Abstract
Sonic hedgehog plays an essential role in maintaining hepatoblasts in a proliferative non-differentiating state during embryogenesis. Transduction of the Hedgehog signaling pathway is dependent on the presence of functional primary cilia and hepatoblasts, therefore, must require primary cilia for normal function. In congenital syndromes in which cilia are absent or non-functional (ciliopathies) hepatorenal fibrocystic disease is common and primarily characterized by ductal plate malformations which underlie the formation of liver cysts, as well as less commonly, by hepatic fibrosis, although a role for abnormal Hedgehog signal transduction has not been implicated in these phenotypes. We have examined liver, lung and rib development in the talpid3 chicken mutant, a ciliopathy model in which abnormal Hedgehog signaling is well characterized. We find that the talpid3 phenotype closely models that of human short-rib polydactyly syndromes which are caused by the loss of cilia, and exhibit hypoplastic lungs and liver failure. Through an analysis of liver and lung development in the talpid3 chicken, we propose that cilia in the liver are essential for the transduction of Hedgehog signaling during hepatic development. The talpid3 chicken represents a useful resource in furthering our understanding of the pathology of ciliopathies beyond the treatment of thoracic insufficiency as well as generating insights into the role Hedgehog signaling in hepatic development.
Keywords: TALPID3, KIAA0586, short-rib polydactyly, pulmonary hypoplasia, liver, cholestasis, cilia, ciliopathy, SHH, GLI
Introduction
Ciliopathies are multi-organ syndromes in which disorders arise either directly due to a loss of cilia formation, or from abnormal processes downstream of cilia function.1,2 Several organ systems which are affected in human ciliopathies such a Bardet-Biedl and Meckel syndrome, are associated with a loss of Hedgehog (Hh) pathway regulation during embryonic development, including polydactyly and abnormal bone formation.3 This is due to a requirement for primary cilia during Hh signal transduction when components of the Hh pathway such as the receptors PTCH1 and SMO are trafficked to and enriched in the cilia. ENU mutagenesis screens for Hh pathway components and loci mapping in human ciliopathy conditions have determined many proteins which are essential for cilia formation and function.1,3
Short-rib polydactyly (SRP) type III syndromes are a range of functionally, and genetically overlapping ciliopathies, presenting primarily with short ribs, reduced thoracic capacity, and pulmonary hypoplasia, leading to respiratory insufficiency and in severe cases, thoracic asphyxiation and death caused by constricted thoracic volume.4-7 Patients are also characterized by polydactyly, suggesting that Hh signaling is abnormal, as well as shortening of the long bones and metacarpels,8 cystic kidneys,9 liver fibrosis, and cholestasis.10 SRPIII syndromes are highly variable; although fetal lethality is common, children surviving infancy may undergo surgery to increase thoracic volume and live to adulthood, where they may present with combinations of key traits. In these cases, hepatic disease becomes more apparent, with liver transplants reported in patients as early as 7 years old.11
The Hh signaling pathway is well studied in the patterning and development of many organ systems. In the mouse lung Shh is necessary for early lung development12,13 and ablation of signaling with cyclopamine in the chicken, like the mouse, causes a loss of lung epithelial branching.14 Although Hh signaling has been shown to be abnormal in models of asphyxiating ciliopathies,7 a loss of lung morphogenesis has not been shown to be the primary cause of this.15 At the initiation of the developing liver bud, interactions between Shh and FGF signaling in the endoderm have been proposed to specify hepatic endothelial cells.16 Subsequently both Shh and Indian hedgehog ligands are expressed by hepatoblasts between E11.5-E17.517 in the mouse, as well as the Hh responsive genes Gli117 and Ptch1.18 Addition of Hh ligand in vitro causes an increase in hepatoblasts proliferation. Thus the current model for the action of Hh signaling during liver development is that it acts to control the balance between hepatoblast proliferation and the differentiation to hepatocytes.17 There is also a well-documented role for SHH in liver regeneration and repair; Hh responsive cells are observed in the adult liver when damaged.19,20 SHH is activated in response to chronic liver injury, but in addition, increasing Hh signaling through reduction of PTCH1 activity, results in greater damage to the liver.20 Conversely, inhibition of the Hh pathway has also been shown to reverse the development of fibrosis and hepatocarcinoma.21 Hh signaling has therefore become a focal point for understanding liver repair, regeneration and the basis of various liver cancers.21-24 The role of cilia in transduction of the Hh signal within the liver has not been investigated, although ciliated cells correspond to the intrahepatic Hh responsive cells in adult mice.20 We can therefore assume, as in all other cells types investigated, that Hh responsive liver cells require cilia to transduce the Hh signal. Cilia have other functions within the developing liver; a loss of cilia on cholangiocytes, which localize proteins such as the polycystin family, important in mechano-, osmo-, and chemo-sensory functions, leads to cystic and fibrotic liver diseases.25,26 The severity of most ciliopathy models commonly results in embryonic lethality; however, the role of cilia in developmental hepatic phenotypes is particularly under-studied.
The talpid3 chicken provides a classic model for studying human ciliopathies and Hh signaling, exhibiting many ciliopathy phenotypes, including polydactyly,27 polycystic kidneys28 and a loss of endochondral bone ossification29 and has been useful in elucidating the role of Hh signaling in limb and neural tube development.27,30 The TALPID3 protein (KIAA0586) localizes to the centrosome in human, chicken, mouse and zebrafish and is required for the docking of the basal body prior to ciliogenesis.28,31,32 Loss of TALPID3 protein causes a loss both of motile and non-motile primary cilia.28,33 Due to the loss of primary cilia in TALPID3−/− cells, the downstream effectors of Hh signaling, the GLI transcription factors are abnormally processed and localized and their function therefore abrogated. Consequently, as is seen commonly in other ciliopathy models, the expression of PTCH1 is not initiated at sites of high Hh signaling.27,31
Here we propose that the classical talpid3 chicken may act as a model for the SRPIII class of ciliopathies. The talpid3 chicken is able to develop until E7-E12, substantially further than the Talpid3−/− mouse and most other mammalian ciliopathy models, allowing us to extend our analyses to organs not possible in the mouse, and thus to study the role of Hedgehog signaling in the developing lung and liver.
Results
Talpid3 embryos exhibit abnormal liver and lung morphology reminiscent of SRPIII patients
Gross morphological analysis of talpid3 embryos identified clear abnormalities in the liver and lungs (Fig. 1). Birds differ from mammalian lung development, in that they have a parabronchial lung, rather than the alveolar lung found in mammals. However branching events in avians are similar to the mammalian lung and also exhibit conserved signaling pathways.34 By E8 the wt lungs are highly branched, distinct structures (Fig. 1A), whereas talpid3 lungs are smaller, lacking branches, and were typically surrounded by fibrotic mesenchymal tissue (Fig. 1B). The abdominal air sac is however normal (Fig. 1A and B, asterisk).
