Abstract
Background
Our goal is to conduct a multicenter randomized controlled trial (RCT) to investigate whether exercise can reduce incident fractures compared with no intervention among women aged ≥65 years with a vertebral fracture.
Objectives
This pilot study will determine the feasibility of recruitment, retention, and adherence for the proposed trial.
Design
The proposed RCT will be a pilot feasibility study with 1:1 randomization to exercise or attentional control groups.
Setting
Five Canadian sites (1 community hospital partnered with an academic center and 4 academic hospitals or centers affiliated with an academic center) and 2 Australian centers (1 academic hospital and 1 center for community primary care, geriatric, and rehabilitation services).
Participants
One hundred sixty women aged ≥65 years with vertebral fracture at 5 Canadian and 2 Australian centers will be recruited.
Intervention
The Build Better Bones With Exercise (B3E) intervention includes exercise and behavioral counseling, delivered by a physical therapist in 6 home visits over 8 months, and monthly calls; participants are to exercise ≥3 times weekly. Controls will receive equal attention.
Measurements
Primary outcomes will include recruitment, retention, and adherence. Adherence to exercise will be assessed via calendar diary. Secondary outcomes will include physical function (lower extremity strength, mobility, and balance), posture, and falls. Additional secondary outcomes will include quality of life, pain, fall self-efficacy, behavior change variables, intervention cost, fractures, and adverse events. Analyses of feasibility objectives will be descriptive or based on estimates with 95% confidence intervals, where feasibility will be assessed relative to a priori criteria. Differences in secondary outcomes will be evaluated in intention-to-treat analyses via independent Student t tests, chi-square tests, or logistic regression. The Bonferroni method will be used to adjust the level of significance for secondary outcomes so the overall alpha level is .05.
Limitations
No assessment of bone mineral density will be conducted. The proposed definitive trial will require a large sample size.
Conclusions
The viability of a large-scale exercise trial in women with vertebral fractures will be evaluated, as well as the effects of a home exercise program on important secondary outcomes.
Osteoporotic fractures create a substantial human and economic burden.1 Vertebral fractures are a common consequence of osteoporosis that can cause pain and are associated with increased mortality.2 One woman in 5 who have a vertebral fracture will have another fracture within a year; risk of death is 2.7 times higher than in those without fracture.3,4 Osteoporosis management guidelines recommend exercise for those at high risk, such as those with vertebral fractures.5–7 However, no randomized controlled trials (RCTs) of exercise have had fractures as a primary endpoint. Much of the evidence to date regarding the effects of exercise on fracture risk has been indirect in that it examined surrogate outcomes (eg, falls, bone mineral density [BMD]) and was conducted in lower-risk populations (eg, women who are postmenopausal with no history of fracture).8 Meta-analyses have demonstrated a small benefit of exercise for BMD, but trial heterogeneity may alter the conclusions.9–14 For example, estimates of effect change when analyses are performed by exercise type.14 Bone mineral density is an inadequate surrogate for fracture risk.15,16 Exercises that challenge balance may prevent falls,17–20 but generalizability to people with vertebral fractures is unclear. Excessive kyphosis, pain, and altered trunk muscle control can affect adherence to and efficacy of exercise and contribute to falls or fracture risk.21,22 Alterations in posture and spine loading can increase the risk of vertebral fracture without a fall.22,23 Meta-analyses and position papers have called for large RCTs to evaluate whether exercise can prevent fractures.13,24
Research on the efficacy and safety of exercise for people with osteoporotic fractures is scarce. Exercise, especially if unsupervised, might increase fracture risk. Fractures and injuries attributable to exercise in patients with hip and vertebral fractures have been reported.25–28 In some observational studies, physical activity was associated with increased fracture incidence.29,30 Adverse events are more frequently reported in exercise trials of individuals with health conditions and functional limitations.31 In a Cochrane review by Giangregorio et al,32 only 7 trials evaluated the efficacy of exercise after vertebral fracture; many trials were subject to bias and were underpowered, and few trials had long-term follow-up. There are limited data from which to develop exercise recommendations for people with vertebral fractures, and an RCT is needed to determine whether exercise does more good than harm. Physical therapists who provide exercise prescription for individuals with vertebral fractures need evidence or guidelines based on trials that test interventions that are realistic to deliver in practice and provide evidence on the risks and benefits to inform their clinical practice.
A large study sample size is needed to detect benefit or harm of exercise for fracture prevention.13 To enhance the probability of detecting an effect, individuals at high risk for fracture should be studied. The presence of vertebral fracture is associated with an increased risk of hip fracture after 5 years (hazard ratio=2.10, 95% confidence interval [95% CI]=1.58–2.78) and 10 years (hazard ratio=1.41, 95% CI=1.15–1.73) of follow-up.33 Trials of osteoporosis medication often study women with vertebral fractures.5 Furthermore, it is in individuals at high risk for fracture that the question of whether exercise does more good than harm is most relevant. We designed a multicenter trial to test the hypothesis that home exercise, 3 times a week for 1 year, can reduce incident fractures among individuals aged ≥65 years with a history of vertebral fracture compared with no exercise intervention. We will conduct a pilot feasibility, parallel-group RCT with 1:1 allocation ratio to determine:
Study recruitment rates: The study will be considered feasible if we recruit 20 participants per site. Twenty participants per site per year translates to 2,400 participants with 40 sites in 3 years.
Study retention rates: The study will be considered feasible if ≥75% of the sample complete visit 2. A study of exercise after vertebral fracture demonstrated that 81% and 77% of the participants returned at 6 and 12 months, respectively.28
Adherence to the exercise intervention: The intervention will be considered feasible if participants complete ≥60% of the prescribed number of exercises at 12-month follow-up. It is not uncommon for exercise interventions to have average adherence rates of approximately 60% or lower but report positive outcomes.26
Secondary outcomes include physical function, forward head posture, falls, fractures, the intervention cost, adverse events, quality of life, pain, and health resource use.
Method
Study Setting
Eight sites were originally chosen to ensure diversity in city population, structure, services, presence of a medical school, and other variables. Canadian sites include a community hospital partnered with an academic center (St Mary's Hospital–University of Waterloo) and 4 academic hospitals or centers affiliated with an academic center (McMaster University, University of Toronto/Toronto General Hospital, Western University/St Joseph's Health Care, and University of British Columbia). Two Australian centers are included to test feasibility outside of Canada: Broadmeadows Health Service (community primary care, geriatric, and rehabilitation services) and Royal Melbourne Hospital (academic hospital) partnered with University of Melbourne. One additional Canadian site was closed prior to recruitment; the site leader was planning to leave, and there would be no oversight. We retained the original target sample of n=160 for the 5 Canadian sites and 2 Australian sites. The University of Waterloo will be the coordinating site.
Trial Design
A pilot single-blinded, multicenter RCT comparing thrice-weekly exercise with health discussion control is proposed (ClinicalTrials.gov identifier: NCT01761084), with a 1:1 allocation ratio. The web-based randomization scheme will be generated and managed by Empower Health Research Inc and stratified by center, using a random numbers generator, in permuted block sizes of 2 and 4. After the baseline assessment, an unblinded physical therapist will enter participant ID and center into the web-based system to determine group allocation and will make contact with the participant. Allocation will be concealed from all but the physical therapist and the primary investigator (L.M.G.).
Participants
Individuals will be eligible for inclusion if they are female, ≥65 years of age, and have radiographic evidence of a nontraumatic fracture of ≥1 vertebrae between T4 and L4. Vertebral fractures will be defined as radiographic presence of ≥25% reduction in anterior, middle, or posterior height of a vertebra, assessed centrally from lateral thoracic and lumbar spine X-rays using the Genant visual semiquantitative method.34 If fracture history is uncertain, the presence of hyperkyphosis and documented height loss of ≥2 cm or historical height loss of ≥6 cm will be criteria for sending for X-ray verification.35,36 Exclusion criteria include: index vertebral fracture due to trauma; not able to communicate in English; on dialysis; receiving palliative care; current or prior cancer (except basal cell carcinoma); clinically significant kidney, liver, or intestinal disease; exercise participation ≥3 times per week that addresses ≥2 of 5 domains in the Build Better Bones With Exercise (B3E) exercise prescription; progressive neurological disorder or progressive disorder likely to prevent study completion; unable to stand or walk 10 m with or without a gait aid; impaired capacity to give informed consent (positive Mini-Cog37 score and cannot recall what they are being asked to do during the consent process); or contraindication to exercise as determined by a physician. No more than 1 person from each retirement home can be included. We will not exclude individuals with a history of vertebroplasty, kyphoplasty, glucocorticoid use, or use of a pharmaceutical drug approved for osteoporosis treatment in the participant's country or those who are enrolled in research studies that do not include exercise or behavioral counseling interventions. We hypothesize that the direction of response, but not the magnitude, will be the same.38 We will not stratify participants based on these factors for the pilot study due to the potential for strata with low numbers, but we will perform post-stratification analyses to determine the strata and exclusion criteria for the larger trial.39
Intervention
Intervention, comparator, care providers, and standardization are outlined elsewhere.40 The B3E intervention includes the B3E Exercise Prescription and the Motivation to Move Program, a behavioral counseling guide designed by the study team to enhance adherence (Tab. 1) and informed by motivational interviewing,41,42 and the health action process approach.43 Elicit-provide-elicit strategies, agenda setting, reflective listening, summarizing, and affirmations are used. Participants will answer Likert-style questions about the importance of and confidence in making a change and about exercise self-efficacy. Developing implementation intentions, or “action plans,” and contingency plans, or “coping plans,” will be used to enhance adherence to exercise.44,45 Self-monitoring will be implemented via a daily diary that asks:
if they performed aerobic exercise (yes/no); if yes, how many minutes of exercise, and rating of perceived exertion,46 and
if they performed balance, posture, or strengthening exercises; if yes, how many that day.
