Abstract
Purpose: To evaluate the morphological and histological changes induced by PGA scaffold seeded with autologous adipose or muscle derived stem cells implanted on rabbit bladder wall. Material and Methods: Adipose derived stem cells (ADSCs) were obtained from the inguinal fat of eight rabbits and muscle derived stem cells (MDSCs) from the anterior tibial muscle of other eight rabbits. After culture and isolation, the cells were stained with Vybrant Red CM DiI and then implanted at third passage. Two PGA scaffolds were implanted on the bladder submucosa of each animal. On the right bladder side was implanted unseeded PGA scaffold while on the left side was implanted ADSCs or skeletal MDSCs seeded PGA scaffold. ADSCs were implanted in eight animals and MDSC in other eight animals. The animals were sacrificed at four and eight weeks. Histological evaluation was performed with Hematoxylin and Eosin, Masson's Trichrome and smooth muscle α-actin. Results: We observed a mild inflammatory response in all the three groups. Seeded scaffolds induced higher lymphocytes and lower polimorphonuclear migration than controls. Fibrosis was more pronounced in the control groups. Smooth muscle α-actin was positive only in ADSC and MDSC seeded scaffolds. At four and eight weeks ADCSs and skeletal MDSCs labeled cells were found at the implant sites. Conclusions: The implantation of PGA scaffolds seeded with ADSC and MDSC induced less fibrosis than control and smooth muscle regeneration.
Keywords: adipocyte derived stem cells, muscle derived stem cells, PGA scaffold, rabbit bladder
Introduction
Bladder disease affects 400 million individuals worldwide with 1 in every 1000 newborns presenting spinal dysraphism, and more than 50 000 Americans diagnosed annually with bladder cancer. Gastrointestinal tissue is the gold standard when tissue is required for urinary diversion or bladder augmentation; however, the interposition of gastrointestinal segments on the urinary tract can be associated with long-term complications, such as mucous production, metabolic disturbance, and cancer.1
Advances in biomaterial science have led to a flurry of synthetic materials to augment, repair or replace the native tissues. In this context, tissue engineering represents one of the newest innovations in modern-day science and explores the possibility of growing biological substitutes to repair damaged tissue. The premise of this method is that cells can be expanded in vitro, seeded on a scaffold, and then implanted into the host.2
Acellular tissue grafts and synthetic engineered materials have been experimentally used to augment the bladder; however, the results were limited by graft fibrosis, stone formation, and graft failure. Likewise, tissue engineered grafts have been created using host bladder cells to overcome these limitations but the use of bladder cells also entails limitations. Cells from diseased bladders seem to retain their pathological features in vitro.3
The ideal biomaterial should be biodegradable and provide an adequate environment to promote good cell integration and cellular/matrix self-regulation. Polymers such as polyglycolic-acid (PGA) have been widely used in tissue engineering because of their chemical and mechanical properties. They have hydrolytically labile ester-bonds and are degraded by non-enzymatic hydrolysis. The degradation metabolites are non-toxic and eliminated from the body as carbon dioxide and water. Since these polymers are thermoplastics, they can be easily manufactured according to different tissue specifications.4
Adult stem cells are responsible for tissue homeostasis maintenance and integrity. These cells can be found in different tissues, and remain in a quiescent state in specific niches. Once the tissue in injured they are recruited to provide cell replacement to restore the tissue integrity. Mesenchymal stem cells (MSC) are a heterogeneous subset of stromal stem cells that can be isolated from adult tissues and are capable of differentiating into cells of the mesodermal lineage. While reports have shown the ability of MSC to engraft tissues such as lung, liver, heart, and brain, data are still scarce for bladder tissue regeneration.4-6
In this study we investigated the morphological and histological changes induced by PGA scaffolds seeded with autologous ADSC or skeletal MDSC when implanted on rabbit bladder submucosal layer.
Results
The fat and muscle biopsies were harmless and any early or late post-operative complications were observed at four and eight weeks. Three grams of adipose tissue were harvested to obtain approximately 3 × 106 adipose derived stem cells two weeks later. Three grams of skeletal muscle were harvested to obtain 3 × 106 muscle derived stem cells four weeks later.
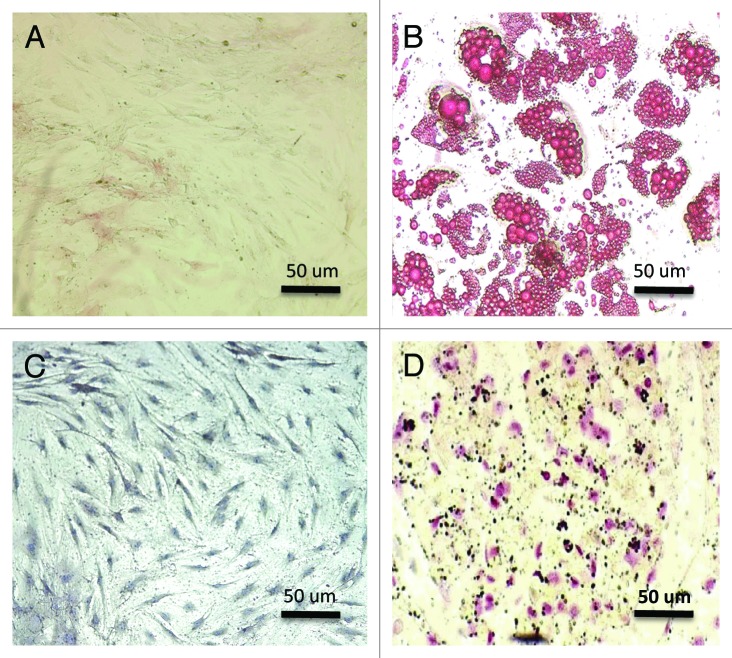
Enrichment of microchimeric cells from bulk populationsspecific inductive media, either ADSCs or MDSCs differentiated into osteogenic and adipogenic tissues. Von Kossa staining showed mineralization in certain areas and Oil-Red-O showed adipose vesicle formation. Figure 1 represents Von Kossa and Oil-Red-O staining.