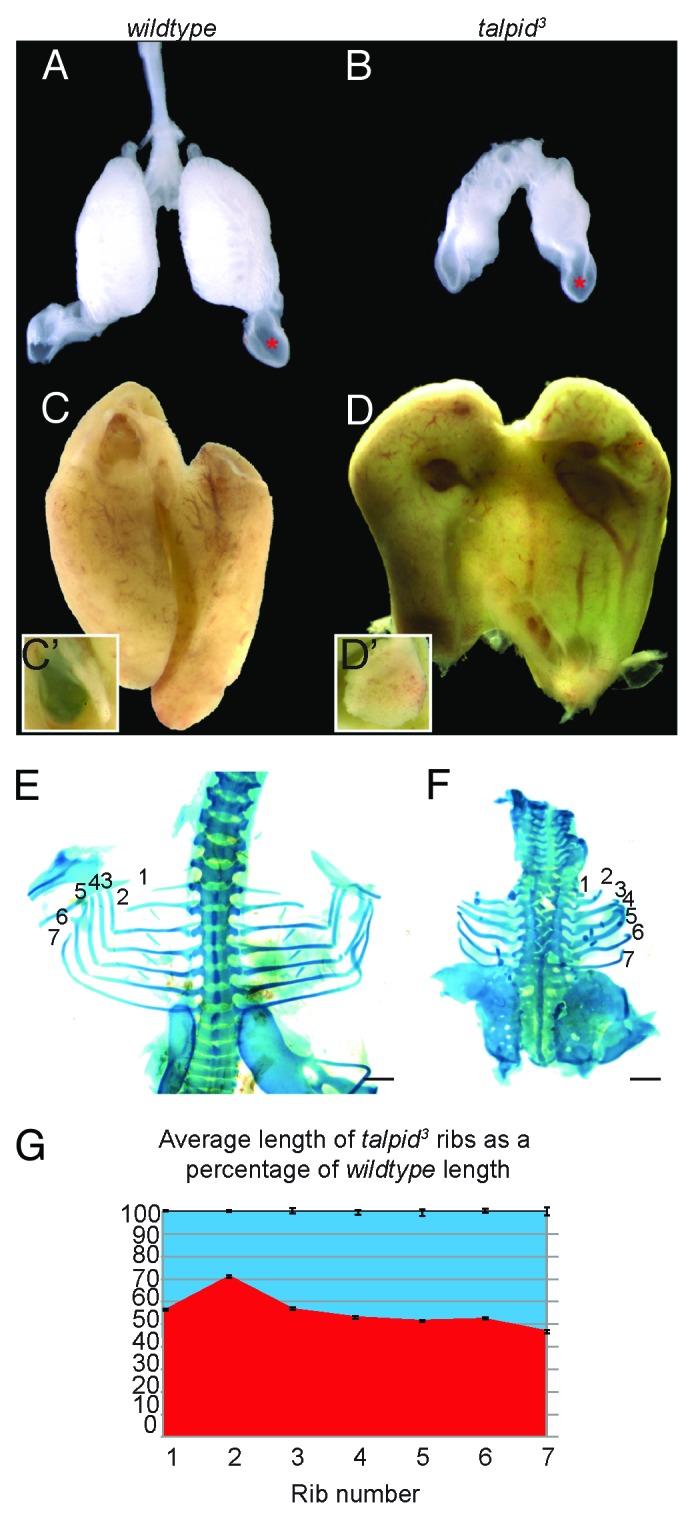
Figure 1. The talpid3 chicken exhibits abnormal lung and liver morphology. Compared with E10 wt lung (A), the talpid3 lung is smaller and poorly branched (B), air sac development was normal (red asterisk) (A andB). Wt liver (C) and talpid3 liver (D) are of similar size, although the talpid3 liver is green. The wt gall bladder is bile filled (green) (C’), while the talpid3 gall bladder lacks bile (D’). At E10 individual ribs were measured in wt (E) and compared with the corresponding rib in talpid3 (F). Average lengths were reduced in the talpid3 chicken; Rib one = 44% reduced, rib two = 28%, rib three = 44%, rib four = 46%, ribs five/six = 48%, rib seven = 53% smaller in talpid3 (G). Magnification is the same between A and B, C and D, and E and F.
All talpid3 embryos dissected had ventral abdominal herniation of viscera. All talpid3 livers were normally patterned with a lobe either side of the midline, the right lobe being larger and the left exhibiting a fissure dividing the right lobe into two parts. However, the talpid3 liver was clearly distinguished from the wt (Fig. 1C) by its green color (Fig. 1D), suggesting increased levels of bile within the talpid3 liver. The gall bladder, while visible in talpid3 and correctly attached to the right liver lobe, did not contain bile (compare Fig. 1C' with Fig. 1D'). SRPIII conditions in humans are associated with abnormal thoracic skeleton development, in particular, short ribs. The talpid3 rib cage was considerably smaller than that of the wt (Fig. 1E and F) and individual ribs measured between 44–53% of the length of their wt counterparts (Fig. 1G).
Lung development in talpid3 chicken is abnormal
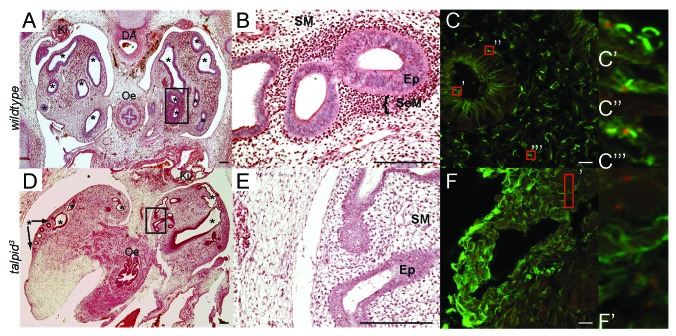
Pulmonary insufficiency is a key feature of SRPIII syndromes, but it is unclear if this is due to a primary effect on lung development or secondary to thoracic restriction. Having identified pulmonary hypoplasia in talpid3 dissections, we characterized the extent of abnormalities in talpid3 pulmonary development by histological analysis (Fig. 2). At E7, the primary wt mesobronchi consist of a thickened epithelium (Ep, Fig. 2B) surrounded by condensed subepithelial mesenchyme (SeM, Fig. 2B). The submesothelial mesenchyme comprising the remaining lung (SM, Fig. 2B) is punctured extensively by epithelial bronchiolar branches (asterisks Fig. 2A). Like SRPIII patients, talpid3 lung development was highly variable; from embryos exhibiting two lungs that resemble small lungs with some mesobronchial branching (Fig. 2D), ranging to absent or extremely reduced lungs with no mesobronchi (not shown). A separated esophagus and trachea were observed in all samples. In less severely affected E7 talpid3 lungs in which epithelia-lined lumen were present (Fig. 2D), bronchiolar lumen were smaller and unevenly distributed through the mesenchyme (arrows, Fig. 2D), the bronchiolar epithelia was thin and disorganized (Ep, Fig. 2E), and SeM condensations were not observed (Fig. 2D and E). Treatment of chick lungs at E8 with SHH pathway inhibitor cyclopamine causes a similar hypoplastic lung phenotype.14 We examined SHH expression in E6 chicken lungs (Fig. S1) and detected SHH in the wt trachea (Fig. S1A and B, arrow) but not in the mesobronchial epithelium, instead observing SHH expression in the wt distal lung mesenchyme (Fig. S1C, arrow). Neither the talpid3 lung epithelium nor mesenchyme expressed SHH at E6 (Fig. S1D–F). WNT5A negatively regulates SHH expression in the chick lung and overexpression of WNT5A causes pulmonary hypoplasia.14 At E8 we observed WNT5A expression in both the distal bronchial epithelia and mesenchyme, whereas expression appeared reduced in the distal mesenchyme of talpid3 lungs. GATA6 is strongly expressed in the distal lung epithelia in humans35 and abnormal expression has been suggested as a cause of respiratory distress in neonates. At E8 GATA6 was strongly expressed in distal epithelium of wt and talpid3 embryos, confirming the distal epithelial identity of the talpid3 bronchioles (Fig. S1G–J). We have previously shown that FOXJ1, a master regulator of motile ciliogenesis, is not expressed in the chicken embryo respiratory tract before E10, suggesting the developing lung epithelia does not have motile cilia at this time.33 We examined the presence and type of cilia at E10; short, primary cilia were identified in the three main tissue types of the wt lung; SeM, SM and epithelial (Fig. 2C). No cilia were identified in talpid3 lungs (Fig. 2F). In summary due to a loss of cilia and cilia transduced pathways such as SHH, lung morphogenesis was severely disrupted, resulting in defects in both the epithelium and mesenchyme, although distal structures were still present.
