Abstract
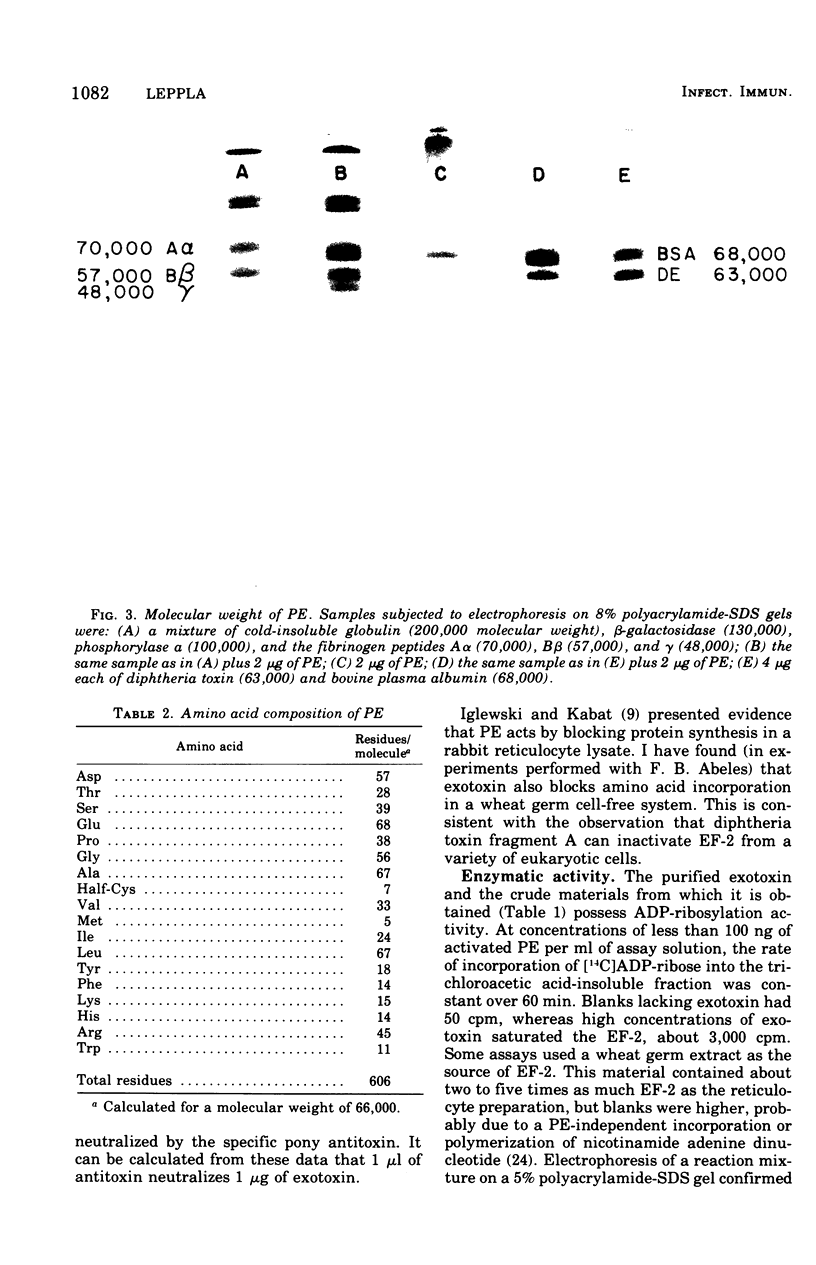
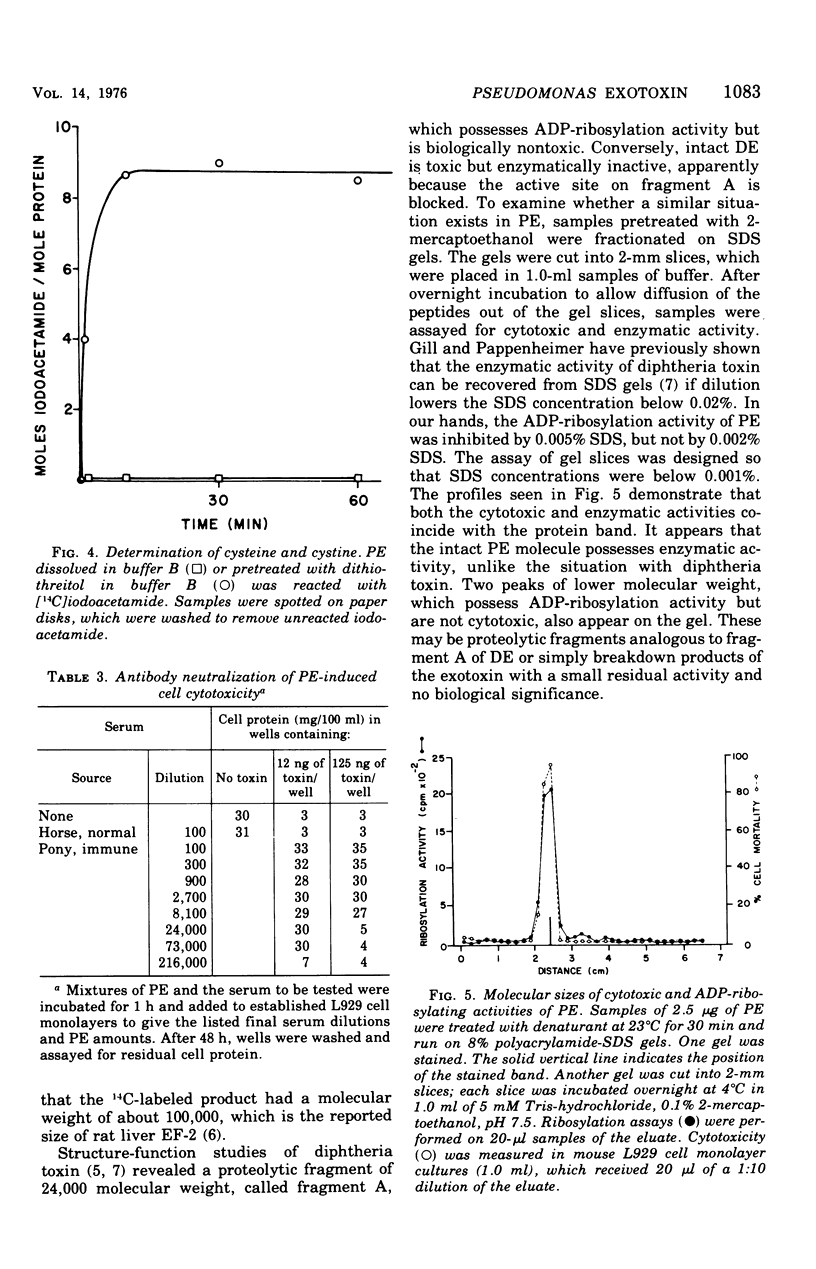
The exotoxin (PE) of Pseudomonas aeruginosa was purified from 50-liter cultures by a simple three-step procedure, yielding 135 mg of essentially homogeneous protein. In Ouchterlony gel diffusion, PE produces a single line which does not interact with a diphtheria toxin-antitoxin precipitin line. The protein has a molecular weight of 66,000, an isoelectric point of 5.1, N-terminal arginine, and four disulfide bridges. The amino acid composition shows no apparent similarity to that of diphtheria toxin. The median lethal dose of this PE preparation in mice weighing 20 g is 0.1 mug. The median lethal dose in 350-g rats is 20 mug. The cytotoxicity of PE for mouse L929 fibroblasts is completely neutralized by small amounts of specific pony antitoxin. The exotoxin possesses adenosine diphosphate-ribosylation activity. Both cytotoxic and adenosine diphosphate-ribosylation activities are shown to be properties of the intact 66,000-dalton protein.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bhatti T., Chambers R. E., Clamp J. R. The gas chromatographic properties of biologically important N-acetylglucosamine derivatives, monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides. Biochim Biophys Acta. 1970 Nov 24;222(2):339–347. doi: 10.1016/0304-4165(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Callahan L. T., 3rd Purification and characterization of Pseudomonas aeruginosa exotoxin. Infect Immun. 1974 Jan;9(1):113–118. doi: 10.1128/iai.9.1.113-118.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J. Diphtheria toxin: mode of action and structure. Bacteriol Rev. 1975 Mar;39(1):54–85. doi: 10.1128/br.39.1.54-85.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier R. J., Kandel J. Structure and activity of diphtheria toxin. I. Thiol-dependent dissociation of a fraction of toxin into enzymically active and inactive fragments. J Biol Chem. 1971 Mar 10;246(5):1496–1503. [PubMed] [Google Scholar]
- Collins J. F., Raeburn S., Maxwell E. S. Aminoacyltransferase II from rat liver. II. Some physical and chemical properties of the purified enzyme and its adenosine diphosphate ribose derivative. J Biol Chem. 1971 Feb 25;246(4):1049–1054. [PubMed] [Google Scholar]