Figure 1. (A and D) The mesenquimal stem cells (ADSCs and MDSCs) appeared elliptical and fusiform before differentiation. (A) ADSCs before differentiation (200×). (B) Oil red O staining of ADSCs after differentiation demonstrating adipose vesicles (200×). (C) MDSCs before differentiation (200×). (D) Von Kossa staining of MDSCs after differentiation demonstrating calcium deposition (200×).
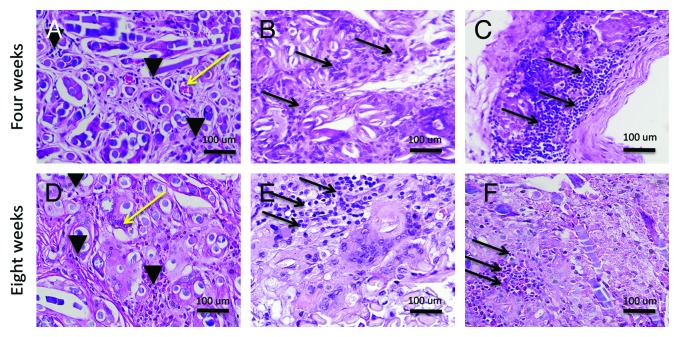
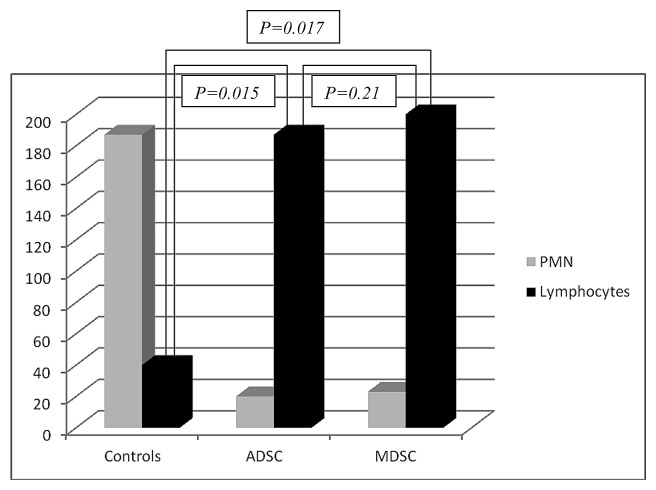
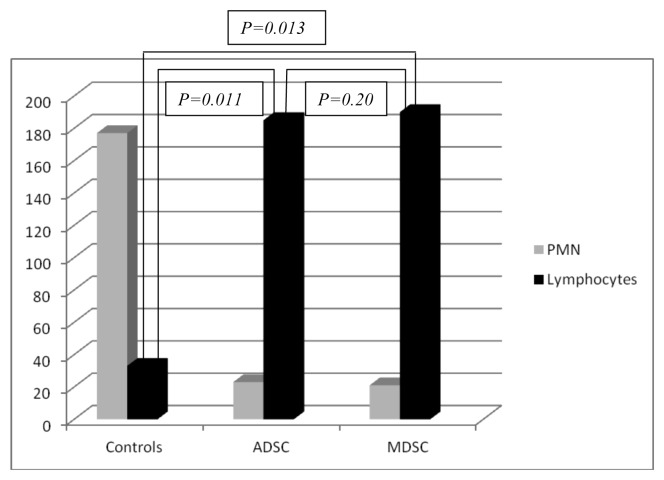
The local inflammatory reaction was mild in the three groups (ADSC, MDSC, and controls) at four and eight weeks. In the control group (right bladder) there was higher polymorphonuclear (PMN) and lower lymphocyte migration than ADSCs and MDSCs seeded scaffolds. (P = 0.015 and 0.017 for ADSCs and MDSCs respectively at four weeks; and P = 0.011 and 0.013 for ADSCs and MDSCs respectively at eight weeks). Figure 2 represents the local inflammatory reaction in the three groups. No differences were observed at four and eight weeks between ADSCs and MDSCs seeded scaffolds (P = 0.21 at four weeks and P = 0.20 at eight weeks). Charts 1 and 2 represent the quantification of local inflammatory in control and seeded scaffolds.