Figure 2. Lung development in the wt and talpid3 chicken. Haematoxylin and eosin staining E7 (A–E). IHC anti-acetylated tubulin (cilia axonemes;green) and anti- γtubulin (centrosomes;red) E10 (CandF). At E7 the epithelial mesobronchi of each lung has branched extensively throughout the wt lung mesenchyme (asterisks) (A). The more mature wt primary bronchi (circled area) (A andB) are surrounded by a condensation of SeM cells (B). Mesobronchi branching in the talpid3 lung is disturbed and differs between lungs within the same embryo (bronchi labeled with asterisk or arrow asterisk) (D), the epithelia is thinner and disorganized and no SeM condensations are seen (E). E10 wt lung exhibit cilia (C) on epithelia (C’), SeM (C”) and SM cells (C”’). E10 talpid3 lung, cilia do not project from centrosomes (red box) (F and F’) Magnification comparable between A–E, C–F.
The talpid3 liver exhibits abnormal biliary tract development
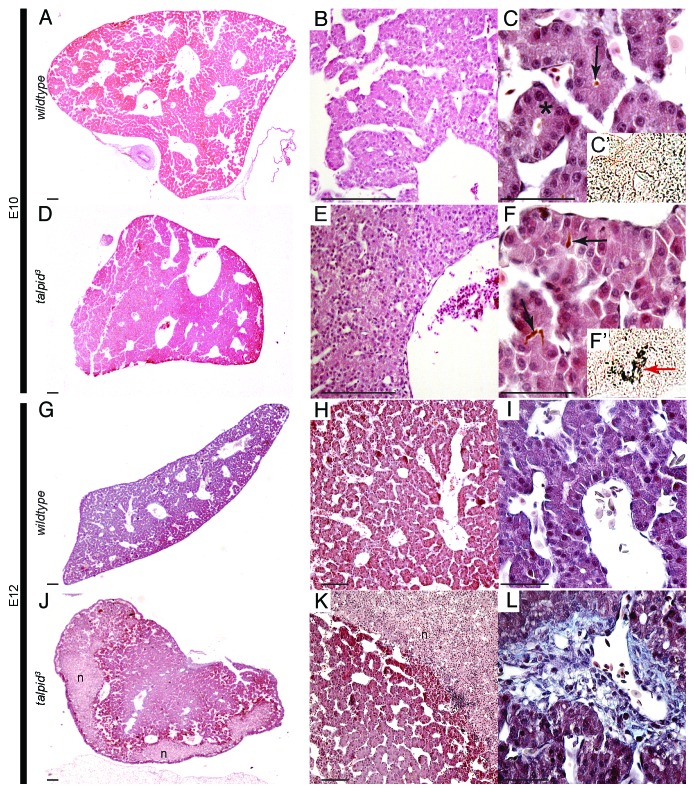
Wt avian livers at E10 are highly organized structures, consisting of compact systems of hepatocytes surrounding blood vessels and sinusoidal spaces in which an immature version of the classic hepatic triumvirate can be observed, with cholangiocytes arranged in a ring, producing early bile ducts (Fig. 3A and B, asterisk C). In comparison, blood vessels are less clearly defined in the talpid3 liver and sinusoidal spaces are considerably smaller, giving the liver a more condensed appearance overall (Fig. 3D). The ductal plate in the talpid3 liver is considerably more cell dense with several layers of cholangiocytes forming along the ductal plate (Fig. 3E and F). Immunohistochemistry for cytokeratin19, expressed in the ductal plate (bile ducts) at E10 shows an increase in the number of epithelial cells in the talpid3 ductal plate, (Fig. 3, red arrow F’ compared with C’) characteristic of a ductal plate malformation (DPM). This may well be caused by hyperplasia or abnormal remodelling and morphogenesis of the talpid3 ductal plate. Bile in the wt liver sits within the lumen of the biliary duct (Fig. 3C, arrow), while bile is found between cholangiocytes and outside of the bile duct in talpid3 (Fig. 3F, arrow). At E12 fibrosis can be observed in talpid3 livers around the ductal plate (Fig. 3L, blue; compare with wtFig. 3I) and necrosis is widespread (n; Fig. 3J and K).
Figure 3. Abnormal liver histology in the talpid3chicken. Haematoxylin and eosin staining E10 (A–F), E12 (G, H, J, K). IHC Cytokeratin19 (C’ andF’). Masson’s trichrome staining at E12 (IandL). E10, wt livers have large spaces throughout (AandB) and exhibit an immature version of the classic hepatic triumvirate with cholangiocytes arranged in a ring to produce early bile ducts (asterisk) (C) with bile (arrow) (C) but no Cytokeratin19 present (C’). E10 talpid3 liver is more compact, with fewer, smaller, spaces (D andE). Bile ducts develop but are overcrowded with cholangiocytes (F) which express Cytokeratin19 (F’) and bile is observed between the cholangiocytes (arrow) (F). E12 wt liver is more compact (G andH). E12 talpid3 liver presents areas of necrosis (J, K) and portal fibrosis (blue stain) (L) compared with wt (I).
