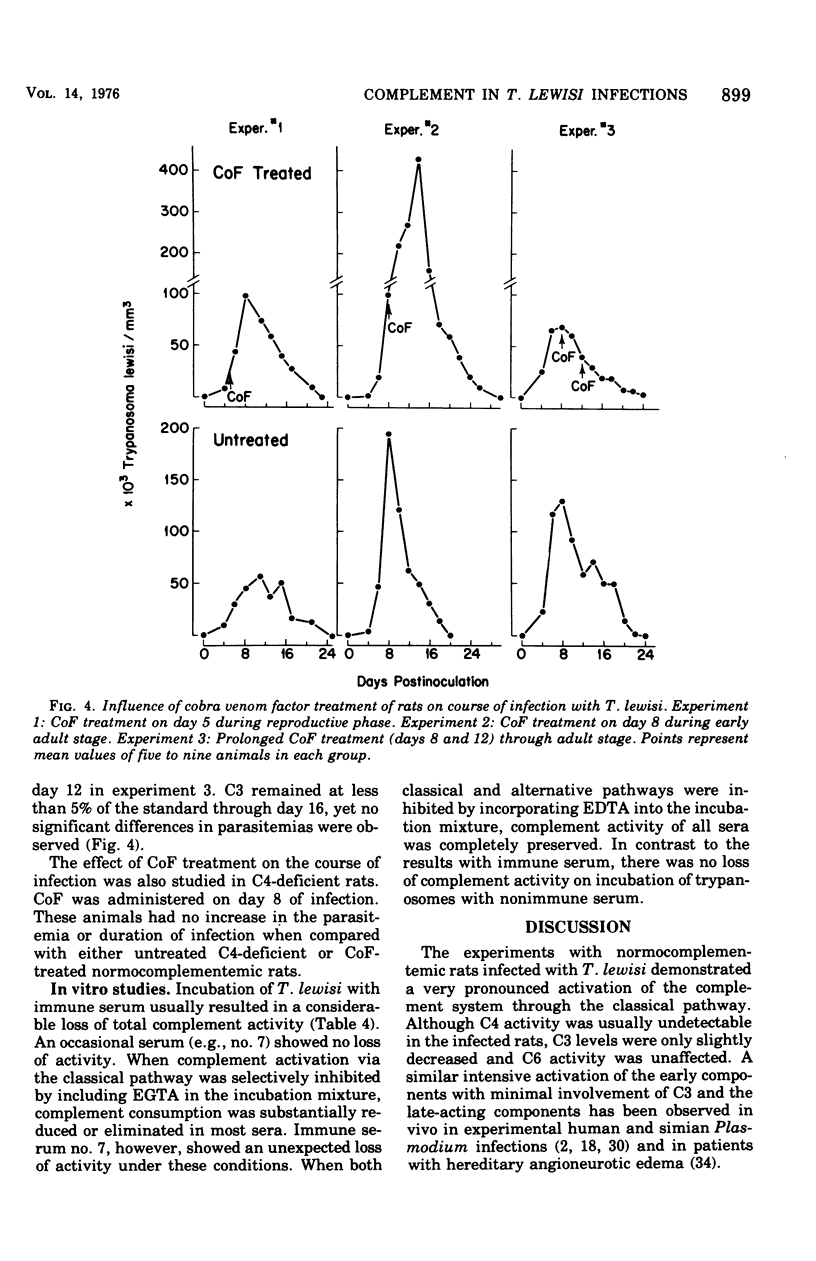
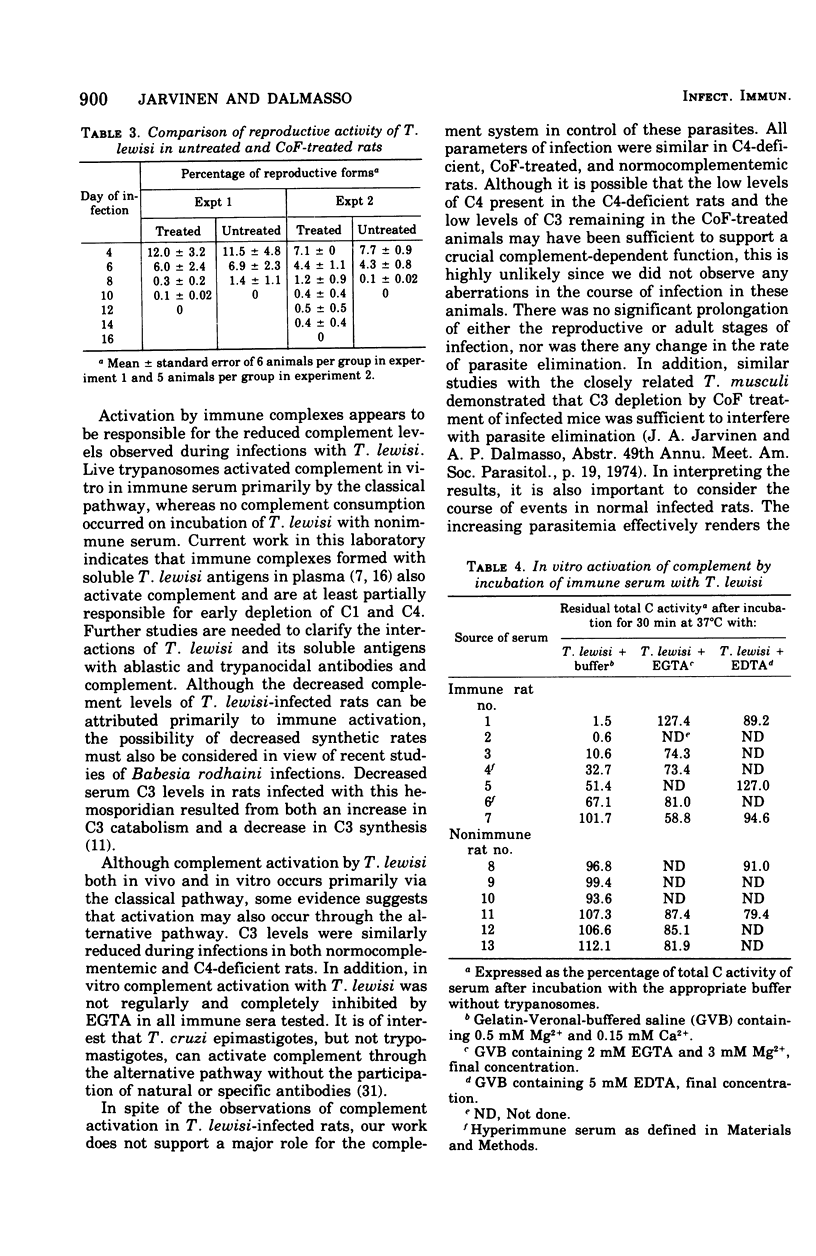
The role of complement in host resistance to infection with Trypanosoma lewisi was studied in normal, C4-deficient, and C3-depleted rats. Complement levels were measured in normal rats throughout the course of infection. A drastic reduction of total complement and C4 hemolytic activities occurred, and C3 levels measured immunochemically were decreased. Although total complement and C4 levels were regularly reduced to less than 10% of preinfection levels regardless of parasite numbers, the degree of C3 consumption correlated with the parasitemia. C3 levels varied from 100% of preinfection in rats with light infections to 35% in animals with heavy parasitemias. Recovery to normal levels followed trypanosome elimination from the peripheral blood. The infection had no significant effect on C6 hemolytic activity. Parasitemias and C3 levels in C4-deficient rats did not differ from those of normocomplementemic controls. Depletion of C3 and late-acting components by cobra venom factor during the reproductive or adult stages of infection did not alter the parasitemias. In addition, T. lewisi and immune serum caused complement activation in vitro, which could be inhibited with ethylene glycol-bis-(beta-aminoethyl ether)N,N'-tetraacetic acid or ethylenediaminetetraacetic acid. It is concluded that T. lewisi infection in rats result in activation of the classical complement pathway with extensive consumption of the early-acting components, as well as a low degree of activation of the alternative pathway. However, complement does not appear to play a major role in the control and termination of the infection.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anziano D. F., Dalmasso A. P., Lelchuk R., Vásquez C. Role of complement in immune lysis of Trypanosoma cruzi. Infect Immun. 1972 Nov;6(5):860–864. doi: 10.1128/iai.6.5.860-864.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson J. P., Glew R. H., Neva F. A., Frank M. M. Serum complement and immunity in experimental simian malaria. II. Preferential activation of early components and failure of depletion of late components to inhibit protective immunity. J Infect Dis. 1975 Jan;131(1):26–33. doi: 10.1093/infdis/131.1.26. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Ballow M., Cochrane C. G. Two anticomplementary factors in cobra venom: hemolysis of guinea pig erythrocytes by one of them. J Immunol. 1969 Nov;103(5):944–952. [PubMed] [Google Scholar]