Figure 2. Local inflammatory reaction. (A) H&E staining of unseeded PGA scaffold (400×). (B) H&E staining of ADSCs seeded scaffold (400×). (C) H&E staining of MDSCs seeded scaffold (400×). (D) H&E staining of unseeded PGA scaffold (400×). (E) H&E staining of ADSCs seeded scaffold (400×). (F) H&E staining of MDSCs seeded scaffold (400×). Black arrowheads, polimorphonuclear cells; black arrows, lymphocytes; yellow arrow, blood vessels.
Chart 1. Local inflammatory response at four weeks. PMN, polymorphonuclear cells.
Chart 2. Local inflammatory response at eight weeks. PMN, polymorphonuclear cells.
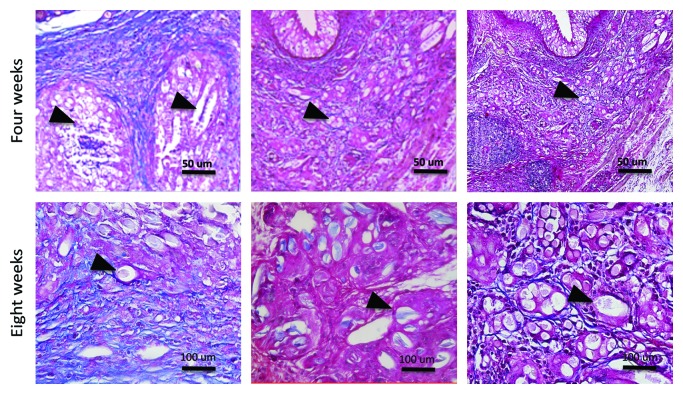
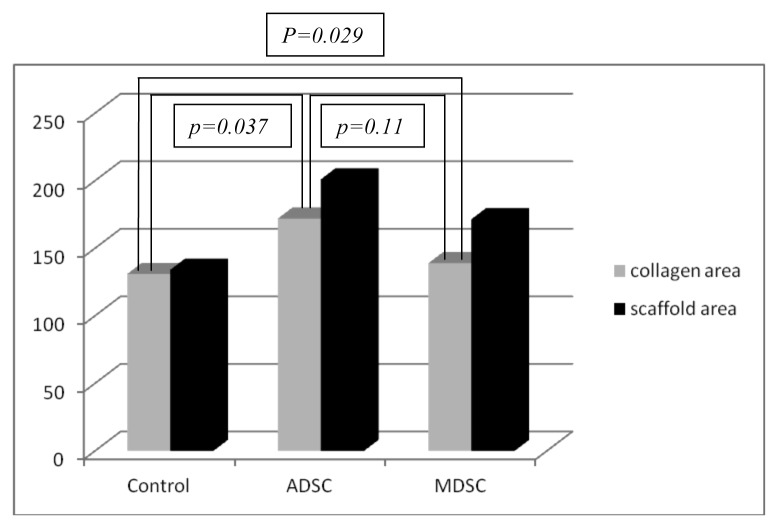
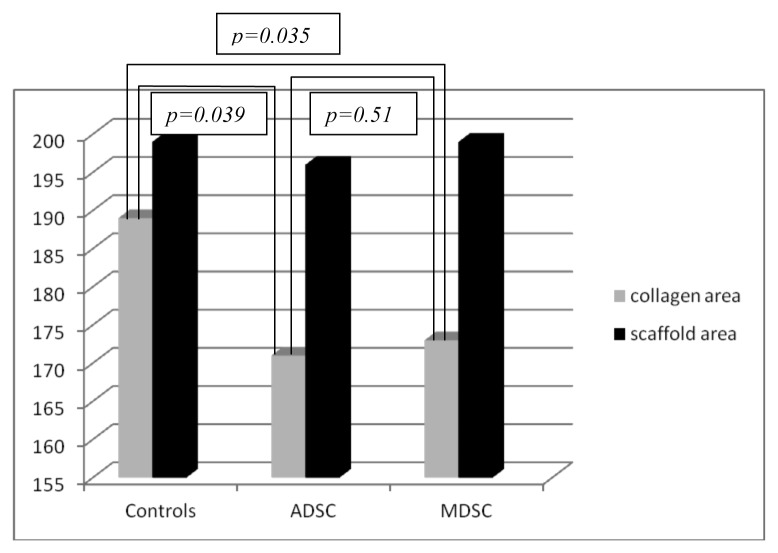
The fibrosis was more pronounced in unseeded scaffolds whether compared to seeded scaffolds (P = 0.037 and 0.029 for ADSCs and MDSCs respectively at four weeks; P = 0.039 and 0.035 for ADSCs and MDSCs respectively at eight weeks). Figure 3 represents Masson’s Trychrome in unseeded and seeded scaffolds. No differences in fibrosis were observed between ADSCs and MDSCs seeded scaffolds. (P = 0.11 at four weeks and P = 0.51 at eight weeks). Charts 3 and 4 represent the fibrosis quantification in the three groups.
Figure 3. Characterization of fibrosis at the implant sites. (A) Masson’s Trychrome staining of control group (200×). (B) Masson’s Trychrome staining of ADSCs seeded scaffold (200×). (C) Masson’s Trychrome staining of MDSCs seeded scaffold (200×). (D) Masson’s Trychrome staining of control group (400×). (E) Masson’s Trychrome staining of ADSCs seeded scaffold (400×). (F) Masson’s Trychrome staining of MDSCs seeded scaffold (400×). Blue, collagen; black arrowheads, PGA scaffold.