PTCH1 is expressed in the normal embryonic liver
Long cilia have been observed on cholangiocytes during human liver development and a loss causes liver defects in fetuses with Meckel syndrome, a severe ciliopathy.36 To observe if cilia are only found on cholangiocytes during development we used immunohistochemistry to observe cilia in the developing liver. Short primary cilia were widely observed in the wt liver as early as E6 (Fig. 4A, circled), resembling the short primary cilia which are required for Hh signaling elsewhere in the embryo, rather than long cilia that have been reported on cholangiocytes.23 In talpid3 liver tissue, although centrosomes were observed (Fig. 4B, circled), cilia were absent. Loss of cilia in the liver suggests that hepatic abnormalities in the talpid3 liver may be due to aberrant SHH signaling. Levels of PTCH1 expression indicate levels of Hh signaling, therefore Real-time qPCR was used to study levels of PTCH1 as a read out for SHH activity in the liver at E6, prior to onset of fibrosis and necrosis. PTCH1 expression was reduced 0.08-fold in the talpid3 liver compared with wt (Fig. 4C), suggesting Hh signaling is greatly abrogated in the talpid3 embryonic liver.
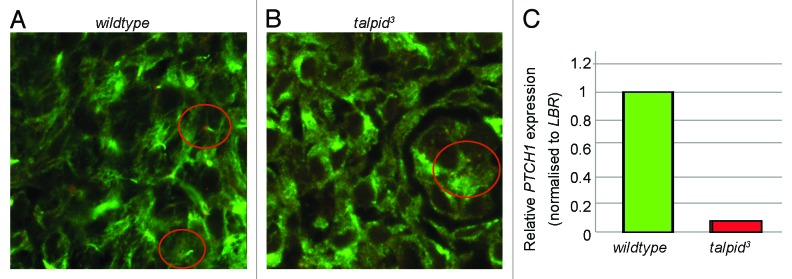
Figure 4. Hedgehog signaling is perturbed in the talpid3 liver during development. IHC anti-acetylated (cilia axonemes, green) and γtubulin (centrosomes, red) E6 (A andB). Wt liver has cilia axonemes projecting from centrosomes (circled) (A) cilia axonemes are not observed projecting from centrosomes in the talpid3 liver (circled) (B). (C) Real-time PCR identified a 0.08-fold reduction in PTCH1 expression in the day 6 talpid3 liver, compared with the wt.
Discussion
The talpid3 chicken models human SRPIII syndromes
Abnormal development in the talpid3 chicken offers an insight into human development and disease. SRPIII syndromes encompass a variable spectrum of developmental disorders, many of which are related to ciliopathies. We suggest that the talpid3 chicken phenocopies a number of these, including short ribs, polydactyly, polycystic kidneys, liver fibrosis and cholestasis, making it a useful model to study SRPIII and related syndromes.
Does chicken lung morphogenesis model mammalian lung morphogenesis?
With some exceptions14,34,37,38 the majority of our understanding of the signaling driving pulmonary development has been investigated by studies in mammalian systems.39,40,65 However, the chicken is a useful alternative to mammalian models, offering shorter gestation times and many alternative options for embryonic manipulations (for review see ref. 41). If we are to fully utilize the chicken as a system for understanding human development and disease, it is important to determine the similarities and differences between them. Shh signaling is essential for early mouse pulmonary development12,13 where it is expressed in the distal epithelial endoderm at the budding tips.42 Likewise, the Shh receptor, PTCH1 is expressed in the distal mesenchyme, in both mouse43 and human44 suggesting that a requirement for SHH signaling in the growth and differentiation of the bronchioles is conserved within mammalian species. Perturbation of the SHH signaling pathway through mutations in SHH and the downstream targets, GLI2 and GLI3, results in highly abnormal tracheal and pulmonary development, in particular loss of asymmetry and pulmonary hypoplasia.45,46 In contrast although we found strong SHH expression in the avian gut, as described previously,47 expression of SHH in the budding epithelium of the lungs was absent from wild type chickens, although we detected SHH expression in distal mesenchyme. This suggests that while the target tissue of SHH action is the same between mouse and chicken (the distal mesenchyme), the chicken alters its expression between an early mesenchymal domain to a later epithelial expression,14 during development. While there are clear differences in the basic development of avian and mammalian lungs, analysis of epithelial and mesenchymal expressed genes at later stages of lung development in the wt and talpid3 chicken also suggest that many of the underlying processes are still comparable to the mammalian model. Previous studies in mouse have shown Nkx2.1, Gata6, Sox2, Wnt5a, and Wnt3a to be expressed in the branching epithelia. We have confirmed this in the avian model and further identified that this expression was not significantly disrupted in the talpid3 mutant. It is however clear that branching is affected in the talpid3 chicken, albeit to a variable extent. Most interestingly, we identified a reduction of mesenchymal WNT5a in talpid3 embryos. In the mouse and chicken, Wnt5a is thought to regulate Shh and Fgf10 in the developing lung14,48 and in turn Fgf10 null mice fail to produce any structures distal to the primary bronchi.49 Loss of WNT5A would be expected to result in an increase in SHH signaling.14 However, in the talpid3 chicken, the loss of WNT5A accompanies a loss of SHH phenotype, hypoplastic lungs. We suggest that this is due to a complex signaling loop that we are only beginning to understand. The increase in SHH caused by loss of WNT5A, previously reported by Loscertales and colleagues,14 is caused by an increase in GLIA and loss of GLIR, producing a hyperplastic lung phenotype. Loss of SHH signaling in the same report, was achieved by cyclopamine, a Hedgehog repressor that inhibits the GLIA pathway, resulting in hypoplasia. The talpid3 chicken phenotype is the result of a loss of GLIA and GLIR,27 producing a hypoplasia phenotype. We propose that a WNT5A/SHH signaling loop acts within a PCP/cilia network to maintain development in the lung. The loss of cilia in the talpid3 mutant has previously been attributed to abnormal basal body migration,33 a PCP phenotype. It is likely that this lack of cilia not only prevents the tissue from responding to loss of SHH signaling, but also to loss of WNT5A, therefore producing a loss of SHH phenotype, despite the apparent loss of WNT5A.