Table 1.
The Motivation to Move Program: A Framework for Standardizing a Behavior Change Intervention Delivered in Conjunction With the Build Better Bones With Exercise (B3E) Exercise Prescriptiona
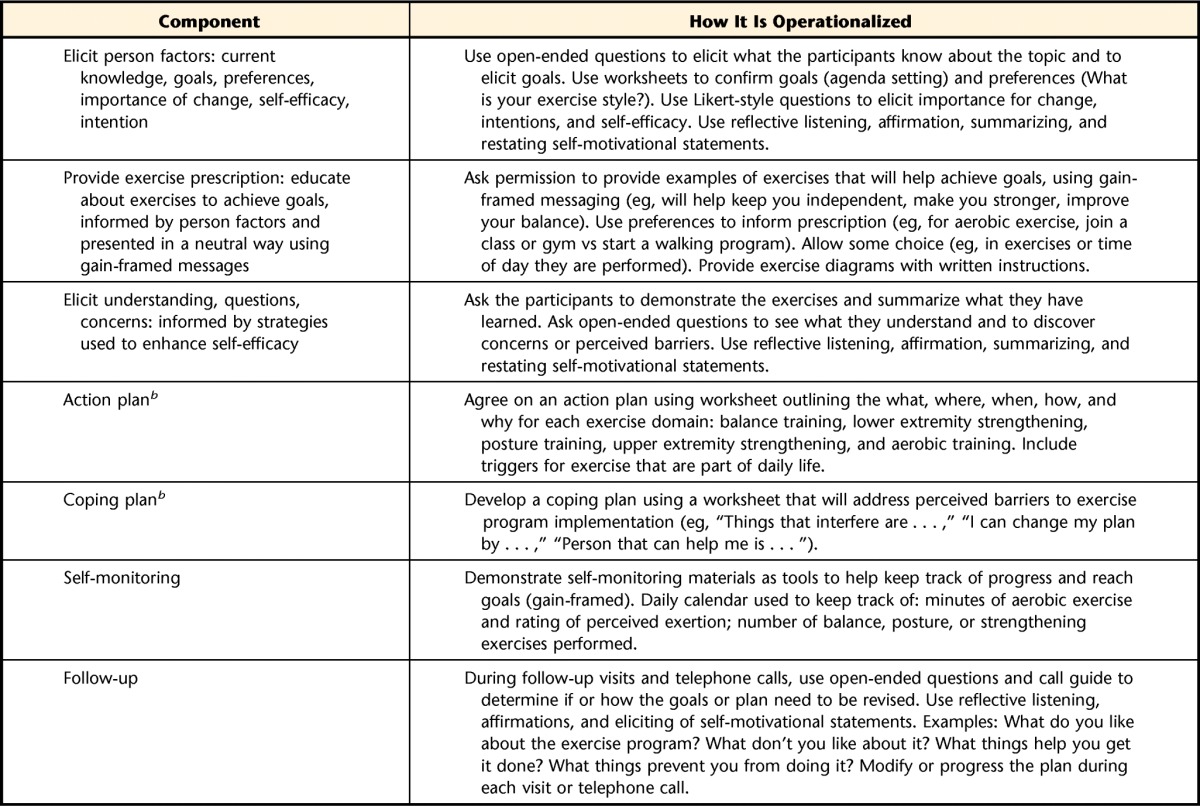
The Motivation to Move Program may not be used or reproduced without written permission from the authors.
b Although a formal framework or sequence of events that enforces planning and a time line for intervention is not consistent with the tenets of motivational interviewing, participants in the study have agreed to participate under the assumption that they will receive an exercise program—they have agreed to make a change, and we have promised that we will provide them with a plan. Therefore, we have used the motivational interviewing approach to inform the strategies that we use to guide behavior change, but we are not delivering a traditional motivational interviewing intervention. We chose to standardize the strategies and provide a structure to the approach to be consistent with the CONSORT guidelines for reporting and to ensure some standardization in the intervention and documentation across sites, given the number of care providers.
Adherence calendars will be reviewed monthly; poor adherence (ie, <3 times/week) will prompt a telephone call or visit from the therapist to provide counsel or modification.
The B3E exercise prescription (Tab. 2) is informed by Bone Fit, a workshop and manual developed by an expert panel in collaboration with Osteoporosis Canada47 to train physical therapists and kinesiologists about exercise for individuals with osteoporosis. In addition to muscle strengthening, aerobic training, and balance training, the B3E exercise prescription includes exercises to address hyperkyphosis, a source of pain, and increased fall risk that may be amenable to exercise.48,49 Exercise goals also are informed by meta-analyses of exercise for reducing falls19,20 or improving BMD14,50 and a Cochrane review of exercise in individuals with vertebral fracture.32 Intervention and control activities will be delivered by certified physical therapists; we will attempt to have participants work with the same therapist throughout the study. At sites that have more than 1 therapist, allocation of therapists will be determined by proximity to participant, modifying factors (eg, therapists' pet allergies), and caseload. Many of the physical therapists have completed the Bone Fit training workshop, and for those who are unable to do so, a 1-hour telephone training session and the Bone Fit manual and training videos are provided. Physical therapists will receive materials and an 80-minute telephone training session on Motivation to Move. All participants will receive vitamin D and be instructed to take 1,000 IU daily. Individuals prescribed a higher daily dose by a physician will continue taking that dosage.
Table 2.