Chart 3. Correlation between collagen formation and scaffold area in the three groups at four weeks.
Chart 4. Correlation between collagen formation and scaffold area in the three groups at eight weeks.
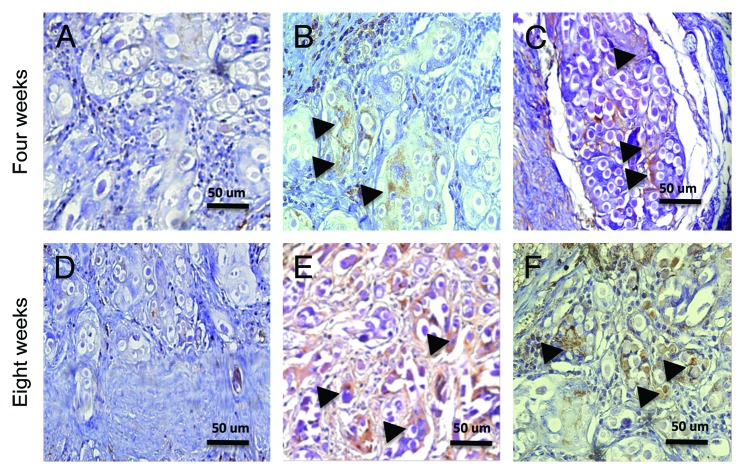
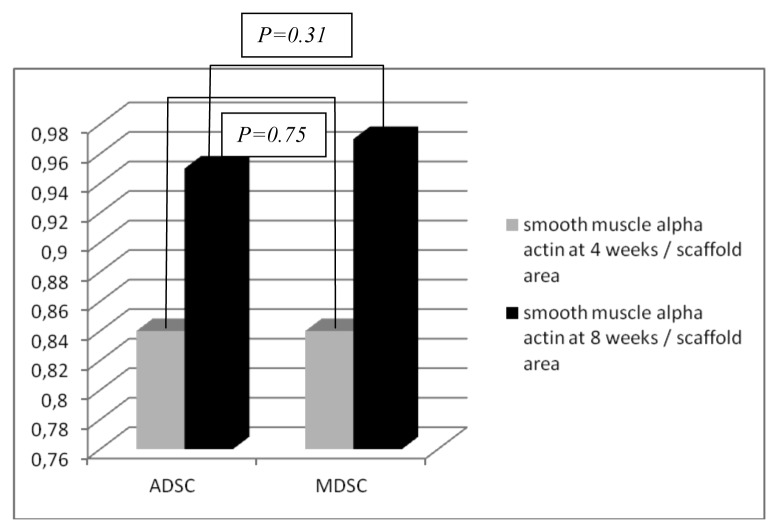
The smooth muscle α-actin was positive on ADSCs and MDSCs seeded scaffolds at the implant site. Control scaffolds did not express smooth muscle α-actin. Figure 4 represents smooth muscle α-actin in the three groups. In a comparative analysis, there were no significant differences between ADSCs and MDSCs seeded scaffolds (P = 0.75 at four weeks and P = 0.31 at eight weeks). Chart 5 represents the smooth muscle α-actin quantification in ADSCs and MDSCs seeded scaffolds.
Figure 4. Characterization of the smooth muscle alpha-actin on the implant sites. (A and D) Unseeded scaffold, control group (200×). (B, E) ADSCs seeded scaffold (200×). (C, F) MDSCs seeded scaffold (200×). Brown color, smooth muscle alpha-actin; arrowheads, cells.
Chart 5. Smooth muscle alpha-actin area and scaffold area in the ADSC and MDSC seeded scaffolds at four and eight weeks.
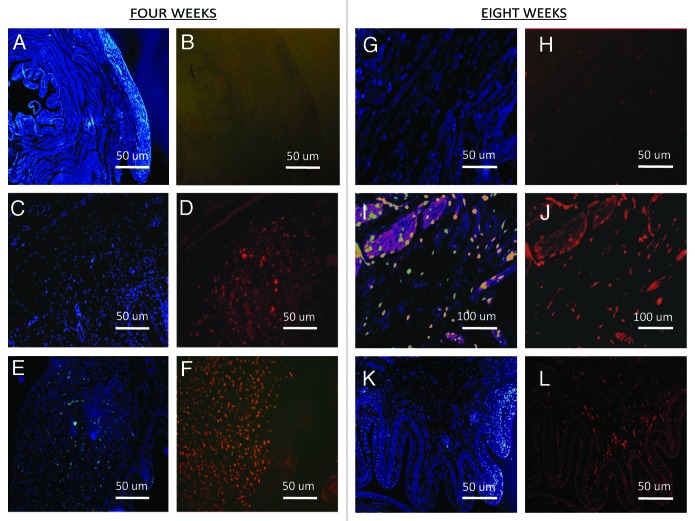
ADSCs and MDSCs labeled cells with Vybrant Red CM DiI were found at four and eight weeks at the implant sites. Figure 5 represents ADSCs and MDSCs labeled with Vybrant CM-DiI Based on our findings we cannot conclude whether the Vybrant CM-DiI positive cells and smooth muscle α-actin positive cells are the same cells.
