Abstract
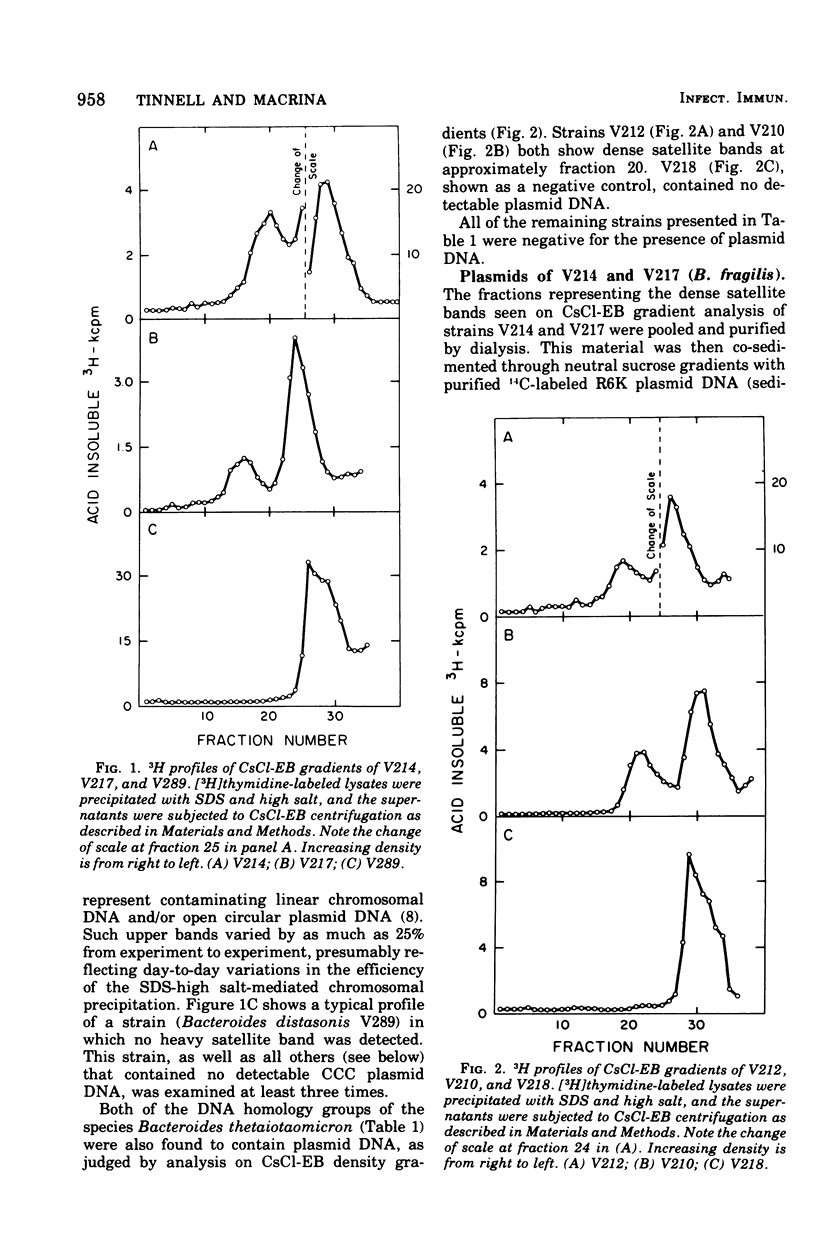
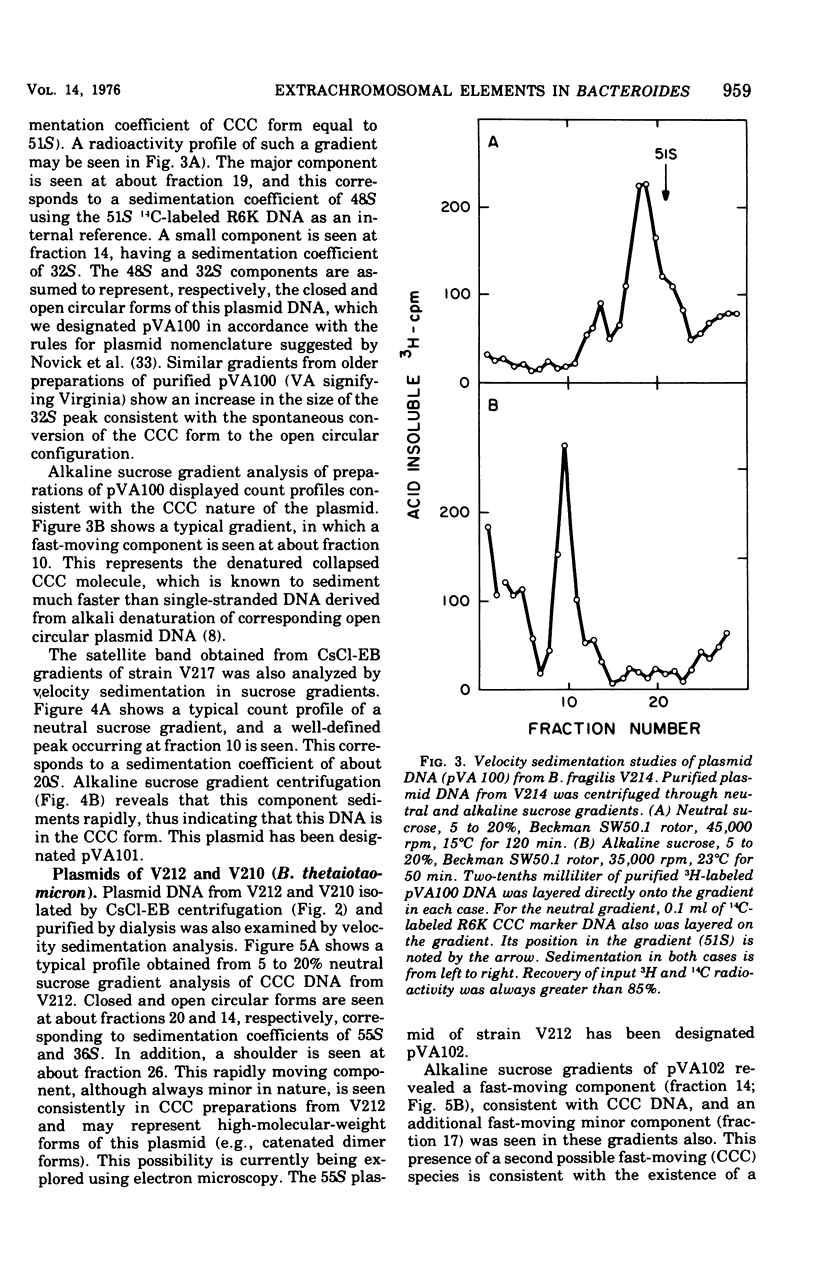
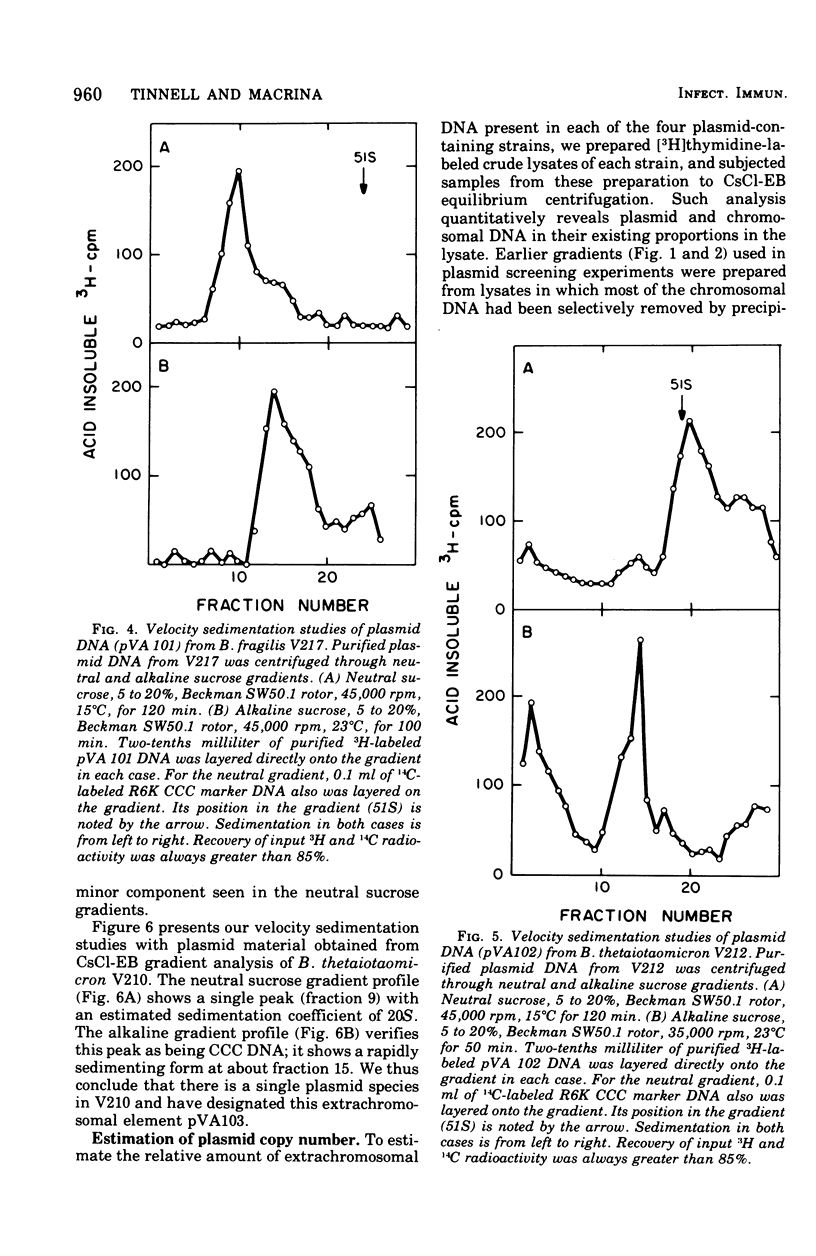
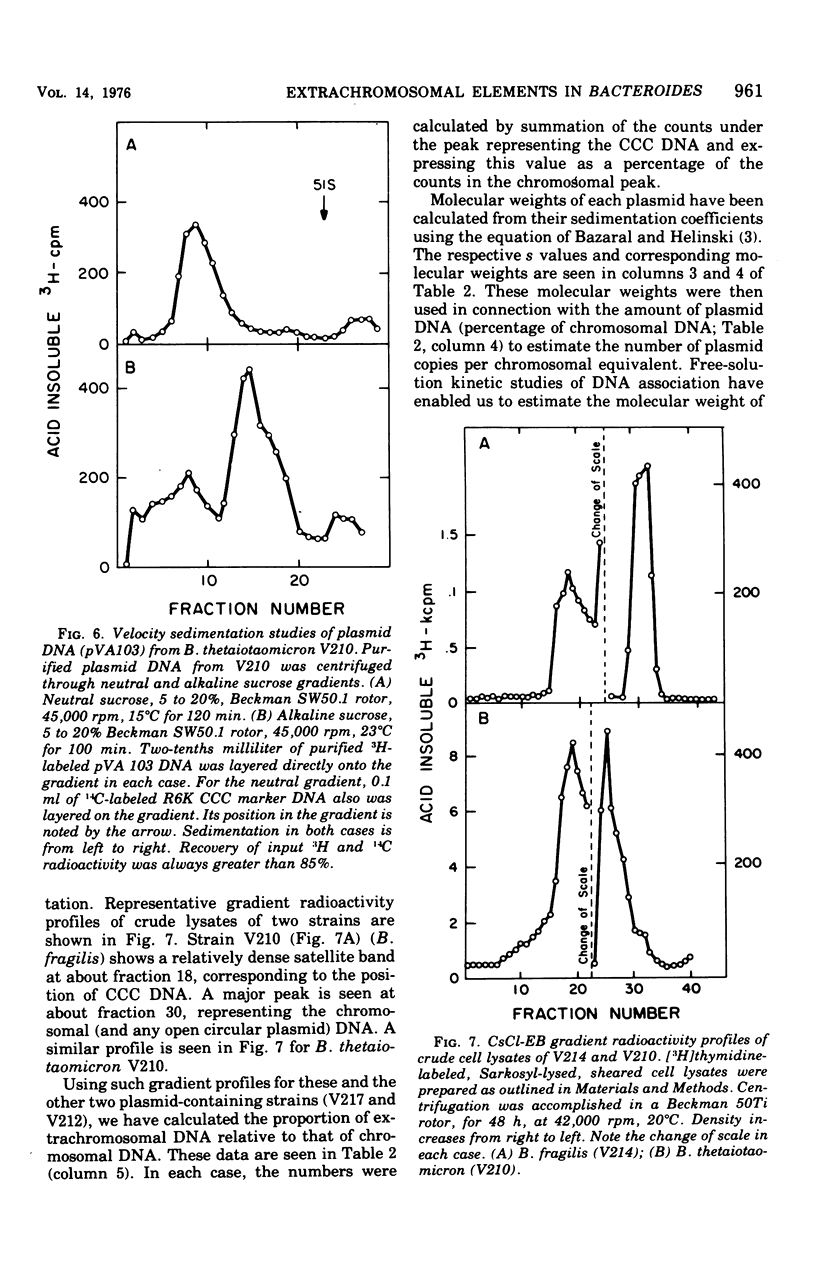
Previous nucleic acid association studies have identified at least nine deoxyribonucleic acid (DNA) homology classes of the Bacteroides fragilis group of organisms. Using these classes as a taxonomic framework, we have screened representative strains of the B. fragilis group for the presence of extrachromosomal (plasmid) DNA. [3H]thymidine-labeled cell lysates were subjected to sodium dodecyl sulfate-salt precipitation, and supernatant fractions from such preparations were analyzed using cesium chloride-ethidium bromide equilibrium centrifugation. One strain from each group was examined in this fashion. Five of the strains were judged to contain no detectable plasmid DNA; however, four strains were observed to yield satellite bands corresponding to covalently closed circular plasmid DNA. Plasmid DNA from such gradients was subjected to velocity sedimentation through both neutral and alkaline sucrose gradients to determine molecular size. A 23 X 10(6)-molecular-weight plasmid was found in a B. fragilis strain representing one DNA homology group of this species, whereas a 3 X 10(6)-molecular-weight plasmid was found in a B. fragilis strain representing a second homology group. Similarly, a 31 X 10(6)-molecular-weight plasmid was found in a Bacteroides thetaiotaomicron strain representing one DNA