Many mouse mutants with a loss of SHH signaling exhibit a tracheoesophageal fistula, whereby the early esophagus fails to split to produce discrete tracheal and esophageal tubes.12 In contrast, in the talpid3 embryo, the trachea was clearly distinct from the esophagus in all animals studied. Either this may be because SHH is not required for avian respiratory tract development, or alternatively may be due to loss of GLI processing observed in talpid3, which in some organs causes loss of Hh phenotype, and in others a gain of Hh phenotype.50 Hh signaling requires interaction with GLI proteins in both activator (A) and repressor (R) form; therefore loss of GLIR in the talpid3 limb results in polydactyly, (due to loss of repression of Hh signaling targets by GLIR), while craniofacial abnormalities can be attributed to a loss of GLIA but are partially rescued by lack of GLIR also, producing variable holoprosencephaly that is less severe than SHH−/− mutants.27,51
Implications for SRPIII patients
The analysis of the molecular basis of lung hypoplasia, a disruption identified in infants with SRPIII syndromes, is unique to this study and has rarely been studied in models for the disease.4 Analysis of SRPIII mouse models caused by mutations in Ift80 and Ift144 (in which lung development is not assessed) indicate, as in talpid3, that aberrant Hh signaling is the cause of many SRPIII associated phenotypes, such as polydactyly.5,15 A further model of SRPIII, the Wdr35 mouse does demonstrate pulmonary hypoplasia which is independent of rib development.52 We therefore suggest that lung hypoplasia identified in the Wdr35 mutant, talpid3 and SRPIII patients may be due to Hh signaling abnormalities and is not solely a secondary consequence of physical constriction due to thoracic dystrophy as is generally assumed in SRPIII patients. Treatment of SRPIII patients often involves expansion of the rib cage to allow pulmonary growth, while this treatment offers a great increase in life expectancy, we know of no studies that have investigated how well pulmonary development is rescued, and we would suggest that surgical intervention will never fully restore pulmonary function in these patients due to inability of the lungs to undergo normal morphogenesis due to Hh signaling defects.
Among the key difficulties facing patients who survive to adulthood is the development of fibrocystic kidneys,53-55 which has previously been described in the talpid3 chicken. Poor liver function has been recorded as early as 3 days of life in patients with the SRPIII syndrome Jeune’s Asphyxiating Dystrophy (JAD) with biopsies indicating portal fibrosis and dilated bile ducts.11 While the key presentation of JAD is asphyxiating thoracic dystrophy, as treatment improves it is important to recognize the range of hepatic abnormalities patients are susceptible to. Alongside fibrosis and DPMs described here, previous clinical reports have identified hepatomegaly9,56 and biliary cirrhosis.11 Fibrocystic liver presentations in Bardet-Biedl syndrome patients are reviewed in depth by Waters et al.,57 while the COACH subset of Joubert syndrome patients exhibit a very mild liver phenotype with portal hypertension, congenital fibrosis and mild DPM (reviewed ref. 58). In this study we have identified that ductal plate malformation (DPM) and cholestasis precede embryonic portal fibrosis in talpid3 embryos. Extrahepatic biliary atresia, most likely caused by abnormal morphogenesis of the extrahepatic bile duct,59 has also been discussed in animal models for situs inversus and abnormal SHH signaling and may be a potential cause of bile retention, and subsequently hepatic necrosis.60-63 We propose however, that reduction in biliary duct lumen, caused by over-proliferation or abnormal remodelling of the ductal plate may be a cause of biliary blockage, while the loss of mechanosensory cholangiocyte cilia may prevent regulation of biliary flow, resulting in cholestasis.25,64 Cholestasis itself may therefore prove useful as either in identification of disease prior to liver damage in patients or as a target for therapy in reducing liver damage.
The talpid3 chicken indicates a requirement for Hh signaling via cilia in liver development
We have shown that short cilia are present and PTCH1 is normally expressed in the developing liver indicating that Hh signaling is active in the embryonic liver and likely to be important in morphogenesis. We would predict a loss of PTCH1 in talpid3 livers, as we see in other talpid3 embryonic tissues, due to a loss of cilia and Hh signal transduction.27,56 In talpid3 embryos we have observed cholestasis, DPM and liver fibrosis. Although liver fibrosis has previously been linked to over-activation of the Hh pathway,20 it is not clear if these phenotypes are caused only through misregulation of Hh signaling in talpid3 or more generally due to a loss of cilia. Cilia play numerous roles in liver homeostasis, including mediating functions of the cholangiocytes through the polycystins and other signaling pathways65 but certainly Hh signal transduction is defective in talpid3 and this may prove a useful model for examining the function of Hh signaling in liver development and function.
Concluding Remarks
A loss of cilia during embryonic development causes cholestasis and liver fibrosis. The talpid3 chicken offers a valuable resource in understanding the role of Hh signaling in liver development and in furthering studies on SRPIII ciliopathies.
Materials and Methods
Embryo incubation, dissection and histology
Eggs from talpid3 flock (MG Davey; talpid3 chicken lines are maintained at the Roslin Institute under UK Home Office license 60/4506 [Dr Paul Hocking], after ethical review) were incubated at 38 °C for 6–12 d, staged as per ref. 66. Embryos were dissected into PBS, fixed 4%PFA.
Histology
Fixed lung and liver samples were embedded in paraffin, and sectioned stained in hematoxylin and eosin, as per ref. 28 and Masson’s trichrome.
Immunohistochemistry
Embryos were dissected into PBS, fixed, and organs of interest removed before sectioning as per reference 27. Immunohistochemistry was then performed as per reference 27. Antibodies used- acetylated α tubulin (Sigma-Aldrich T7451), γtubulin (Sigma-Aldrich T5192), anti-cytokeratin19 (Developmental Studies Hybridoma Bank Troma-III) anti-mouse (Life Technologies A11017), anti-rabbit (Life Technologies A21207).
Alcian green staining
E10 embryos were dissected in ice-cold PBS, decapitated and eviscerated, and fixed overnight in 5% trichloroacetic acid. Embryos were then transferred into 0.1% alcian green/70% ethanol/1% HCl for 24hrs. Post dehydration, tissue was cleared using methyl salicylate. The rib cage was dissected, photographed and rib measurements taken using Image J. Rib one is often missing from the talpid3 chicken, or too small to be measured.
Whole-mount in situ hybridization
RNA probe synthesis and whole-mount RNA in situ hybridization was performed on lungs as per references 67 and 68. Photography was performed using a Leica M28 microscope. Chicken ESTs were obtained from previously used cDNA sequences or the BBSRC ChickEST Database69 collection held by ARK Genomics corresponding to- GATA6 (ChEST944e13), NKX2.1 (ChEST763 g11), SOX2 (ChEST878b12), WNT3A (ChEST36j7), and WNT5A (ChEST378m15), SHH.68
Real-time PCR
RNA was isolated from E6 livers using tri reagent (Sigma) and reverse transcription performed using High Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Real-time PCR was performed using Brilliant III ultrafast SYBR green QPCR master mix (Agilent Technologies) and analysis performed using the stratagene MX3000 and MxPro software.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
The authors would like to Dr P Beard for her help with analysis and Mr Neil McIntyre of the Veterinary Pathology Unit at the University of Edinburgh for assistance with Masson trichrome staining.
Financial Support
M.G.D., L.A.S., and L.M. are supported by BBSRC Career Track Fellowship funding to MGD (BB/F024347/1). A.B. was supported by a summer project studentship from the Nuffield Foundation. L.J.F. is supported by a grant from the Wellcome Trust 094182/Z/10/Z. M.G.D. is supported by funding to The Roslin Institute via Institute Strategic Grant funding from the BBSRC.