Figure 5. Cell procurement on the implant sites. (A, B, G, H, K, and L) DAPI and Vybrant CM-Dil staining of control scaffolds (200×). (C and D) DAPI and Vybrant CM-Dil staining of ADSC seeded scaffolds (200×). (E and F) DAPI and Vybrant CM-Dil staining of MDSC seeded scaffolds (200×). (I, J) DAPI and Vybrant CM-Dil staining of ADSC seeded scaffolds (400×).
Discussion
To our knowledge this is the first study to investigate the behavior of ADSCs and MDSCs when transplanted onto synthetic scaffolds on the rabbit’s bladder submucosal layer. It is a proof of concept for future bladder reconstruction using synthetic scaffolds and autologous adult stem cells. The seeded and unseeded PGA scaffold induced a mild localized inflammatory response when implanted on rabbit submucosal bladder. Cell seeded scaffolds stimulated higher lymphocyte migration and less fibrosis than unseeded scaffolds. The smooth muscle α-actin was positive only in the seeded scaffolds. We could find the implanted stem cells four and eight weeks later.
Since mesenchymal stem cells are a self-renewing population, they carry the potential of differentiating into multiple cell types. Adult stem cells that can differentiate into mesodermal tissues, such as adipose, cartilage, bone and smooth muscle, have been identified in the adipose tissue and skeletal muscle. These cells are easily harvested with little morbidity, creating tissue abundance, making them promising candidates for tissue regeneration. Furthermore, their ability to differentiate into smooth muscle cells as well as vascular and neurological structures makes them a potential source of cells for genitourinary regenerative medicine.2,5,8
There is a lack of studies correlating the immune response, stem cells, and bladder regeneration.9,10 It has been demonstrated that MSC inhibit the action of T, B and natural killer cells through a paracrine secretion mechanism. Such effects can be observed in experimental studies in which neural, pulmonary and renal injuries were improved using intravenous administration of MSCs. Almost 70% of the injected MSCs are retained in the lungs. Once in the blood stream, the MSCs are attracted to the injured organs through the activation of specific receptors that facilitate cell migration and adhesion. Evren and colleagues have shown that autologous acellular bladder implants induced migration of TH-2 lymphocytes, increased IL-4, and decreased TGF β-1 production. When these matrixes were treated with hyaluronic acid or vascular endothelium growth factor (VEGF), the lymphocytes migration was reduced and the TGF β-1 production was increased.10,11
Several cell sources have been used for cell therapy, including adult stem cells, embryonic stem cells, induced pluripotent stem cells, etc. Studies have shown that ADSCs and MDSCs stimulate pro-angiogenic cytokines such as hepatocytic growth factor (HGF), TGF-β, VEGF, fibroblastic growth factor type 2, angiopoietin-1, placenta growth factor, and play an essential part in the antifibrosis effect. Among the growth factors, HGF plays an essential part in the angiogenesis and regeneration of the tissue, and act as a potent antifibrotic agent. We believe that angiogenesis and fibrosis reduction in the seeded scaffold occurred due to paracrine mechanisms instead of cell incorporation.12-15
Vessels were observed within the scaffold fibers; however, we could not demonstrate differences between the seeded and unseeded scaffolds due to the small scaffold size. The unseeded scaffold implant created a defect that triggered a regenerative response, inducing the tissue needed to repair the defect possibly leading to cell migration and vascular formation. The role of the transplanted stem cells in the regeneration process is not clear. Theoretically, these cells could differentiate or induce a better regeneration response in the transplanted tissue.13
Zuk et al. have shown the presence of mesenchymal stem cells in the vascular fraction of human adipose tissue.14 These cells expressed actin, calponin, caldesmon, SM-22 and myosin heavy chain, then when they were exposed to the pharmacological agents, they were able to contract and relax.13 In another study, the same population of cells was obtained from human lipoaspiration and after being injected around the urethra of incontinent mice, they recovered the continence.2 In our study, we used ADSC derived from rabbit inguinal fat pad, because this region is easily accessible in rabbits and have a lot of adipose tissue. These cells expressed some mesenchymal stem cell markers and may have stimulated bladder smooth muscle regeneration.
Stem cells from the skeletal muscles also carry the potential to differentiate into multiple cell lines. These cells are easily obtained by biopsies conducted under local anesthesia. The MDSCs are morphologically very similar to the fibroblasts, and they merge to form myotubes and myofiber.6 In an animal model of urinary incontinence, MDSCs or fibroblasts were injected around the urethra and urodynamic improvement within four weeks was observed in the animals receiving MDSCs, while deterioration was observed in those receiving only fibroblasts.15 In 2008, results of eight women with urinary incontinence treated with periurethral injection of autologous MDSCs were published. Cells were obtained from thigh biopsies and 70% of participants became continent within one year.16 In our study, we used MDSCs harvested from the anterior tibial muscle. These cells also expressed some stem cell markers and may have stimulated bladder smooth muscle regeneration.