homology group of this species, whereas a 3 X 10(6)-molecular-weight plasmid was found in a B. thetaiotaomicron strain representing a second homology group. In all instances, the small-molecular weight plasmids were present to the extent of about 15 copies per chromosomal equivalent, whereas the large plasmids were present to the extent of approximately 1 copy per chromosomal equivalent. The biological function of these plasmids is unknown.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. D., Sykes R. B. Characterisation of a -lactamase obtained from a strain of Bacteroides fragilis resistant to -lactam antibiotics. J Med Microbiol. 1973 May;6(2):201–206. doi: 10.1099/00222615-6-2-201. [DOI] [PubMed] [Google Scholar]
- Bak A. L., Christiansen G., Christiansen C., Stenderup A., Orskov I., Orskov F. Circular DNA molecules controlling synthesis and transfer of the surface antigen (K88) in Escherichia coli. J Gen Microbiol. 1972 Nov;73(2):373–385. doi: 10.1099/00221287-73-2-373. [DOI] [PubMed] [Google Scholar]
- Bazaral M., Helinski D. R. Characterization of multiple circular DNA forms of colicinogenic factor E-1 from Proteus mirabilis. Biochemistry. 1968 Oct;7(10):3513–3520. doi: 10.1021/bi00850a028. [DOI] [PubMed] [Google Scholar]
- Chakrabarty A. M., Chou G., Gunsalus I. C. Genetic regulation of octane dissimilation plasmid in Pseudomonas. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1137–1140. doi: 10.1073/pnas.70.