Glossary
Abbreviations:
- PTCH1
patched 1
- SMO
smoothened
- Hh
hedgehog
- SRPIII
short-rib polydactyly type III syndrome
- SHH
sonic hedgehog
- ENU
N-ethyl-N-nitrosourea
- FGF
fibroblast growth factor
- ChEST
chicken expressed sequence tag
- wt
wildtype
- Oe
oesophagus
- Ki
kidney
- DA
dorsal aorta
- Ep
epithelium
- SeM
subepithelial mesenchyme
- SM
submesothelial mesenchyme
- AS
air sac
- IHC
immunohistochemistry
- DPM
ductal plate malformation
- Ift80/144
intraflagella transport protein 80/144
- JAD
Jeune Asphyxiating Dystrophy
- COACH
cerebellar vermis hypoplasia, oligophrenia, ataxia, colobomas, and hepatic fibrosis
References
- 1.Fliegauf M, Benzing T, Omran H. When cilia go bad: cilia defects and ciliopathies. Nat Rev Mol Cell Biol. 2007;8:880–93. doi: 10.1038/nrm2278. [DOI] [PubMed] [Google Scholar]
- 2.Hildebrandt F, Benzing T, Katsanis N. Ciliopathies. N Engl J Med. 2011;364:1533–43. doi: 10.1056/NEJMra1010172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nat Rev Genet. 2010;11:331–44. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho NC, Francomano CA, van Allen M. Jeune asphyxiating thoracic dystrophy and short-rib polydactyly type III (Verma-Naumoff) are variants of the same disorder. Am J Med Genet. 2000;90:310–4. doi: 10.1002/(SICI)1096-8628(20000214)90:4<310::AID-AJMG9>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 5.Rix S, Calmont A, Scambler PJ, Beales PL. An Ift80 mouse model of short rib polydactyly syndromes shows defects in hedgehog signalling without loss or malformation of cilia. Hum Mol Genet. 2011;20:1306–14. doi: 10.1093/hmg/ddr013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dagoneau N, Goulet M, Geneviève D, Sznajer Y, Martinovic J, Smithson S, Huber C, Baujat G, Flori E, Tecco L, et al. DYNC2H1 mutations cause asphyxiating thoracic dystrophy and short rib-polydactyly syndrome, type III. Am J Hum Genet. 2009;84:706–11. doi: 10.1016/j.ajhg.2009.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J, et al. IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet. 2007;39:727–9. doi: 10.1038/ng2038. [DOI] [PubMed] [Google Scholar]
- 8.Bredrup C, Saunier S, Oud MM, Fiskerstrand T, Hoischen A, Brackman D, Leh SM, Midtbø M, Filhol E, Bole-Feysot C, et al. Ciliopathies with skeletal anomalies and renal insufficiency due to mutations in the IFT-A gene WDR19. Am J Hum Genet. 2011;89:634–43. doi: 10.1016/j.ajhg.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shokeir MH, Houston CS, Awen CF. Asphyxiating thoracic chondrodystrophy. Association with renal disease and evidence for possile heterozygous expression. J Med Genet. 1971;8:107–12. doi: 10.1136/jmg.8.1.107. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitley CB, Schwarzenberg SJ, Burke BA, Freese DK, Gorlin RJ. Direct hyperbilirubinemia and hepatic fibrosis: a new presentation of Jeune syndrome (asphyxiating thoracic dystrophy) Am J Med Genet Suppl. 1987;3:211–20. doi: 10.1002/ajmg.1320280525. [DOI] [PubMed] [Google Scholar]
- 11.Yerian LM, Brady L, Hart J. Hepatic manifestations of Jeune syndrome (asphyxiating thoracic dystrophy) Semin Liver Dis. 2003;23:195–200. doi: 10.1055/s-2003-39950. [DOI] [PubMed] [Google Scholar]
- 12.Pepicelli CV, Lewis PM, McMahon AP. Sonic hedgehog regulates branching morphogenesis in the mammalian lung. Curr Biol. 1998;8:1083–6. doi: 10.1016/S0960-9822(98)70446-4. [DOI] [PubMed] [Google Scholar]
- 13.Miller L-AD, Wert SE, Clark JC, Xu Y, Perl A-KT, Whitsett JA. Role of Sonic hedgehog in patterning of tracheal-bronchial cartilage and the peripheral lung. Dev Dyn. 2004;231:57–71. doi: 10.1002/dvdy.20105. [DOI] [PubMed] [Google Scholar]
- 14.Loscertales M, Mikels AJ, Hu JK-H, Donahoe PK, Roberts DJ. Chick pulmonary Wnt5a directs airway and vascular tubulogenesis. Development. 2008;135:1365–76. doi: 10.1242/dev.010504. [DOI] [PubMed] [Google Scholar]
- 15.Ashe A, Butterfield NC, Town L, Courtney AD, Cooper AN, Ferguson C, Barry R, Olsson F, Liem KF, Jr., Parton RG, et al. Mutations in mouse Ift144 model the craniofacial, limb and rib defects in skeletal ciliopathies. Hum Mol Genet. 2012;21:1808–23. doi: 10.1093/hmg/ddr613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deutsch G, Jung J, Zheng M, Lóra J, Zaret KS. A bipotential precursor population for pancreas and liver within the embryonic endoderm. Development. 2001;128:871–81. doi: 10.1242/dev.128.6.871. [DOI] [PubMed] [Google Scholar]
- 17.Hirose Y, Itoh T, Miyajima A. Hedgehog signal activation coordinates proliferation and differentiation of fetal liver progenitor cells. Exp Cell Res. 2009;315:2648–57. doi: 10.1016/j.yexcr.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 18.Sicklick JK, Li Y-X, Melhem A, Schmelzer E, Zdanowicz M, Huang J, Caballero M, Fair JH, Ludlow JW, McClelland RE, et al. Hedgehog signaling maintains resident hepatic progenitors throughout life. Am J Physiol Gastrointest Liver Physiol. 2006;290:G859–70. doi: 10.1152/ajpgi.00456.2005. [DOI] [PubMed] [Google Scholar]
- 19.Ochoa B, Syn W-K, Delgado I, Karaca GF, Jung Y, Wang J, Zubiaga AM, Fresnedo O, Omenetti A, Zdanowicz M, et al. Hedgehog signaling is critical for normal liver regeneration after partial hepatectomy in mice. Hepatology. 2010;51:1712–23. doi: 10.1002/hep.23525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grzelak CA, Martelotto LG, Sigglekow ND, Patkunanathan B, Ajami K, Calabro SR, Dwyer BJ, Tirnitz-Parker JEE, Neil Watkins D, Warner FJ, et al. The Intrahepatic Signalling Niche of Hedgehog is Defined by Primary Cilia Positive Cells During Chronic Liver Injury. J Hepatol. 2014;60:143–51. doi: 10.1016/j.jhep.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 21.Philips GM, Chan IS, Swiderska M, Schroder VT, Guy C, Karaca GF, Moylan C, Venkatraman T, Feuerlein S, Syn W-K, et al. Hedgehog signaling antagonist promotes regression of both liver fibrosis and hepatocellular carcinoma in a murine model of primary liver cancer. PLoS One. 2011;6:e23943. doi: 10.1371/journal.pone.0023943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omenetti A, Diehl AM. The adventures of sonic hedgehog in development and repair. II. Sonic hedgehog and liver development, inflammation, and cancer. Am J Physiol Gastrointest Liver Physiol. 2008;294:G595–8. doi: 10.1152/ajpgi.00543.2007. [DOI] [PubMed] [Google Scholar]