- Barret-Connor E., Ugoretz R. J., Braude A. I. Disseminated intravascular coagulation in trypanosomiasis. Arch Intern Med. 1973 Apr;131(4):574–577. doi: 10.1001/archinte.131.4.574. [DOI] [PubMed] [Google Scholar]
- Borsos T., Rapp H. J. Immune hemolysis: a simplified method for the preparation of EAC'4 with guinea pig or with human complement. J Immunol. 1967 Aug;99(2):263–268. [PubMed] [Google Scholar]
- Budzko D. B., Pizzimenti M. C., Kierszenbaum F. Effects of complement depletion in experimental chagas disease: immune lysis of virulent blood forms of Trypanosoma cruzi. Infect Immun. 1975 Jan;11(1):86–91. doi: 10.1128/iai.11.1.86-91.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell J. R., Ruddy S., Austen K. F. Assay of complement components C1, C4, C2, C3 and C9 in whole rat serum. Int Arch Allergy Appl Immunol. 1972;43(6):887–897. doi: 10.1159/000230906. [DOI] [PubMed] [Google Scholar]
- Chapman W. E., Ward P. A. Changes in C3 metabolism during protozoan infection (Babesia rodhaini) in rats. J Immunol. 1976 May;116(5):1284–1288. [PubMed] [Google Scholar]
- Cochrane C. G., Müller-Eberhard H. J., Aikin B. S. Depletion of plasma complement in vivo by a protein of cobra venom: its effect on various immunologic reactions. J Immunol. 1970 Jul;105(1):55–69. [PubMed] [Google Scholar]
- D'ALESANDRO P. A. Electrophoretic and ultracentrifugal studies of antibodies to Trypanosoma lewisi. J Infect Dis. 1959 Jul-Aug;105(1):76–95. doi: 10.1093/infdis/105.1.76. [DOI] [PubMed] [Google Scholar]
- D'ALESANDRO P. A. In vitro studies of ablastin, the reproduction-inhibiting antibody to Trypanosoma lewisi. J Protozool. 1962 Aug;9:351–358. doi: 10.1111/j.1550-7408.1962.tb02633.x. [DOI] [PubMed] [Google Scholar]
- D'Alesandro P. A. Trypanosoma lewisi: production of exoantigens during infection in the rat. Exp Parasitol. 1972 Aug;32(1):149–164. doi: 10.1016/0014-4894(72)90020-3. [DOI] [PubMed] [Google Scholar]
- Dusanic D. G. Trypanosoma musculi infections in complement-deficient mice. Exp Parasitol. 1975 Apr;37(2):205–210. doi: 10.1016/0014-4894(75)90071-5. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Atkinson J. P., Frank M. M., Collins W. E., Neva F. A. Serum complement and immunity in experimental simian malaria. I. Cyclical alterations in C4 related to schizont rupture. J Infect Dis. 1975 Jan;131(1):17–25. doi: 10.1093/infdis/131.1.17. [DOI] [PubMed] [Google Scholar]
- Khachoian V. I., Arakelian L. A. Izmenenie kolichestva komplementa (C') pri éksperimental'nom tripanosomoze. Med Parazitol (Mosk) 1974 Mar-Apr;43(2):224–226. [PubMed] [Google Scholar]