- Gill D. M., Pappenheimer A. M., Jr Structure-activity relationships in diphtheria toxin. J Biol Chem. 1971 Mar 10;246(5):1492–1495. [PubMed] [Google Scholar]
- Hosoda J., Cone R. Analysis of T4 phage proteins. I. Conversion of precursor proteins into lower molecular weight peptides during normal capsid formation. Proc Natl Acad Sci U S A. 1970 Aug;66(4):1275–1281. doi: 10.1073/pnas.66.4.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski B. H., Kabat D. NAD-dependent inhibition of protein synthesis by Pseudomonas aeruginosa toxin,. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2284–2288. doi: 10.1073/pnas.72.6.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. D., Metzger J. F., Spero L. Production, purification, and chemical characterization of Staphylococcus aureus exfoliative toxin. Infect Immun. 1975 Nov;12(5):1206–1210. doi: 10.1128/iai.12.5.1206-1210.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschenbaum D. M. A compilation of amino acid analyses of proteins VI residues per molecule. IV. Anal Biochem. 1974 Oct;61(2):567–609. doi: 10.1016/0003-2697(74)90425-4. [DOI] [PubMed] [Google Scholar]
- Kirschenbaum D. M. A compilation of amino acid analyses of proteins. VII. Residues per molecule-5. Anal Biochem. 1975 May 26;66(1):123–150. doi: 10.1016/0003-2697(75)90732-0. [DOI] [PubMed] [Google Scholar]
- Liu P. V. Exotoxins of Pseudomonas aeruginosa. I. Factors that influence the production of exotoxin A. J Infect Dis. 1973 Oct;128(4):506–513. doi: 10.1093/infdis/128.4.506. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Hsieh H. Exotoxins of Pseudomonas aeruginosa. 3. Characteristics of antitoxin A. J Infect Dis. 1973 Oct;128(4):520–526. doi: 10.1093/infdis/128.4.520. [DOI] [PubMed] [Google Scholar]
- Liu P. V. The roles of various fractions of Pseudomonas aeruginosa in its pathogenesis. 3. Identity of the lethal toxins produced in vitro and in vivo. J Infect Dis. 1966 Oct;116(4):481–489. doi: 10.1093/infdis/116.4.481. [DOI] [PubMed] [Google Scholar]