- 23.Omenetti A, Diehl AM. Hedgehog signaling in cholangiocytes. Curr Opin Gastroenterol. 2011;27:268–75. doi: 10.1097/MOG.0b013e32834550b4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Syn W-K, Jung Y, Omenetti A, Abdelmalek M, Guy CD, Yang L, Wang J, Witek RP, Fearing CM, Pereira TA, et al. Hedgehog-mediated epithelial-to-mesenchymal transition and fibrogenic repair in nonalcoholic fatty liver disease. Gastroenterology. 2009;137:1478–, e8. doi: 10.1053/j.gastro.2009.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology. 2006;131:911–20. doi: 10.1053/j.gastro.2006.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergmann C, Weiskirchen R. It’s not all in the cilium, but on the road to it: genetic interaction network in polycystic kidney and liver diseases and how trafficking and quality control matter. J Hepatol. 2012;56:1201–3. doi: 10.1016/j.jhep.2011.10.014. [DOI] [PubMed] [Google Scholar]
- 27.Davey MG, Paton IR, Yin Y, Schmidt M, Bangs FK, Morrice DR, Smith TG, Buxton P, Stamataki D, Tanaka M, et al. The chicken talpid3 gene encodes a novel protein essential for Hedgehog signaling. Genes Dev. 2006;20:1365–77. doi: 10.1101/gad.369106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yin Y, Bangs F, Paton IR, Prescott A, James J, Davey MG, Whitley P, Genikhovich G, Technau U, Burt DW, et al. The Talpid3 gene (KIAA0586) encodes a centrosomal protein that is essential for primary cilia formation. Development. 2009;136:655–64. doi: 10.1242/dev.028464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macrae VE, Davey MG, McTeir L, Narisawa S, Yadav MC, Millan JL, Farquharson C. Inhibition of PHOSPHO1 activity results in impaired skeletal mineralization during limb development of the chick. Bone. 2010;46:1146–55. doi: 10.1016/j.bone.2009.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cruz C, Ribes V, Kutejova E, Cayuso J, Lawson V, Norris D, Stevens J, Davey M, Blight K, Bangs F, et al. Foxj1 regulates floor plate cilia architecture and modifies the response of cells to sonic hedgehog signalling. Development. 2010;137:4271–82. doi: 10.1242/dev.051714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bangs F, Antonio N, Thongnuek P, Welten M, Davey MG, Briscoe J, Tickle C. Generation of mice with functional inactivation of talpid3, a gene first identified in chicken. Development. 2011;138:3261–72. doi: 10.1242/dev.063602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andersen JS, Wilkinson CJ, Mayor T, Mortensen P, Nigg EA, Mann M. Proteomic characterization of the human centrosome by protein correlation profiling. Nature. 2003;426:570–4. doi: 10.1038/nature02166. [DOI] [PubMed] [Google Scholar]
- 33.Stephen LA, Davis GM, McTeir KE, James J, McTeir L, Kierans M, Bain A, Davey MG. Failure of centrosome migration causes a loss of motile cilia in talpid(3) mutants. Dev Dyn. 2013;242:923–31. doi: 10.1002/dvdy.23980. [DOI] [PubMed] [Google Scholar]
- 34.Maina JN, Madan AK, Alison B. Expression of fibroblast growth factor-2 (FGF-2) in early stages (days 3-11) of the development of the avian lung, Gallus gallus variant domesticus: an immunocytochemical study. J Anat. 2003;203:505–12. doi: 10.1046/j.1469-7580.2003.00236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Vähätalo R, Asikainen TM, Karikoski R, Kinnula VL, White CW, Andersson S, Heikinheimo M, Myllärniemi M. Expression of Transcription Factor GATA-6 in Alveolar Epithelial Cells Is Linked to Neonatal Lung Disease. Neonatology. 2011;99:231–40. doi: 10.1159/000317827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clotman F, Libbrecht L, Killingsworth MC, Loo CCK, Roskams T, Lemaigre FP. Lack of cilia and differentiation defects in the liver of human foetuses with the Meckel syndrome. Liver Int. 2008;28:377–84. doi: 10.1111/j.1478-3231.2007.01617.x. [DOI] [PubMed] [Google Scholar]
- 37.Gleghorn JP, Kwak J, Pavlovich AL, Nelson CM. Inhibitory morphogens and monopodial branching of the embryonic chicken lung. Dev Dyn. 2012;241:852–62. doi: 10.1002/dvdy.23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sakiyama J, Yokouchi Y, Kuroiwa A. Coordinated expression of Hoxb genes and signaling molecules during development of the chick respiratory tract. Dev Biol. 2000;227:12–27. doi: 10.1006/dbio.2000.9880. [Available from] [DOI] [PubMed] [Google Scholar]
- 39.Cardoso WV, Lü J. Regulation of early lung morphogenesis: questions, facts and controversies. Development. 2006;133:1611–24. doi: 10.1242/dev.02310. [DOI] [PubMed] [Google Scholar]
- 40.Maeda Y, Davé V, Whitsett JA. Transcriptional control of lung morphogenesis. Physiol Rev. 2007;87:219–44. doi: 10.1152/physrev.00028.2006. [DOI] [PubMed] [Google Scholar]
- 41.Davey MG, Tickle C. The chicken as a model for embryonic development. Cytogenet Genome Res. 2007;117:231–9. doi: 10.1159/000103184. [DOI] [PubMed] [Google Scholar]
- 42.Miller LA, Wert SE, Whitsett JA. Immunolocalization of sonic hedgehog (Shh) in developing mouse lung. J Histochem Cytochem. 2001;49:1593–604. doi: 10.1177/002215540104901213. [DOI] [PubMed] [Google Scholar]
- 43.Weaver M, Batts L, Hogan BL. Tissue interactions pattern the mesenchyme of the embryonic mouse lung. Dev Biol. 2003;258:169–84. doi: 10.1016/S0012-1606(03)00117-9. [DOI] [PubMed] [Google Scholar]
- 44.Zhang M, Wang H, Teng H, Shi J, Zhang Y. Expression of SHH signaling pathway components in the developing human lung. Histochem Cell Biol. 2010;134:327–35. doi: 10.1007/s00418-010-0738-2. [DOI] [PubMed] [Google Scholar]