Sharma and collegues conducted a study using a synthetic polymer consisting of 1,8-octanediol-co-citrate to reconstruct bladder in animal model. After 10 wk, the implants seeded with bone marrow stem cells had positive immunohistochemical results for gamma human anti-tubulin, calponin, smooth muscle α actin and elastin.17
Atala et al. published the data from seven patients with myelomeningocele and end stage bladder disease undergoing bladder augmentation with homologous bladder acellular matrix or synthetic PGA scaffolds. These scaffolds were seeded with autologous smooth muscle and urothelium and after 46 wk of implantation the bladders remained viable with good efficacy and function.1 This study was a pioneer study regarding bladder regeneration in humans; however, the critical point is that they used autologous cells harvested from a disease bladder. In this context, MSCs are an attractive source of cells due to their high replicative capacity, self-renewal, and multilineage potential.
Regenerative medicine aims to reestablish structure and function of organs and tissues. The use of mesenchymal stem cells and the development of scaffolds are promising, but their clinical application is still under investigation. Regenerative centers around the world have been striving to translate the results of basic science into clinical studies. In this context, our study opens an attractive alternative for bladder regeneration using autologous stem cells.
This study has some limitations that should be considered. First of all, we implanted two scaffolds in the same bladder. Second, we used smooth muscle α-actin instead of more specific markers such as myosin heavy chain. Third, the scaffold size was small, which precludes a more detailed analysis. Fourth, we did not do any functional studies to evaluate the potential for bladder augmentation. Fifth, the follow up was short.
We believe that in the field of regenerative medicine there are many challenges to be achieved. The ultimate goal is to provide a feasible and reliable therapy for patients with bladder dysfunctions. Although there are many improvements on bladder regeneration, there are still many hurdles to be overcome. With a better comprehension of bladder physiology and stem cell biomolecular mechanisms, we will be able in the future to help many patients to have a better quality of life.
Material and Methods
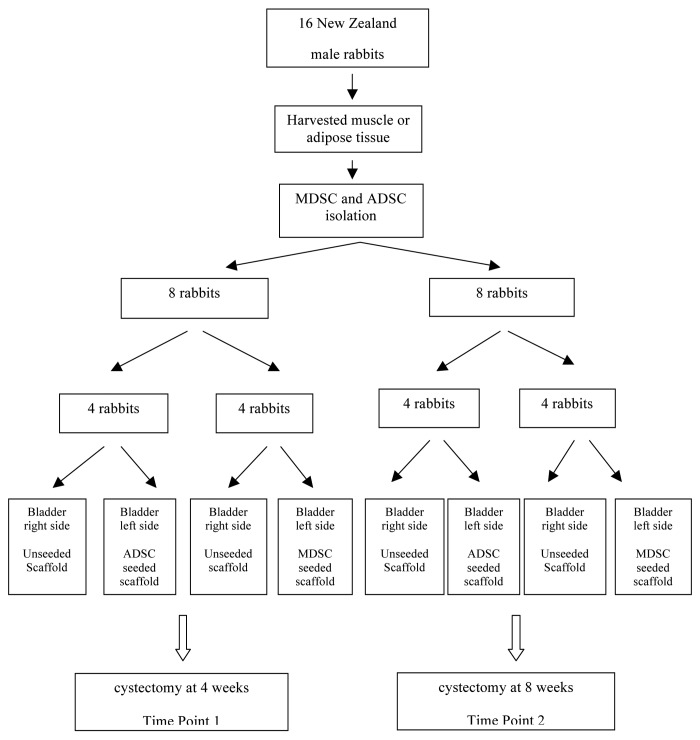
Animal studies were performed in accordance with guidelines set forth by the Animal Research Committee at our Institution. Sixteen New Zealand white male rabbits were used in this study. Two sections of previous embedded Type I bovine collagen PGA scaffolds were implanted on the submucosal bladder layer of each animal. On the left bladder side was implanted seeded PGA scaffold (ADSC or MDSC), whereas on the right bladder side was implanted unseeded PGA scaffold. Eight animals were sacrificed at four weeks and eight weeks respectively.
Study design
The full study design is diagrammed in Scheme 1. Eight male rabbits were anesthetized with 2% isoflurane, and then three grams of adipose tissue were obtained from the rabbit’s left inguinal area. After harvesting, fat was extensively washed with phosphate buffered saline solution (PBS), minced with surgical scissors, digested, and centrifuged at 5000 rpm for 5 min. The obtained pellet was incubated overnight in non-inductive medium consisting of Dulbecco’s Modified Eagle’s Medium (DMEM, Sigma-Aldrich), 10% fetal bovine serum (FBS; Gibco), and 1% antimicrobial solution (Penicillin G/ Amphotericin B/ Streptomycin) (Sigma–Aldrich). This isolated purified cell population, referred to as adipose derived stem cells (ADSCs), was seeded into 100 mm2 culture dishes at a density of 1 × 106 cells per plate and split routinely when they achieved 80% confluence.
Scheme 1. Culture of autologous adipose derived stem cells (ADSCs) and skeletal muscle derived stem cells (MDSCs) and flow cytometry analysis.