- Kierszenbaum F. Cross-reactivity of lytic antibodies against blood forms of Trypanosoma cruzi. J Parasitol. 1976 Feb;62(1):134–135. [PubMed] [Google Scholar]
- Kloetzel J., Deane M. P. Adherence of sensitized trypanosomes to peritoneal cells. Rev Inst Med Trop Sao Paulo. 1970 Nov-Dec;12(6):383–387. [PubMed] [Google Scholar]
- LANGE D. E., LYSENKO M. G. In vitro phagocytosis of Trypanosoma lewisi by rat exudative cells. Exp Parasitol. 1960 Sep;10:39–42. doi: 10.1016/0014-4894(60)90081-3. [DOI] [PubMed] [Google Scholar]
- MARDINEY M. R., Jr, MUELLER-EBERHARD H. J. MOUSE BETA-1C-GLOBULIN: PRODUCTION OF ANTISERUM AND CHARACTERIZATION IN THE COMPLEMENT REACTION. J Immunol. 1965 Jun;94:877–882. [PubMed] [Google Scholar]
- MULLER-EBERHARD H. J. A new supporting medium for preparative electrophoresis. Scand J Clin Lab Invest. 1960;12:33–37. [PubMed] [Google Scholar]
- Mancini G., Carbonara A. O., Heremans J. F. Immunochemical quantitation of antigens by single radial immunodiffusion. Immunochemistry. 1965 Sep;2(3):235–254. doi: 10.1016/0019-2791(65)90004-2. [DOI] [PubMed] [Google Scholar]
- Nagle R. B., Ward P. A., Lindsley H. B., Sadun E. H., Johnson A. J., Berkaw R. E., Hildebrandt P. K. Experimental infections with African Trypanosomes. VI. Glomerulonephritis involving the alternate pathway of complement activation. Am J Trop Med Hyg. 1974 Jan;23(1):15–26. [PubMed] [Google Scholar]
- Nelson R. A., Jr, Jensen J., Gigli I., Tamura N. Methods for the separation, purification and measurement of nine components of hemolytic complement in guinea-pig serum. Immunochemistry. 1966 Mar;3(2):111–135. doi: 10.1016/0019-2791(66)90292-8. [DOI] [PubMed] [Google Scholar]
- Neva F. A., Howard W. A., Glew R. H., Krotoski W. A., Gam A. A., Collins W. E., Atkinson J. P., Frank M. M. Relationship of serum complement levels to events of the malarial paroxysm. J Clin Invest. 1974 Aug;54(2):451–460. doi: 10.1172/JCI107781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nogueira N., Bianco C., Cohn Z. Studies on the selective lysis and purification of Trypanosoma cruzi. J Exp Med. 1975 Jul 1;142(1):224–229. doi: 10.1084/jem.142.1.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruddy S., Austen K. F. A stoichiometric assay for the fourth component of complement in whole human serum using EAC'la-gp and functionally pure human second component. J Immunol. 1967 Dec;99(6):1162–1172. [PubMed] [Google Scholar]