- 45.Litingtung Y, Lei L, Westphal H, Chiang C. Sonic hedgehog is essential to foregut development. Nat Genet. 1998;20:58–61. doi: 10.1038/1717. [DOI] [PubMed] [Google Scholar]
- 46.Motoyama J, Liu J, Mo R, Ding Q, Post M, Hui CC. Essential function of Gli2 and Gli3 in the formation of lung, trachea and oesophagus. Nat Genet. 1998;20:54–7. doi: 10.1038/1711. [DOI] [PubMed] [Google Scholar]
- 47.Roberts DJ, Johnson RL, Burke AC, Nelson CE, Morgan BA, Tabin C. Sonic hedgehog is an endodermal signal inducing Bmp-4 and Hox genes during induction and regionalization of the chick hindgut. Development. 1995;121:3163–74. doi: 10.1242/dev.121.10.3163. [DOI] [PubMed] [Google Scholar]
- 48.Li C, Hu L, Xiao J, Chen H, Li JT, Bellusci S, Delanghe S, Minoo P. Wnt5a regulates Shh and Fgf10 signaling during lung development. Dev Biol. 2005;287:86–97. doi: 10.1016/j.ydbio.2005.08.035. [DOI] [PubMed] [Google Scholar]
- 49.Min H, Danilenko DM, Scully SA, Bolon B, Ring BD, Tarpley JE, DeRose M, Simonet WS. Fgf-10 is required for both limb and lung development and exhibits striking functional similarity to Drosophila branchless. Genes Dev. 1998;12:3156–61. doi: 10.1101/gad.12.20.3156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis KE, Drossopoulou G, Paton IR, Morrice DR, Robertson KE, Burt DW, Ingham PW, Tickle C. Expression of ptc and gli genes in talpid3 suggests bifurcation in Shh pathway. Development. 1999;126:2397–407. doi: 10.1242/dev.126.11.2397. [DOI] [PubMed] [Google Scholar]
- 51.Buxton P, Davey MG, Paton IR, Morrice DR, Francis-West PH, Burt DW, Tickle C. Craniofacial development in the talpid3 chicken mutant. Differentiation. 2004;72:348–62. doi: 10.1111/j.1432-0436.2004.07207006.x. [DOI] [PubMed] [Google Scholar]
- 52.Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MA, Keighren M, Bahlo M, Bromhead CJ, Budd P, et al. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet. 2011;88:508–15. doi: 10.1016/j.ajhg.2011.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kajantie E, Andersson S, Kaitila I. Familial asphyxiating thoracic dysplasia: clinical variability and impact of improved neonatal intensive care. J Pediatr. 2001;139:130–3. doi: 10.1067/mpd.2001.114701. [DOI] [PubMed] [Google Scholar]
- 54.Gunay-Aygun M. Liver and kidney disease in ciliopathies. Am J Med Genet C Semin Med Genet. 2009;151C:296–306. doi: 10.1002/ajmg.c.30225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.de Vries J, Yntema JL, van Die CE, Crama N, Cornelissen EA, Hamel BC. Jeune syndrome: description of 13 cases and a proposal for follow-up protocol. Eur J Pediatr. 2010;169:77–88. doi: 10.1007/s00431-009-0991-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Oberklaid F, Danks DM, Mayne V, Campbell P. Asphyxiating thoracic dysplasia. Clinical, radiological, and pathological information on 10 patients. Arch Dis Child. 1977;52:758–65. doi: 10.1136/adc.52.10.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Waters AM, Beales P. Bardet–Biedl and Jeune Syndromes. Fibrocystic Diseases of the Liver. 2010: 257-285. [Google Scholar]
- 58.Doherty D. Joubert syndrome: insights into brain development, cilium biology, and complex disease. Semin Pediatr Neurol. 2009;16:143–54. doi: 10.1016/j.spen.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sokol RJ, Mack CL. Biliary atresia and the ductal plate. In: Gastroenterology: Fibrocystic Diseases of the Liver, Murray KF and Larson AM (eds.). Humana Press: 2010, 179-200 [Google Scholar]
- 60.Norris DP. Cilia, calcium and the basis of left-right asymmetry. BMC Biol. 2012;10:102. doi: 10.1186/1741-7007-10-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Omenetti A, Bass LM, Anders RA, Clemente MG, Francis H, Guy CD, McCall S, Choi SS, Alpini G, Schwarz KB, et al. Hedgehog activity, epithelial-mesenchymal transitions, and biliary dysmorphogenesis in biliary atresia. Hepatology. 2011;53:1246–58. doi: 10.1002/hep.24156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shimadera S, Iwai N, Deguchi E, Kimura O, Fumino S, Yokoyama T. The inv mouse as an experimental model of biliary atresia. J Pediatr Surg. 2007;42:1555–60. doi: 10.1016/j.jpedsurg.2007.04.018. [DOI] [PubMed] [Google Scholar]
- 63.Cui S, Leyva-Vega M, Tsai EA, EauClaire SF, Glessner JT, Hakonarson H, Devoto M, Haber BA, Spinner NB, Matthews RP. Evidence from human and zebrafish that GPC1 is a biliary atresia susceptibility gene. Gastroenterology. 2013;144:1107–, e3. doi: 10.1053/j.gastro.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang BQ, Masyuk TV, Muff MA, Tietz PS, Masyuk AI, Larusso NF. Isolation and characterization of cholangiocyte primary cilia. Am J Physiol Gastrointest Liver Physiol. 2006;291:G500–9. doi: 10.1152/ajpgi.00064.2006. [DOI] [PubMed] [Google Scholar]
- 65.Strazzabosco M, Somlo S. Polycystic liver diseases: congenital disorders of cholangiocyte signaling. Gastroenterology. 2011;140:1855–9, e1. doi: 10.1053/j.gastro.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hamburger V, Hamilton HL. A series of normal stages in the development of the chick embryo. J Morphol. 1951;88:49–92. doi: 10.1002/jmor.1050880104. [DOI] [PubMed] [Google Scholar]
- 67.Acloque H, Wilkinson DG, Nieto MA. In Situ Hybridization Analysis of Chick Embryos in Whole Mount and Tissue Sections. Methods Cell Biol. 2008;87:169–85. doi: 10.1016/S0091-679X(08)00209-4. [DOI] [PubMed] [Google Scholar]
- 68.Dunn IC, Paton IR, Clelland AK, Sebastian S, Johnson EJ, McTeir L, Windsor D, Sherman A, Sang H, Burt DW, et al. The chicken polydactyly (Po) locus causes allelic imbalance and ectopic expression of Shh during limb development. Dev Dyn. 2011;240:1163–72. doi: 10.1002/dvdy.22623. [DOI] [PubMed] [Google Scholar]
- 69.Boardman PE, Sanz-Ezquerro J, Overton IM, Burt DW, Bosch E, Fong WT, Tickle C, Brown WR, Wilson SA, Hubbard SJ. A comprehensive collection of chicken cDNAs. Curr Biol. 2002;12:1965–9. doi: 10.1016/S0960-9822(02)01296-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.