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou S. C., Pan H. Y., Heu P., Conklin K. A. Differentiation of DNA and RNA on filter paper discs. Anal Biochem. 1974 Jan;57(1):62–69. doi: 10.1016/0003-2697(74)90050-5. [DOI] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- Gillis M., De Ley J., De Cleene M. The determination of molecular weight of bacterial genome DNA from renaturation rates. Eur J Biochem. 1970 Jan;12(1):143–153. doi: 10.1111/j.1432-1033.1970.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Goebel W., Schrempf H. Isolation and characterization of supercoiled circular deoxyribonucleic acid from beta-hemolytic strains of Escherichia coli. J Bacteriol. 1971 May;106(2):311–317. doi: 10.1128/jb.106.2.311-317.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbach S. L., Bartlett J. G. Anaerobic infections. 1. N Engl J Med. 1974 May 23;290(21):1177–1184. doi: 10.1056/NEJM197405232902106. [DOI] [PubMed] [Google Scholar]
- Gorbach S. L. Intestinal microflora. Gastroenterology. 1971 Jun;60(6):1110–1129. [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiney D. G., Davis C. E. Isolation of plasmid deoxyribonucleic acid from two strains of Bacteroides. J Bacteriol. 1975 Oct;124(1):503–510. doi: 10.1128/jb.124.1.503-510.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gyles C., So M., Falkow S. The enterotoxin plasmids of Escherichia coli. J Infect Dis. 1974 Jul;130(1):40–49. doi: 10.1093/infdis/130.1.40. [DOI] [PubMed] [Google Scholar]