The other eight male rabbits were also anesthetized with 2% isoflurane, and then 3 g of skeletal muscle were biopsied from each rabbit’s left anterior tibial muscle. The MDSCs were prepared according to the protocol described by Qu-Peterson, et al.7 After harvesting, the connective tissues was removed and the muscle was minced using razor blades. The tissue was transferred into a 50 ml centrifugation tube, doubling the volume of collagenase type XI (Sigma-Aldrich), which was added and centrifuged for 1h. Temperature was kept constant at 37 °C and the enzymatic dissociation was performed on a shaking table set. The suspension was then centrifuged for 5 min at 5000 rpm and the supernatant was transferred into a fresh 15 ml tube. One volume of dispase (grade II, 2.4 U/ml, Roche, Basel, Switzerland) was added and allowed to dissociate for 30 min under the same conditions as the first digestion. The suspension was centrifuged for 5 min at 5000 rpm and the supernatant was discarded. The pellet was then resuspended with growth medium. After being filtered through a 75μm mesh, the cells were seeded in a 25cm2 culture flask and labeled as Pre-plate 1 (PP1). Cells were incubated at 37 °C with 5% CO2 for 24 h, and any cells that had not adhered to the flask were transferred to another culture flask and labeled as Pre-plate 2 (PP2). The same steps were repeated until Pre-plate 6 (PP6) was achieved. The cells from PP6 were cultured with DMEM low-glucose, 10% fetal bovine serum and 1% antimicrobial solution (Penicillin G/ Amphotericin B/ Streptomycin) (Sigma–Aldrich). They were seeded into 100 mm2 culture dishes at a density of 1 × 106 cells per plate and split routinely when they achieved 80% confluence.
For flow cytometry analysis, third passage ADSCs and MDSCs (1 × 106 cells) were incubated with 10 microliters of primary monoclonal antibodies coupled to fluorescein isothiocyanate (FITC) in 50 microliters of PBS for 30 min in the dark at room temperature. Then cells were washed twice with 1 ml PBS, resuspended, diluted in 200 microliters of PBS, and analyzed with FACScan flow cytometer (Becton-Dickinson). Antibodies for CD45, CD73, and CD90 were obtained from BD Biosciences. Furthermore, ADSCs and MDSCs were cultures in osteogenic and adipogenic inductive media to induce cell differentiation.
Cell labeling and tracking
Cultured ADSCs and MDSCs cells at passage 3 were rinsed and incubated with 1:200 dilution of dialkylcarbocyanine fluorescent solution (VybrantR DiI) in accordance with the manufacturer’s protocol, and then rinsed and cultured in control medium 24 h before bladder implantation. The cell viability was confirmed by Trypan blue staining.
Each PGA scaffold was seeded with 1 × 106 ADSCs or MDSCs on the same day of implantation.
Implantation of PGA scaffolds on rabbit’s submucosal bladder layer
The PGA scaffolds were manufactured with 0.3 × 0.7 × 0.1 cm and 10 µm porous size. Bladder implants were performed under general anesthesia with 2% isoflurane. A suprapubic incision was made and the rabbit bladder exposed. The detrusor dissection was performed with microsurgical instruments under optical magnification (3×). After exposure, the PGA scaffolds were implanted on the submucosal bladder layer and recovered by detrusor, which was stitched over the scaffold using separated Vicryl 5.0 suture. On the right bladder side it was implanted unseeded PGA scaffold, while on the left bladder side it was implanted ADSC or MDSC seeded PGA scaffold. In order to identify the implantation sites at the first and second time points (4 and 8 wk), two sutures of Nylon 6.0 were placed at 6 h and 12 h. Figure 6 represents the PGA scaffold on the bladder submucosa.
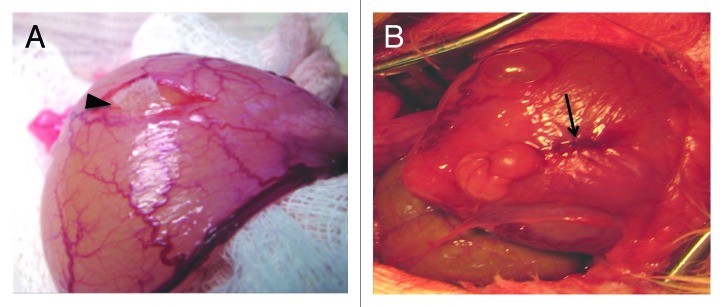
Figure 6. (A) Unseeded PGA scaffold implantation on the rabbit bladder submucosa. Black arrowhead: unseeded PGA scaffold. (B) Detrusor stitched over the PGA scaffold (black arrow).
Tissue procurement and sample analysis
Cystectomy was performed under general anesthesia with 2% isoflurane four or eight weeks later according to the protocol. The bladder was separated from the urethra at the bladder neck; the implantation site was identified and then sectioned transversely. The samples were fixed in formalin and embedded in paraffin. Five microns tissue sections were performed for histological examination. Excised tissues were analyzed for the presence of ADSC and MDSC cells. All slides were imaged using a color digital camera along with imaging software Image-pro plus (Media Cybernetics Inc.). Localization of Vybrant DiI labeled ADSCs or MDSCs was performed using an epifluorescence microscope (Carl Zeiss) filtered for excitation/emission at 546/590 nm. Cell nuclei were counterstained with DAPI (4,6-diamidino-2-phenylindole).