Build Better Bones With Exercise (B3E) Intervention Frameworka
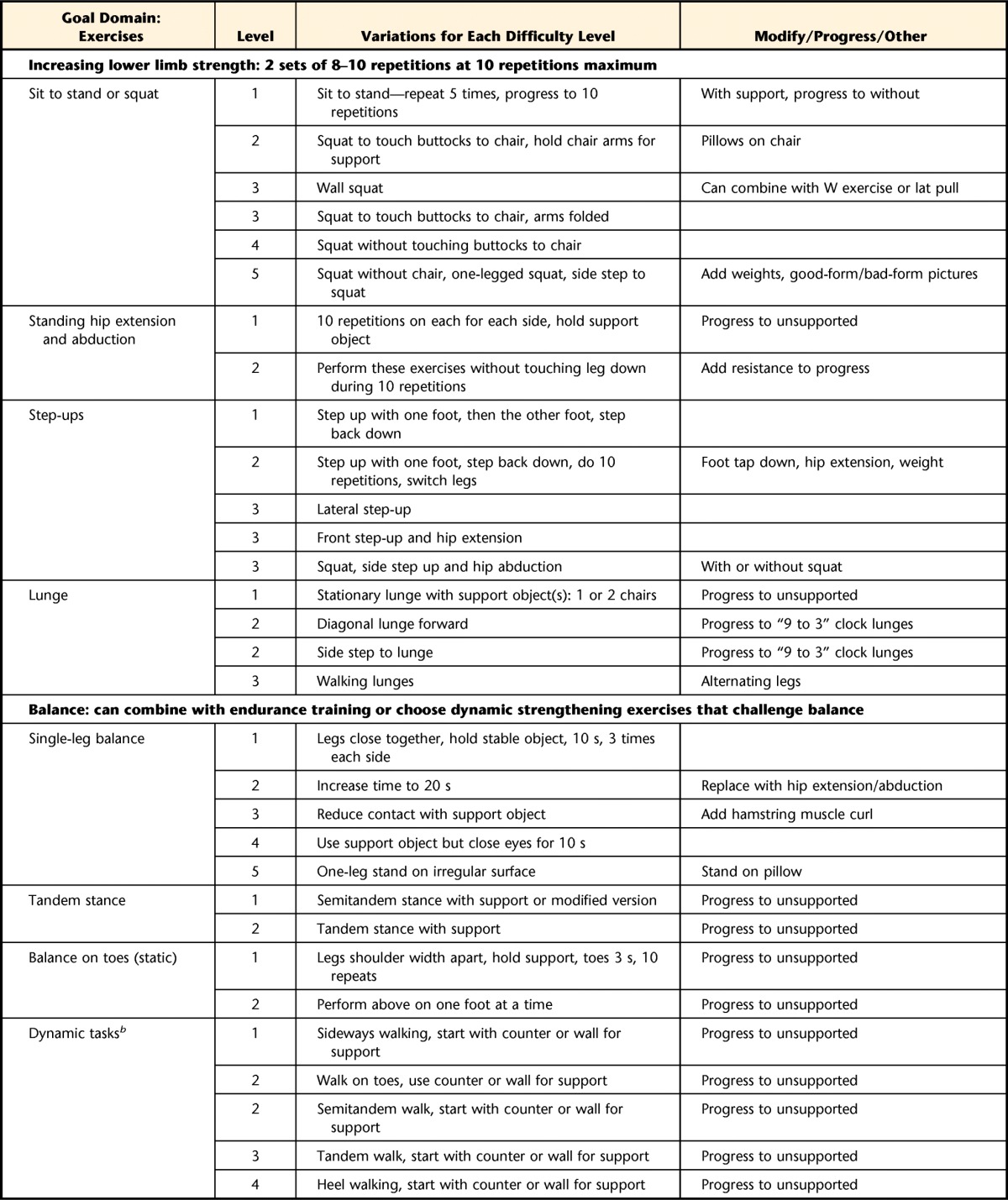
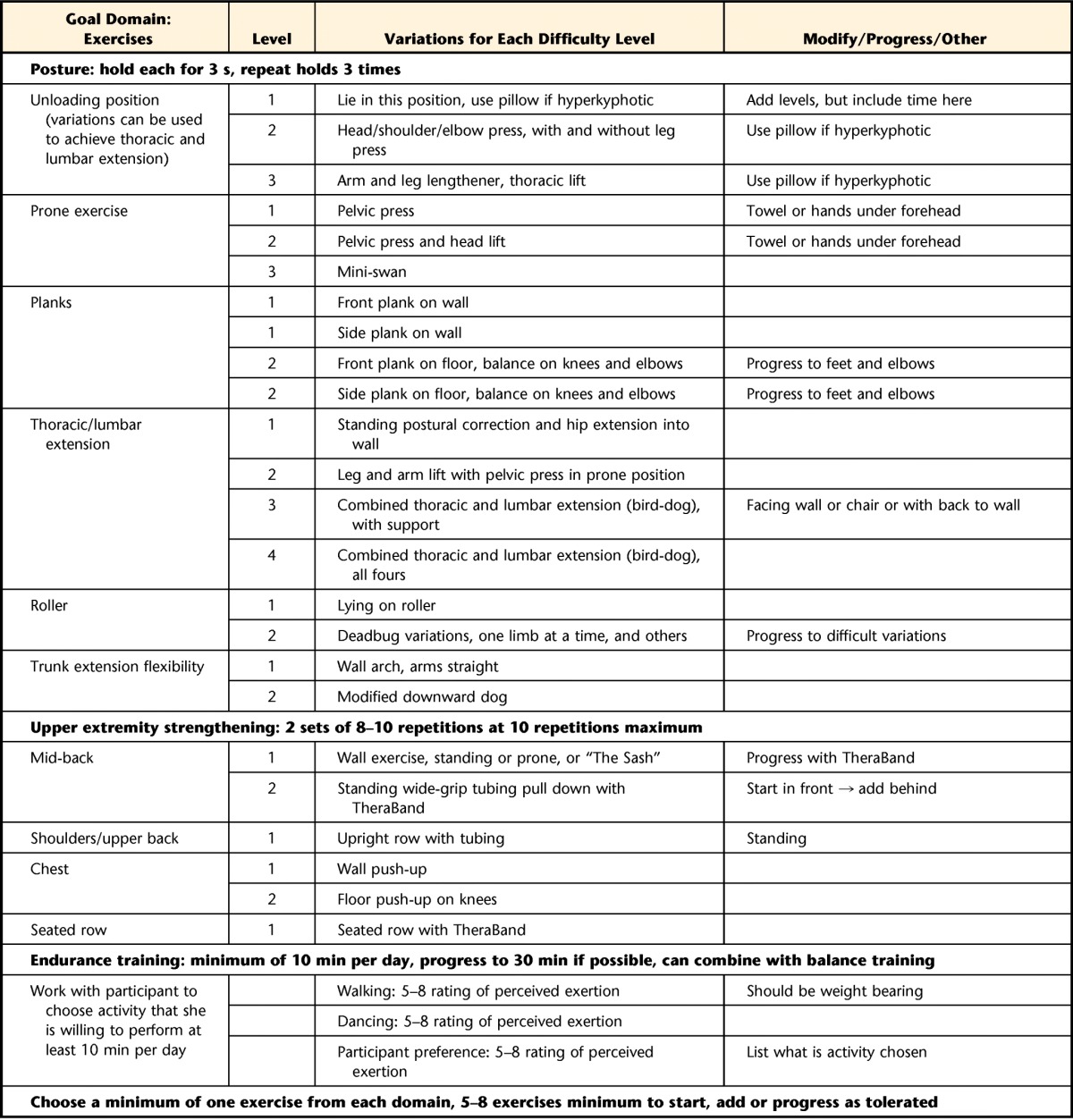
The Build Better Bones With Exercise (B3E) intervention framework may not be used or reproduced without written permission from the authors.
b Dynamic tasks are preferred unless static tasks are more appropriate for or preferred by the participant.
Setting and supervision.
A home-based intervention with intermittent supervision was chosen so participants could integrate exercise into daily activities.28,51,52 A physical therapist visits the participant at home, engages the participant in discussion about behavior change, and provides diagrams of exercises with instructions. The physical therapist tailors the goals and exercises to the patient and develops written action and coping plans (Tab. 1). Biweekly telephone calls and 3 follow-up home visits by the physical therapist will occur in the first 2 months to ensure safety and progression; modifications will be tailored to individual needs (eg, adverse events, participant request, improving or worsening condition). After 2 months, telephone calls will occur monthly, and additional visits will occur at 6 and 8 months. The number and timing of home visits were informed by the Otago Exercise Program, originally delivered by a physical therapist via home visits to individuals approximately 80 years of age or older with impaired balance and strength.52–56
Goals and exercise modes.
The exercise program has 5 main goal domains to target concerns (eg, low bone mass, thoracic hyperkyphosis, impaired balance) specific to individuals with fractures: (1) aerobic exercise including weight bearing, (2) lower extremity muscle strengthening, (3) balance training, (4) optimal postural alignment, and (5) strengthening muscles of trunk and upper extremities.
Frequency and duration.
Participants are given at least one exercise from each domain. Participants are encouraged to perform balance, aerobic and posture training daily, and strengthening 3 times weekly. Aerobic training is prescribed for 10 to 30 minutes daily; duration is progressively increased. The strength training dose is consistent with those in previous studies.26,28,57–59
Intensity.
Participants will be asked to perform aerobic exercise at an intensity of 5 to 8 on the Borg Rating of Perceived Exertion Scale. For lower and upper extremity strengthening and posture training, participants will use body weight, the floor or wall, or a lightweight TheraBand exercise band (The Hygenic Corp, Akron, Ohio) as resistance. For strengthening, the maximum resistance with which 10 repetitions can be completed with good form (10-RM) will be determined for each exercise. Participants will begin with 2 sets of 8 to 10 repetitions at 10-RM resistance for each exercise or with assigned difficulty level if not using resistance.60 For posture exercises, participants will hold each position for 3 seconds and repeat 3 times. For balance training, the difficulty will be individually tailored. Methods for progression include increasing repetitions (up to 12), frequency, duration, or difficulty; training volume will not increase more than 2.5% to 5% per week.60
Time.
The intervention group will be asked to continue the exercise prescription for 1 year.
Comparator.
The control group was designed so that the participants will receive equal attention but will not receive any exercise advice or behavioral counseling. Control participants will receive the same number and duration of visits and calls, focused on health or social discussion. A guide facilitates consistent follow-up calls. Written materials on health-related topics will be used for discussion. Topics include, but are not limited to: osteoporosis diagnosis, osteoporosis treatment, safe gardening tips, and nutrition. The intervention group also will receive the materials.