- Liu P. V., Yoshii S., Hsieh H. Exotoxins of Pseudomonas aeruginosa. II. Concentration, purification, and characterization of exotoxin A. J Infect Dis. 1973 Oct;128(4):514–519. doi: 10.1093/infdis/128.4.514. [DOI] [PubMed] [Google Scholar]
- MCRIPLEY R. J., GARRISON D. W. INCREASED SUSCEPTIBILITY OF BURNED RATS TO PSEUDOMONAS AERUGINOSA. Proc Soc Exp Biol Med. 1964 Feb;115:336–338. doi: 10.3181/00379727-115-28906. [DOI] [PubMed] [Google Scholar]
- MORIHARA K., TSUZUKI H., OKA T., INOUE H., EBATA M. PSEUDOMONAS AERUGINOSA ELASTASE. ISOLATION, CRYSTALLIZATION, AND PRELIMINARY CHARACTERIZATION. J Biol Chem. 1965 Aug;240:3295–3304. [PubMed] [Google Scholar]
- Metzger J. F., Johnson A. D., Spero L. Intrinsic and chemically produced microheterogeneity of Staphylococcus aureus enterotoxin type C. Infect Immun. 1975 Jul;12(1):93–97. doi: 10.1128/iai.12.1.93-97.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A., Zanen J., Monier C., Crispeels C., Dirkx J. Partial characterization of diphtheria toxin and its subunits. Biochim Biophys Acta. 1972 Feb 29;257(2):249–256. doi: 10.1016/0005-2795(72)90276-0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Ueda K., Nakazawa K., Hayaishi O. Studies on the polymer of adenosine diphosphate ribose. I. Enzymic formation from nicotinamide adenine dinuclotide in mammalian nuclei. J Biol Chem. 1967 Jul 10;242(13):3164–3171. [PubMed] [Google Scholar]
- OUCHTERLONY O. Antigen-antibody reactions in gels. IV. Types of reactions in coordinated systems of diffusion. Acta Pathol Microbiol Scand. 1953;32(2):230–240. [PubMed] [Google Scholar]
- Pavlovskis O. R., Gordon F. B. Pseudomonas aeruginosa exotoxin: effect on cell cultures. J Infect Dis. 1972 Jun;125(6):631–636. doi: 10.1093/infdis/125.6.631. [DOI] [PubMed] [Google Scholar]
- Tapper M. L., Armstrong D. Bacteremia due to Pseudomonas aeruginosa complicating neoplastic disease: a progress report. J Infect Dis. 1974 Nov;130 (Suppl)(0):S14–S23. doi: 10.1093/infdis/130.supplement.s14. [DOI] [PubMed] [Google Scholar]
- Weiner A. M., Platt T., Weber K. Amino-terminal sequence analysis of proteins purified on a nanomole scale by gel electrophoresis. J Biol Chem. 1972 May 25;247(10):3242–3251. [PubMed] [Google Scholar]