Local inflammatory response assessment
Local inflammatory response was assessed at the implant`s site using Hematoxylin and Eosin staining. Lymphocytes and polymorphonuclear cells were identified and counted using a manual cell counter (Digitimer). Twenty randomized microscopic fields with 400× optical magnification at the implant site were considered for statistical analysis in each specimen.
Quantification of fibrosis and smooth muscle at the implantation site
In order to quantify the fibrosis at the implant site we used the software Image-Pro plus version 6.0 for Windows. (Media Cybernetics Inc.).
Fibrosis was evaluated using Massom’s Trichrome staining. Statistical analysis was calculated using the ratio between fibrosis and the scaffold area on 20 randomized microscopic fields with 200× optical magnification.
The quantification of smooth muscle α-actin was performed at the implant site with the same software. Statistical analysis was calculated using the ratio between smooth muscle α-actin and scaffold area on 20 randomized microscopic fields with 200× optical magnification.
Statistical analysis
Statistical analysis was performed with the Statistical Package for Social Sciences (IBM Corporation). Statistical significance was established at the 5% level and 95% confidence interval. Kruskall-Wallis Test and Bonferroni Test were used.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006;367:1241–6. doi: 10.1016/S0140-6736(06)68438-9. [DOI] [PubMed] [Google Scholar]
- 2.Jack GS, Almeida FG, Zhang R, Alfonso ZC, Zuk PA, Rodríguez LVA. Processed lipoaspirate cells for tissue engineering of the lower urinary tract: implications for the treatment of stress urinary incontinence and bladder reconstruction. J Urol. 2005;174:2041–5. doi: 10.1097/01.ju.0000176489.96993.84. [DOI] [PubMed] [Google Scholar]
- 3.Pariente JL, Kim BS, Atala A. In vitro biocompatibility evaluation of naturally derived and synthetic biomaterials using normal human bladder smooth muscle cells. J Urol. 2002;167:1867–71. doi: 10.1016/S0022-5347(05)65251-2. [DOI] [PubMed] [Google Scholar]
- 4.Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999;17:149–55. doi: 10.1038/6146. [DOI] [PubMed] [Google Scholar]
- 5.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue Eng. 2001;7:211–28. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 6.Huard J, Cao B, Qu-Petersen Z. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res C Embryo Today. 2003;69:230–7. doi: 10.1002/bdrc.10020. [DOI] [PubMed] [Google Scholar]
- 7.Qu-Petersen Z, Deasy B, Jankowski R, Ikezawa M, Cummins J, Pruchnic R, Mytinger J, Cao B, Gates C, Wernig A, et al. Identification of a novel population of muscle stem cells in mice: potential for muscle regeneration. J Cell Biol. 2002;157:851–64. doi: 10.1083/jcb.200108150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lo KC, Chuang WW, Lamb DJ. Stem cell research: the facts, the myths and the promises. J Urol. 2003;170:2453–8. doi: 10.1097/01.ju.0000087170.97532.ff. [DOI] [PubMed] [Google Scholar]
- 9.Duffy MM, Ritter T, Ceredig R, Griffin MD. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Evren S, Loai Y, Antoon R, Islam S, Yeger H, Moore K, Wong K, Gorczynski R, Farhat WA. Urinary bladder tissue engineering using natural scaffolds in a porcine model: role of Toll-like receptors and impact of biomimetic molecules. Cells Tissues Organs. 2010;192:250–61. doi: 10.1159/000317332. [DOI] [PubMed] [Google Scholar]
- 11.Leite MTC, Freitas-Filho LG, Oliveira AS, Semedo-Kuriki P, Laks M, Arias VEA, Peixoto PS. The use of mesenchymal stem cells in bladder augmentation. Pediatr Surg Int. 2014;30:361–70. doi: 10.1007/s00383-014-3465-2. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Lee SR, Song YS, Lee HJ. Stem cell therapy in bladder dysfunction: where are we? And where do we have to go? Biomed Res Int. 2013;2013:930713. doi: 10.1155/2013/930713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodríguez LV, Alfonso Z, Zhang R, Leung J, Wu B, Ignarro LJ. Clonogenic multipotent stem cells in human adipose tissue differentiate into functional smooth muscle cells. Proc Natl Acad Sci U S A. 2006;103:12167–72. doi: 10.1073/pnas.0604850103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–95. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Becker C, Jakse G. Stem cells for regeneration of urological structures. Eur Urol. 2007;51:1217–28. doi: 10.1016/j.eururo.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 16.Huard J, Cao B, Qu-Petersen Z. Muscle-derived stem cells: potential for muscle regeneration. Birth Defects Res C Embryo Today. 2003;69:230–7. doi: 10.1002/bdrc.10020. [DOI] [PubMed] [Google Scholar]
- 17.Kwon D, Kim Y, Pruchnic R, Jankowski R, Usiene I, de Miguel F, Huard J, Chancellor MB. Periurethral cellular injection: comparison of muscle-derived progenitor cells and fibroblasts with regard to efficacy and tissue contractility in an animal model of stress urinary incontinence. Urology. 2006;68:449–54. doi: 10.1016/j.urology.2006.03.040. [DOI] [PubMed] [Google Scholar]