Data Collection and Management
Standard operating procedures, information and consent form (eAppendix 1), scripts, data forms, and checklists for visits and follow-up calls are in the B3E Study Manual, version date May 20, 2014. The study schedule and the hypotheses and analyses are shown in Tables 3 and 4, respectively. A research assistant/ coordinator will manage the trial at each site, obtain consent, and perform assessments. Assessors and data analysts will be blind to group allocation. An alternate assistant will collect outcomes that may cause unblinding (eg, exercise participation on diaries, physical activity level). Unblinding will be permitted only as required by regulatory authorities. Research staff will receive ≥120 minutes of telephone training and videos of physical assessments. They are asked to pilot test assessments on ≥2 people. On-site training is provided to Ontario and British Columbia sites. Participants can opt out of assessments (except X-rays) or complete questionnaires by telephone. An online protected data management system has been developed and will be managed by Empower Health Research Inc; centers can input data, and the central site can monitor it. Physical therapists access a separate area of the data management system for reporting so research assistants remain blind to group allocation. Quality assurance of intervention implementation will be performed for each therapist after ≥2 participants have received 1 intervention visit: (1) goal-setting and planning documents are uploaded to the data management system for central site review, and (2) a team telephone meeting will be held to discuss successes and challenges in implementation.
Table 3.
Schedule of Enrollment, Interventions, and Assessmentsa
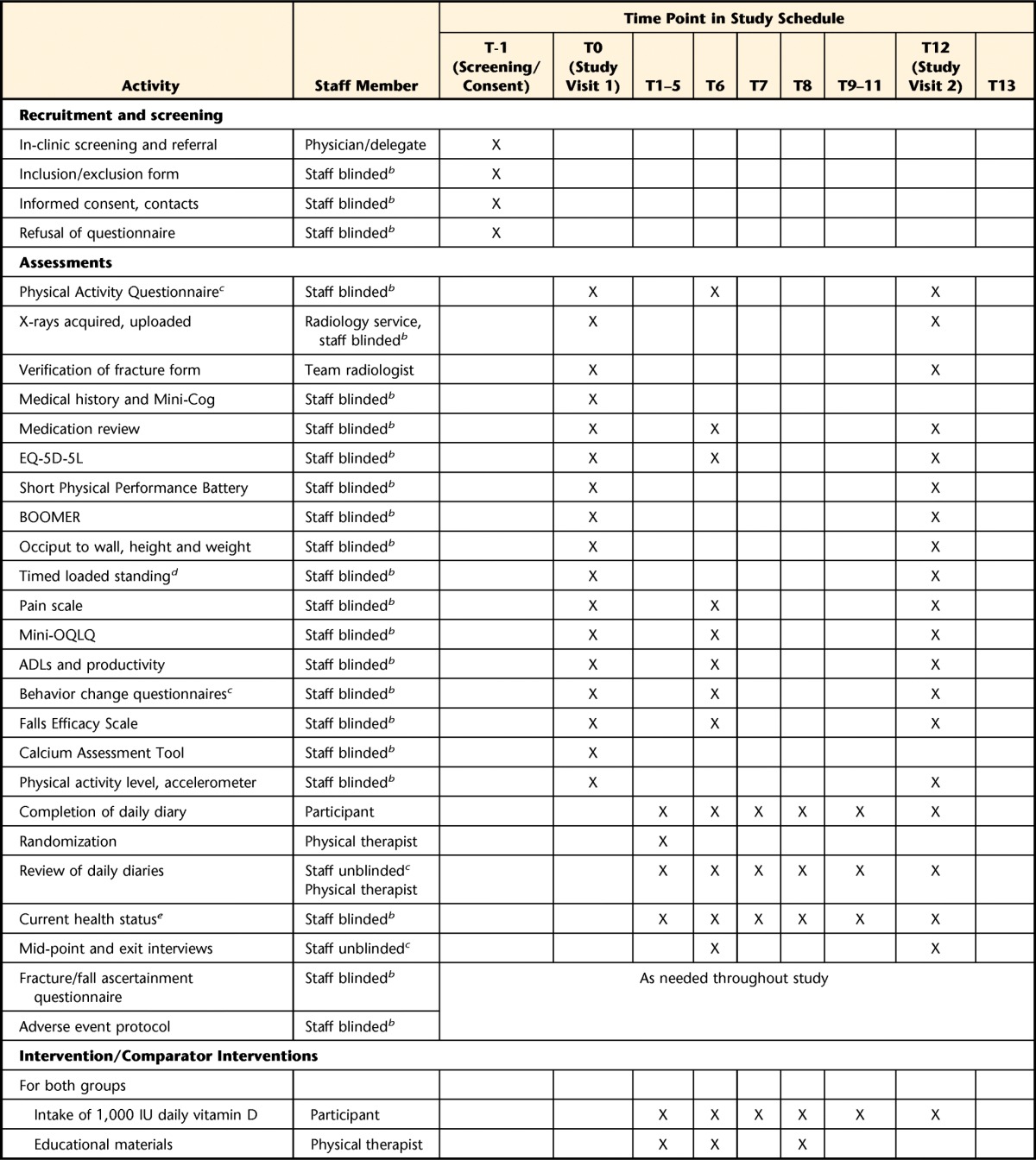
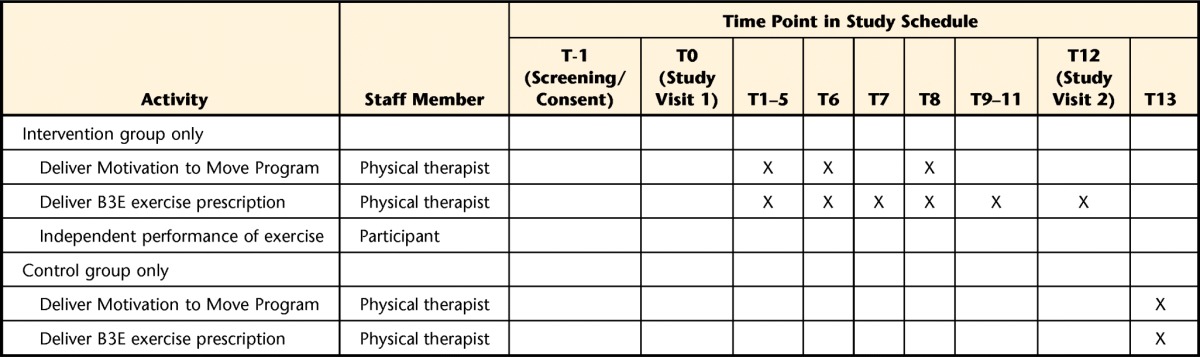
T=time (month), BOOMER=Balance Outcome Measure for Elder Rehabilitation, mini-OQLQ=mini-Osteoporosis Quality of Life Questionnaire, ADLs=activities of daily living, B3E=Build Better Bones With Exercise.
b Staff refers to a research coordinator or research assistant. One main assistant or coordinator will be appointed at each site to perform outcome assessments and will be blinded to group allocation.
c An additional research coordinator or research assistant will be appointed for tasks that require someone who is not blind to group allocation.
d The Timed Loaded Standing Test and accelerometer assessment will be performed at Toronto and Waterloo sites only.
e The current health status assessment at 6 and 12 months will include inquiries about medical problems, illnesses or injuries, current assistive device use, and living arrangements.
Table 4.