- Helinski D. R. Plasmid determined resistance to antibiotics: molecular properties of R factors. Annu Rev Microbiol. 1973;27:437–470. doi: 10.1146/annurev.mi.27.100173.002253. [DOI] [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Hawksworth G., Aries V., Crowther J. S., Williams R. E. Bacteria and aetiology of cancer of large bowel. Lancet. 1971 Jan 16;1(7690):95–100. doi: 10.1016/s0140-6736(71)90837-3. [DOI] [PubMed] [Google Scholar]
- Jacob A. E., Douglas G. J., Hobbs S. J. Self-transferable plasmids determining the hemolysin and bacteriocin of Streptococcus faecalis var. zymogenes. J Bacteriol. 1975 Mar;121(3):863–872. doi: 10.1128/jb.121.3.863-872.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch-Portnoy Y. M., Miklos G. L., Helinski D. R. Properties of the relaxation complexes of supercoiled deoxyribonucleic acid and protein of the R plasmids R64, R28K, and R6K. J Bacteriol. 1974 Oct;120(1):545–548. doi: 10.1128/jb.120.1.545-548.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Weatherly G. G., Curtiss R., 3rd R6K plasmid replication: influence of chromosomal genotype in minicell-producing strains of Escherichia coli K-12. J Bacteriol. 1974 Dec;120(3):1387–1400. doi: 10.1128/jb.120.3.1387-1400.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. J., Gardner M., Washington J. A., 2nd In vitro antimicrobial susceptibility of anaerobic bacteria isolated from clinical specimens. Antimicrob Agents Chemother. 1972 Feb;1(2):148–158. doi: 10.1128/aac.1.