Variables, Hypotheses, Outcomes and Methods of Analysisa
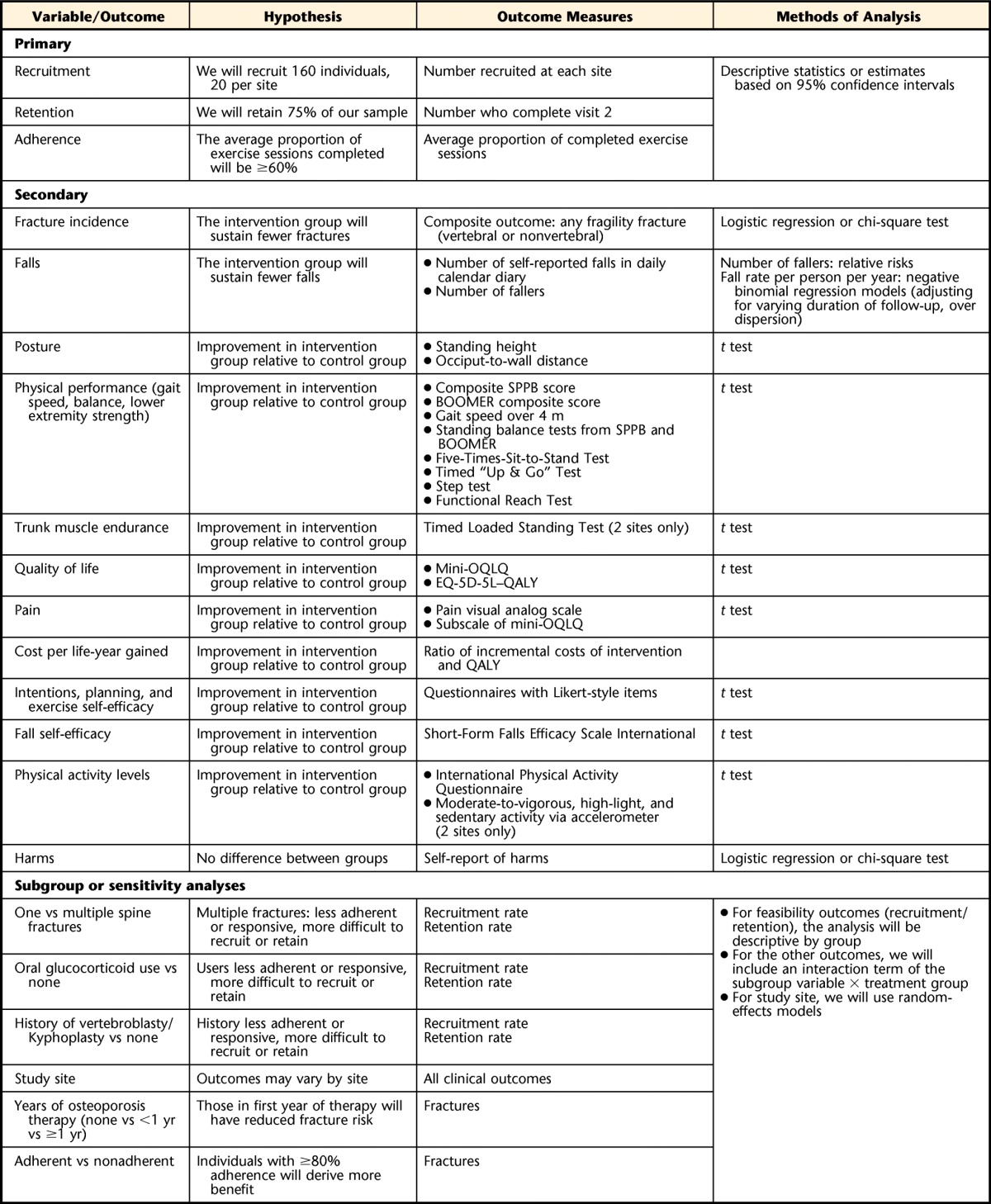
SPPB=Short Physical Performance Battery, BOOMER=Balance Outcome Measure for Elder Rehabilitation, mini-OQLQ=mini-Osteoporosis Quality of Life Questionnaire, QALY=quality-adjusted life-year.
Primary Outcome: Feasibility
Feasibility outcomes are the number of participants recruited and retained and the proportion of exercise sessions completed. We will use a calendar-style diary for all participants to record any exercise participation daily; participants will record whether they did aerobic training (yes/no), how many minutes of aerobic training, how hard it was (Borg scale rating),46 whether they did resistance, posture, or balance training (yes/no), and how many exercises they did. The postage-paid diary will be mailed in monthly and will be used for assessing falls, osteoporosis medication adherence, and health service use. Partial sessions will be counted as a proportion (eg, 0.75 of session). Adherence is defined by the number of sessions of strength, posture, and balance exercises: 100% adherence=≥3 sessions of exercise weekly or required number of exercises completed by weeks' end. Canadian participants will have postage-paid envelopes mailed directly to central site, and Australian diaries will be faxed, emailed, or entered locally by a separate research assistant. Activity will be monitored for potential contamination or cointerventions.61 Time spent in 4 categories of activity (sedentary, low-light, high-light, and moderate-vigorous physical activity) will be measured with an accelerometer (ActiGraph GT3x, ActiGraph, Pensacola, Florida) at 2 study sites over 1 week at baseline and 12 months.
Secondary Outcomes
A consensus report on clinical trials for preventing functional decline and disability in frail older adults suggests that secondary outcomes: (1) include functional outcomes due to their impact on health service use and quality of life, (2) inform the causal pathway from the intervention target to risk of disability, and (3) include effects on well-being.62 Secondary analyses will not influence the decision to move to a larger trial. Secondary outcomes will be assessed at baseline and 12 months, with some outcomes also assessed at 6 months and others assessed monthly (Tab. 3).
Fractures.
Incident fracture will be a composite outcome of any fragility fracture (ie, nontraumatic vertebral, hip, or other nonvertebral fracture, excluding skull, ankle, or fingers). Protocols for verifying fractures will be modeled after those used in the Canadian Multicentre Osteoporosis Study.3 Questionnaires will be used to ascertain fracture cause and timing. Written consent will be obtained for health record data abstraction to verify fracture occurrence, location, and severity. Lateral thoracic and lumbar spine X-rays will be performed at baseline and follow-up. Participants with fractures will maintain randomization and can continue exercise with physician approval and modification as necessary. Two blinded investigators will assess fractures independently to confirm whether they are fragility fractures. We will determine whether the degree of agreement differs between exercise group and control group participants.61 Discordance will be resolved by a third reviewer.
Falls.
The Prevention of Falls Network Europe and Outcomes Consensus Group recommends a monthly falls calendar to assess falls.63 A fall will be defined as “an unexpected event in which the participant comes to rest on the ground, floor, or other lower level.”64(p198) Monthly fall calendars have been shown to be superior to recall methods in some studies.65,66 Participants will be asked to report falls in the postage-paid calendar diaries daily and return calendars monthly.
Posture and physical performance.
Standing height and occiput-to-wall distance will be used as measures of posture. The Five-Times-Sit-to-Stand Test will represent functional leg muscle strength.67,68 We will measure timed static stance in feet together, semitandem and tandem with eyes open, and gait speed in a 4-m walk test to determine a score on the Short Physical Performance Battery.69 Balance will be assessed using the Balance Outcome Measure for Elder Rehabilitation (BOOMER), which comprises 4 measures: step test, Timed “Up & Go” Test, Functional Reach Test, and Timed Static-Stance Feet-Together Eyes-Closed Test.70,71 At 2 study sites, we will use the Timed Loaded Standing Test to assess trunk muscle extensor endurance.72
Quality of life, pain, and health service use.
The disease-specific mini-Osteoporosis Quality of Life Questionnaire will be used to measure health-related quality of life and pain.28,73 A visual analog scale will be used to measure pain with activities and at rest. An economic evaluation will be performed from the health system and societal perspectives. Direct medical resources, direct nonmedical resources, and indirect resources will be collected. Resource use (ie, health care visits, diagnostic tests) will be collected daily via diary. Monthly, a “current health status” telephone interview will be used to inquire about hospitalizations, purchases of assistive devices or supplements, and falls, fractures, and adverse events. Questionnaires about workplace or volunteer work productivity and activities of daily living will be completed monthly. We will estimate the cost per individual of the intervention. The EQ-5D-5L is a health-related quality-of-life instrument that will be used as a measure of morbidity with the score multiplied by mortality to achieve a quality-adjusted life-year (QALY) estimate.74 Multiplying resources collected by jurisdictional unit costs in Canadian dollars will determine the total cost per exercise program. Cost adjustments will be made by country using currency conversions.75 The ratio of incremental costs, as determined by trial resources, between the intervention and clinical outcome (QALY) will be calculated to achieve an incremental cost per life-year gained outcome.
Behavior change variables and fall self-efficacy.
Participants will be asked a number of Likert-style questions to discern self-efficacy, action and coping planning abilities, and implementation intentions.76 Participants will answer on a 5-point scale from 1 (“not at all confident”) to 5 (“completely confident”). The Short-Form Falls Efficacy Scale International (FES-I) will be used to represent fear of falling.77,78
Harms.
Participants will be instructed to report adverse events or injuries, and will be asked about them during monthly calls. Three types of adverse events will represent secondary outcomes: (1) serious adverse events (Health Canada definition: death or event that is life-threatening, requires hospitalization, or results in disability); (2) events linked to intervention; and (3) events leading to study withdrawal or intervention cessation. The research assistant/coordinator will confirm the event date, circumstances, and details and associated health service use and will report them to the ethics boards.
Descriptive data.
Questionnaires will be used to collect demographic data and medical history (eg, medications, Mini-Cog). Physical activity will be estimated using the International Physical Activity Questionnaire at baseline and after 6 and 12 months. Calcium intake will be assessed using the Calcium Assessment Tool.79 The following will be collected to inform future trials: screening to recruitment ratios, number of potentially eligible male participants, and timing and completion of assessments. Midpoint and exit interviews will be used to obtain feedback on the experience.
Recruitment
We plan to recruit a minimum of 20 individuals per site, with over-recruitment allowed, and a total target of 160 individuals. Women with a history of or suspected vertebral fracture who meet eligibility criteria attending specialty clinics will be informed about the study. The research assistant/coordinator will call interested individuals to enroll them. At the University of British Columbia, we can notify individuals in a database of >11,000 people willing to be contacted for research. Local family practice physicians or specialists can be notified.
Strategies to Enhance Recruitment
If, after 6 months of recruitment, we are not nearing our target, we will consider: (1) informing local radiologists of the study and asking them to note women with spine fractures and (2) advertising to patient support, community, or advocacy groups.
Strategies to Enhance Retention
The control group will receive 1 physical therapist home visit, the B3E intervention materials, and an exercise prescription at study end. Participants will receive telephone calls and holiday cards throughout the study and feedback on the results.
Sample Size Estimation
A meta-analysis of observational trials estimated that an exercise trial with hip fracture as primary endpoint would need 9,000 individuals, likely why calls for a trial have been unheeded.13 However, the sample size for a definitive trial may be reduced by: (1) studying a high-risk group and (2) including a composite primary outcome of any fragility fracture. Feasibility will be increased by recruiting participants who are and are not on anti-osteoporosis therapy. Among women with vertebral fractures on alendronate, 8.0% and 11.9% experienced a new vertebral or a clinical nonvertebral fracture, respectively, over 2.9 years, versus 15% and 14.7%, respectively, in the placebo group.80 If all participants were receiving therapy, we would estimate that 6% of the participants would experience a new fracture within 1 year80; to observe a fracture risk reduction of 30%, the larger trial would require about 4,400 participants. However, in untreated women, we anticipate 10% would experience any fracture, resulting in a required sample size of 2,500 individuals. Some people choose not to accept therapy or are nonadherent; the required sample size may lie between the 2 estimates. The sample size for the pilot study (N=160) will provide estimates of recruitment, adherence, and the number of participants with certain covariates to inform future sample size calculations and stratification variables.
Analyses
The protocol was drafted in accordance with the SPIRIT 2013 Statement81 (eAppendix 2). Reporting will be in accordance with CONSORT.82 Analyses of feasibility objectives will be descriptive or based on estimates with 95% CI values. Participant characteristics and outcomes will be summarized using descriptive measures: mean (standard deviation) or median (minimum-maximum or interquartile range) for continuous variables, number (%) for categorical variables, and percentage of change. Intention-to-treat analyses will be performed. Differences in secondary outcomes will be tested using independent Student t tests (continuous variables) or chi-square tests or logistic regression (binary outcomes). Number of fallers will be compared by calculating relative risks. The fall rate per person per year in each group will be compared using negative binomial regression models (including all falls, adjusting for varying duration of follow-up, over dispersion). Multiple imputation will be used to impute missing data.83 We will perform a sensitivity analysis, adjusting for potential residual imbalance in age or pain at baseline. The Bonferroni method will be used to adjust the level of significance for multiple testing of secondary outcomes so the overall alpha level is .05. Probability values will be reported to 3 decimal places. All analyses will be performed with SAS version 9.2 (SAS Institute Inc, Cary, North Carolina). No statistical warning rules will be applied to the pilot study.
We hypothesize that individuals with a history of multiple fractures, oral glucocorticoid use, or vertebroplasty or kyphoplasty, because of the presence of pain or comorbid conditions, or who have a positive Mini-Cog score may be less adherent to the intervention, more difficult to recruit and retain, and less responsive to the intervention compared with those without such a history, so we will perform stratified subgroup and sensitivity analyses by poststratification of participants based on these variables, study site, and years of osteoporosis therapy (none versus <1 year versus ≥1 year). Sensitivity analyses will be used to determine whether individuals with ≥80% adherence derive more benefit. A meta-analytic random effects approach will be used to aggregate estimates of effect across strata.
Trial Steering Committee
The principal investigator will be responsible for trial conduct, the B3E Study Manual, and chairing Steering Committee meetings. Lead investigators will facilitate recruitment or contribute expertise in physical therapy, design and analysis, or economic evaluation and contribute to study progress as part of the Steering Committee.
Data Safety Monitoring Committee
A Data Safety Monitoring Committee (DSMC) of 3 arms' length members will review adverse events after all participants reach 6 months of follow-up and at study end to determine whether they were due to intervention. The DSMC will have unblinded access to all data, will report to the primary investigator, and will guide how we might roll participants into the larger trial. No interim analyses are planned, and there are no stopping guidelines for the pilot trial.
Ethics and Confidentiality
The research will be conducted according to the Tri-Council Policy Statement.84 The study has received approval from the research ethics boards at all sites. We initiated a trial run of the baseline assessments (excluding X-rays) and intervention delivery at the University of Waterloo in May 2013. Protocol amendments were made after initial ethics approval and trial run and have been approved by ethics committees. Future amendments will be submitted to ethics committees by the research assistant or principal investigator and updated in the registered protocol. A protocol deviation tracker has been created to note deviations from the protocol. Participants will be assigned an ID to be used on forms and in the data management system. De-identified data will be stored in a secured area at the study site. Hard copies of records with personal identifiers will be kept separately from the data. Data will be entered into the data management system by a research assistant/coordinator. Only the site research assistant/coordinator, the site physical therapist, and the data management service provider will be able to view both participant data and identifiers in the system. Audits of the trial dataset and protocol deviations will be performed by the principal investigator or his or her delegate. Site visits may be performed to address concerns (eg, low recruitment, frequent withdrawals), with advance notification of ≥2 weeks. Each site can view only their data. The complete dataset will reside with the central site; access to it must be approved by the Steering Committee. Trial investigators have no relevant financial or competing interests. Study results will be presented at conferences and published in academic journals. Authorship guidelines are outlined in the B3E Study Manual, version date August 15, 2013; professional writers will not be used.
Discussion and Dissemination
We propose to evaluate the feasibility of a large, multicenter trial of exercise in women with vertebral fracture. Our study is designed as a pilot trial, or “small-scale” version of a larger trial that represents a new direction for bone and exercise research—a move away from surrogate outcomes for fracture risk to studying the effects on fractures directly. A recent consensus process identified a definitive trial with fracture endpoints as a priority for the field.85 As discussed in our section on sample size, the future trial with fracture as a primary outcome may need a very large sample size, resulting in a large cost. If our pilot study suggests that the trial may be feasible, a future definitive trial could adapt our trial protocol and engage collaborators in multiple countries to conduct a large-scale, ground-breaking multicenter RCT to determine whether exercise does more harm than good with respect to osteoporotic fractures, with fracture as a primary endpoint.
On its own, our pilot study will be one of the largest trials of exercise in women with osteoporotic vertebral fractures to date and will provide key insights on the effects of exercise on key patient-centered outcomes. We will address concerns in Canada's Strategy for Patient-Oriented Research86 by involving patient advocates, choosing outcomes important to patients, and designing interventions that could be translated into existing health care systems. Our aim is integrated knowledge translation: members of the Canadian Osteoporosis Patient Network (COPN), physical therapists, and physicians have provided input on the study proposal. Discussions with COPN members have revealed that exercise information and services are among the top 3 things that their members want, but these are limited resources because of the lack of good-quality evidence on what is effective for older adults at high risk for fracture. Our study will inform whether a physical therapist–led exercise intervention developed based on available evidence and substantive expert consultation has an effect on a number of outcomes important for decision making (eg, quality of life, physical functioning) in an understudied patient population—women with osteoporotic vertebral fractures. In addition, we incorporated both prescriptive elements and behavior change strategies, which could be used as a template for how to combine these elements and strategies in clinical practice.
In addition to informing a future trial with fracture as a primary endpoint and evaluating the effect of exercise on quality of life and physical functioning, our trial may inform health services–related research questions. When an individual has a myocardial infarction, he or she often receives cardiac rehabilitation that is, in some jurisdictions, funded by the health care system. However, when someone fractures a vertebra due to osteoporosis, he or she often is sent home with pain medication, which ironically might increase the risk of falls and fractures. Our study will test a model of exercise delivery that could be adapted into existing home care models or implemented in outpatient physical therapy clinics. We have intentionally included health service use, both out-of-pocket and paid for by government-funded health care, as an outcome to begin to capture information that could be used to inform policy decisions. Our pilot study also will provide resource utilization data among women with vertebral fracture. Any future definitive trial will evaluate the cost/benefit of delivering a home exercise program for individuals with vertebral fracture. Future research also could examine willingness to pay for exercise services to determine what future models of care need to provide. It is our vision that individuals who have an osteoporotic vertebral fracture will be able to access an individually tailored home exercise program as part of their health care.
To ensure that the results of our study will inform physical therapist practice and have an impact on patient care, Osteoporosis Canada will evolve the Bone Fit program and the clinical practice guidelines for the diagnosis and management of osteoporosis7 to include new research findings and will disseminate them to patient advocacy groups and professional groups. Bone Fit is a workshop created by a physical therapist and expert advisory group that is “designed for healthcare professionals and exercise practitioners to provide training on the most appropriate, safe and effective methods to prescribe and progress exercise for people with osteoporosis.”47 We plan to build on the results of this trial, our Cochrane review,32 and our new exercise recommendations8,87 by developing and evaluating knowledge translation strategies to integrate evidence-based exercise prescription into clinical practice for adults with osteoporosis.