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore W. E., Holdeman L. V. Human fecal flora: the normal flora of 20 Japanese-Hawaiians. Appl Microbiol. 1974 May;27(5):961–979. doi: 10.1128/am.27.5.961-979.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Clowes R. C., Cohen S. N., Curtiss R., 3rd, Datta N., Falkow S. Uniform nomenclature for bacterial plasmids: a proposal. Bacteriol Rev. 1976 Mar;40(1):168–189. doi: 10.1128/br.40.1.168-189.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overman S., Lambe D. W., Jr, Bennett J. V. Proposed standardized method for testing and interpreting susceptibility of Bacteroides fragilis to tetracycline. Antimicrob Agents Chemother. 1974 Apr;5(4):357–361. doi: 10.1128/aac.5.4.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiffler P. W., Keller R., Traub N. Isolation and characterization of several cryptic plasmids from clinical isolates of Bacteroides fragilis. J Infect Dis. 1974 Nov;130(5):544–548. doi: 10.1093/infdis/130.5.544. [DOI] [PubMed] [Google Scholar]
- Varel V. H., Bryant M. P. Nutritional features of Bacteroides fragilis subsp. fragilis. Appl Microbiol. 1974 Aug;28(2):251–257. doi: 10.1128/am.28.2.251-257.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R., Rogolsky M., Wiley B. B., Glasgow L. A. Isolation of extrachromosomal deoxyribonucleic acid for exfoliative toxin production from phage group II Staphylococcus aureus. J Bacteriol. 1975 Apr;122(1):99–105. doi: 10.1128/jb.122.1.99-105.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