Our study is not without limitations. We chose not to measure bone strength outcomes, such as BMD, because the cost was prohibitive, and we wanted to minimize participant burden. We modeled the design of our trial after what we envisioned the larger definitive trial to be, and it would be unrealistic to measure BMD in all participants in the larger trial. However, it would be valuable to determine whether exercise has an impact on BMD of the spine or hip in individuals with established osteoporosis, as many of the trials to date have excluded individuals on osteoporosis medication or with osteoporosis or prevalent fractures. We are only testing feasibility in 2 English-speaking countries; a larger trial may need to engage multiple countries, including those where English is not the official language. The proposed definitive trial is very ambitious and will require extensive collaboration, a strong communication nework, and funds to achieve our goal. However, the pilot trial will provide rich data on the effect of exercise in women with osteoporotic spine fractures, an area where there is little research to guide practice.
In summary, our goal is to move toward a trial of exercise with fracture as a primary endpoint, conducted in individuals at risk for fracture. The current trial will provide important feasibility information that can be used to design a definitive future trial and, in the meantime, provide insight on the effect of exercise on physical functioning, quality of life, and other outcomes important for patient and provider decision making.
Supplementary Material
Footnotes
The study was conceived by Dr Giangregorio, Dr Thabane, Dr Adachi, Dr Ashe, Dr Cheung, Dr Hill, Dr Hodsman, Dr Kendler, Dr Wark, and Dr Papaioannou. The final proposal approved for funding was developed by Dr Giangregorio, Dr Thabane, Dr Adachi, Dr Ashe, Dr Cheung, Dr Hill, Dr Hodsman, Dr Kendler, Dr Mittmann, and Dr Wark. Dr Thabane is the trial biostatistician. Dr Mittmann is the trial health economist. Dr Braun, Dr Prasad, and Mr Scherer provided essential contributions in the finalization of the protocol as it pertains to logistics and outcome assessment at sites without research infrastructure. Dr Fraser replaced Dr Hodsman as a site leader after it received funding and had input on the protocol as it has evolved. Dr Bleakney, Dr Kendler, and Dr Giangregorio finalized the X-ray protocol and fracture definitions for inclusion and for outcome assessment. Dr Ashe, Dr Hill, and Dr Giangregorio developed the standardized intervention frameworks. The first draft of the protocol manuscript was produced by Dr Giangregorio with input from all other authors. All authors reviewed, edited, and approved the final version of the manuscript.
The authors acknowledge the contributions of Osteoporosis Canada, Ravi Jain, and Dr Judi Laprade in the form of input on the trial protocol, provision of Bone Fit materials and training to physical therapists, and provision of control group materials. They thank Ina Ilse and Sheila O'Brien for reviewing the protocol and providing the patient perspective on behalf of the Canadian Osteoporosis Patient Network. They are grateful to Ddrops (Woodbridge, Ontario, Canada) and Swisse Wellness (Melbourne, Victoria, Australia) for providing vitamin D supplements to the study participants. The authors acknowledge Dr Norma MacIntyre for her intellectual contributions during the development of the proposal and Dr Brian Lentle for providing advice on the fracture outcome and a standard operating procedure for X-ray acquisition. The authors thank the study physical therapists: Mark Gary Anunciacion, Frances Batchelor, Nicole Hincks, Alexandra Ilich, Darien Lazowski, Danijela Les, Caitlin McArthur, Jennifer Roberts, Sue Williams, Joanne Hill, and Surabhi Venkatesh. They also thank Lesley Beaumont, Jemma Christie, Alice Demaras, Pauline Fisher, Skye Maclean, Jade Poll, Ramya Ponnuswamy, Christine Tsao, Sigrid Vinson, and Eugenia Wu for their ongoing work on the study and Dr Judi Laprade for expertise related to intervention implementation. Many thanks to Symron Bansal, Eric Bowman, Aidan Giangregorio, Caitlin McArthur, Helen Ng, and Carly Skidmore for collecting pilot data and assisting with study start-up.
The Build Better Bones With Exercise (B3E) trial was funded by the Canadian Institutes of Health Research (CIHR) (http://www.cihr-irsc.gc.ca/e/193.html). This funding source had no role in the design of the study and will not have any role during its execution, analyses, interpretation of the data, or decision to submit results. Dr Giangregorio is a CIHR New Investigator. She received funding from the CIHR Randomized Controlled Trials Mentoring Program to support activities related to the development of the protocol and an Early Researcher Award from the Ontario Ministry of Research and Innovation, as well as infrastructure funding from the Canadian Foundation for Innovation and the Ontario Research Fund. Dr Ashe is supported by career awards from the Michael Smith Foundation for Health Research and the CIHR.
ClinicalTrials.gov identifier NCT01761084 (Protocol: B3E Study Manual, version date August 15, 2013; trial sponsor: CIHR).
References
- 1. Hernlund E, Svedbom A, Ivergard M, et al. Osteoporosis in the European Union: medical management, epidemiology and economic burden—a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cauley JA, Thompson DE, Ensrud KC, et al. Risk of mortality following clinical fractures. Osteoporos Int. 2000;11:556–561 [DOI] [PubMed] [Google Scholar]
- 3. Ioannidis G, Papaioannou A, Hopman WM, et al. Relation between fractures and mortality: results from the Canadian Multicentre Osteoporosis Study. CMAJ. 2009;181:265–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lindsay R, Silverman SL, Cooper C, et al. Risk of new vertebral fracture in the year following a fracture. JAMA. 2001;285:320–323 [DOI] [PubMed] [Google Scholar]
- 5. MacLean C, Newberry S, Maglione M, et al. Systematic review: comparative effectiveness of treatments to prevent fractures in men and women with low bone density or osteoporosis. Ann Intern Med. 2008;148:197–213 [DOI] [PubMed] [Google Scholar]
- 6. Nevitt MC, Cummings SR, Stone KL, et al. Risk factors for a first-incident radiographic vertebral fracture in women > or = 65 years of age: the study of osteoporotic fractures. J Bone Miner Res. 2005;20:131–140 [DOI] [PubMed] [Google Scholar]
- 7. Papaioannou A, Morin S, Cheung AM, et al. ; Scientific Advisory Council of Osteoporosis Canada. 2010 clinical practice guidelines for the diagnosis and management of osteoporosis in Canada: summary. CMAJ. 2010;182:1864–1873 Available at: http://www.osteoporosis.ca/health-care-professionals/guidelines/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Giangregorio LM, Papaioannou A, Macintyre NJ, et al. Too fit to fracture: exercise recommendations for individuals with osteoporosis or osteoporotic vertebral fracture. Osteoporos Int. 2014;25:821–835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bonaiuti D, Shea B, Iovine R, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2002;(3):CD000333. [DOI] [PubMed] [Google Scholar]
- 10. Kelley GA. Exercise and regional bone mineral density in postmenopausal women: a meta-analytic review of randomized trials. Am J Phys Med Rehabil. 1998;77:76–87 [PubMed] [Google Scholar]
- 11. Martyn-St James M, Carroll S. Meta-analysis of walking for preservation of bone mineral density in postmenopausal women. Bone. 2008;43:521–531 [DOI] [PubMed] [Google Scholar]
- 12. Martyn-St James M, Carroll S. A meta-analysis of impact exercise on postmenopausal bone loss: the case for mixed loading exercise programmes. Br J Sports Med. 2009;43:898–908 [DOI] [PubMed] [Google Scholar]
- 13. Moayyeri A. The association between physical activity and osteoporotic fractures: a review of the evidence and implications for future research. Ann Epidemiol. 2008;18:827–835 [DOI] [PubMed] [Google Scholar]
- 14. Howe TE, Shea B, Dawson LJ, et al. Exercise for preventing and treating osteoporosis in postmenopausal women. Cochrane Database Syst Rev. 2011;(7):CD000333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kanis JA, Johnell O, Oden A, et al. Ten year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–995 [DOI] [PubMed] [Google Scholar]
- 16. Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med. 2004;164:1108–1112 [DOI] [PubMed] [Google Scholar]
- 17. Gillespie LD, Gillespie WJ, Robertson MC, et al. Interventions for preventing falls in elderly people. Cochrane Database Syst Rev. 2003;(4):CD000340. [DOI] [PubMed] [Google Scholar]
- 18. Sherrington C, Whitney JC, Lord SR, et al. Effective exercise for the prevention of falls: a systematic review and meta-analysis. J Am Geriatr Soc. 2008;56:2234–2243 [DOI] [PubMed] [Google Scholar]
- 19. Gillespie LD, Robertson MC, Gillespie WJ, et al. Interventions for preventing falls in older people living in the community. Cochrane Database Syst Rev. 2012;(9):CD007146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sherrington C, Tiedemann A, Fairhall N, et al. Exercise to prevent falls in older adults: an updated meta-analysis and best practice recommendations. N S W Public Health Bull. 2011;22:78–83 [DOI] [PubMed] [Google Scholar]
- 21. Faber MJ, Bosscher RJ, Chin A Paw MJ, van Wieringen PC. Effects of exercise programs on falls and mobility in frail and pre-frail older adults: a multicenter randomized controlled trial. Arch Phys Med Rehabil. 2006;87:885–896 [DOI] [PubMed] [Google Scholar]
- 22. Briggs AM, Greig AM, Wark JD. The vertebral fracture cascade in osteoporosis: a review of aetiopathogenesis. Osteoporos Int. 2007;18:575–584 [DOI] [PubMed] [Google Scholar]
- 23. Briggs AM, Wrigley TV, van Dieen JH, et al. The effect of osteoporotic vertebral fracture on predicted spinal loads in vivo. Eur Spine J. 2006;15:1785–1795 [DOI] [PubMed] [Google Scholar]
- 24. Jarvinen TL, Sievanen H, Khan KM, et al. Shifting the focus in fracture prevention from osteoporosis to falls. BMJ. 2008;336:124–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Binder EF, Brown M, Sinacore DR, et al. Effects of extended outpatient rehabilitation after hip fracture: a randomized controlled trial. JAMA. 2004;292:837–846 [DOI] [PubMed] [Google Scholar]
- 26. Gold DT, Shipp KM, Pieper CF, et al. Group treatment improves trunk strength and psychological status in older women with vertebral fractures: results of a randomized, clinical trial. J Am Geriatr Soc. 2004;52:1471–1478 [DOI] [PubMed] [Google Scholar]
- 27. Hongo M, Itoi E, Sinaki M, et al. Effects of reducing resistance, repetitions, and frequency of back-strengthening exercise in healthy young women: a pilot study. Arch Phys Med Rehabil. 2005;86:1299–1303 [DOI] [PubMed] [Google Scholar]
- 28. Papaioannou A, Adachi JD, Winegard K, et al. Efficacy of home-based exercise for improving quality of life among elderly women with symptomatic osteoporosis-related vertebral fractures. Osteoporos Int. 2003;14:677–682 [DOI] [PubMed] [Google Scholar]
- 29. Nikander R, Gagnon C, Dunstan DW, et al. Frequent walking, but not total physical activity, is associated with increased fracture incidence: a 5-year follow-up of an Australian population-based prospective study (AusDiab). J Bone Miner Res. 2011;26:1638–1647 [DOI] [PubMed] [Google Scholar]
- 30. Rikkonen T, Salovaara K, Sirola J, et al. Physical activity slows femoral bone loss but promotes wrist fractures in postmenopausal women: a 15-year follow-up of the OSTPRE study. J Bone Miner Res. 2010;25:2332–2340 [DOI] [PubMed] [Google Scholar]
- 31. Liu CJ, Latham N. Adverse events reported in progressive resistance strength training trials in older adults: 2 sides of a coin. Arch Phys Med Rehabil. 2010;91:1471–1473 [DOI] [PubMed] [Google Scholar]
- 32. Giangregorio LM, Macintyre NJ, Thabane L, et al. Exercise for improving outcomes after osteoporotic vertebral fracture. Cochrane Database Syst Rev. 2013;(1):CD008618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schousboe JT, Fink HA, Lui LY, et al. Association between prior non-spine non-hip fractures or prevalent radiographic vertebral deformities known to be at least 10 years old and incident hip fracture. J Bone Miner Res. 2006;21:1557–1564 [DOI] [PubMed] [Google Scholar]
- 34. Schousboe JT, Vokes T, Broy SB, et al. Vertebral fracture assessment: the 2007 ISCD official positions. J Clin Densitom. 2008;11:92–108 [DOI] [PubMed] [Google Scholar]
- 35. Siminoski K, Jiang G, Adachi JD, et al. Accuracy of height loss during prospective monitoring for detection of incident vertebral fractures. Osteoporos Int. 2005;16:403–410 [DOI] [PubMed] [Google Scholar]
- 36. Siminoski K, Warshawski RS, Jen H, Lee K. The accuracy of historical height loss for the detection of vertebral fractures in postmenopausal women. Osteoporos Int. 2006;17:290–296 [DOI] [PubMed] [Google Scholar]
- 37. Mini-Cog. Available at: http://www.alz.org/documents_custom/minicog.pdf
- 38. Yusuf S, Collins R, Peto R. Why do we need some large, simple randomized trials? Stat Med. 1984;3:409–422 [DOI] [PubMed] [Google Scholar]
- 39. Kernan WN, Viscoli CM, Makuch RW, et al. Stratified randomization for clinical trials. J Clin Epidemiol. 1999;52:19–26 [DOI] [PubMed] [Google Scholar]
- 40. Extension of the CONSORT Statement. Available at: http://www.consort-statement.org/extensions/interventions/non-pharmacologic-treatment-interventions/
- 41. Britt E, Hudson SM, Blampied NM. Motivational interviewing in health settings: a review. Patient Educ Couns. 2004;53:147–155 [DOI] [PubMed] [Google Scholar]
- 42. Rubak S, Sandbaek A, Lauritzen T, Christensen B. Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract. 2005;55:305–312 [PMC free article] [PubMed] [Google Scholar]
- 43. Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the Health Action Process Approach (HAPA). Rehabil Psychol. 2011;56:161–170 [DOI] [PubMed] [Google Scholar]
- 44. Scholz U, Schuz B, Ziegelmann JP, et al. Beyond behavioural intentions: planning mediates between intentions and physical activity. Br J Health Psychol. 2008;13(pt 3):479–494 [DOI] [PubMed] [Google Scholar]
- 45. Sniehotta FF, Scholz U, Schwarzer R. Action plans and coping plans for physical exercise: a longitudinal intervention study in cardiac rehabilitation. Br J Health Psychol. 2006;11(pt 1):23–37 [DOI] [PubMed] [Google Scholar]
- 46. Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381 [PubMed] [Google Scholar]
- 47. BoneFit. Available at: http://www.bonefit.ca/
- 48. Kado DM. The rehabilitation of hyperkyphotic posture in the elderly. Eur J Phys Rehabil Med. 2009;45:583–593 [PubMed] [Google Scholar]
- 49. Kado DM, Huang MH, Nguyen CB, et al. Hyperkyphotic posture and risk of injurious falls in older persons: the Rancho Bernardo Study. J Gerontol A Biol Sci Med Sci. 2007;62:652–657 [DOI] [PubMed] [Google Scholar]
- 50. Kelley GA, Kelley KS, Kohrt WM. Exercise and bone mineral density in men: a meta-analysis of randomized controlled trials [erratum in: Bone. 2013;56:30.]. Bone. 2013;53:103–111 [DOI] [PubMed] [Google Scholar]
- 51. Ashworth NL, Chad KE, Harrison EL, et al. Home versus center based physical activity programs in older adults. Cochrane Database Syst Rev. 2005;(1):CD004017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Campbell AJ, Robertson MC, Gardner MM, et al. Randomised controlled trial of a general practice programme of home based exercise to prevent falls in elderly women. BMJ. 1997;315:1065–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Campbell AJ, Robertson MC, Gardner MM, et al. Falls prevention over 2 years: a randomized controlled trial in women 80 years and older. Age Ageing. 1999; 28:513–518 [DOI] [PubMed] [Google Scholar]
- 54. Gardner MM, Buchner DM, Robertson MC, Campbell AJ. Practical implementation of an exercise-based falls prevention programme. Age Ageing. 2001;30:77–83 [DOI] [PubMed] [Google Scholar]
- 55. Gardner MM, Phty M, Robertson MC, et al. Application of a falls prevention program for older people to primary health care practice. Prev Med. 2002;34:546–553 [DOI] [PubMed] [Google Scholar]
- 56. Thomas S, Mackintosh S, Halbert J. Does the “Otago exercise programme” reduce mortality and falls in older adults: a systematic review and meta-analysis. Age Ageing. 2010;39:681–687 [DOI] [PubMed] [Google Scholar]
- 57. Bennell KL, Matthews B, Greig A, et al. Effects of an exercise and manual therapy program on physical impairments, function and quality-of-life in people with osteoporotic vertebral fracture: a randomised, single-blind controlled pilot trial. BMC Musculoskelet Disord. 2010;11:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Nikander R, Sievanen H, Heinonen A, et al. Targeted exercise against osteoporosis: a systematic review and meta-analysis for optimising bone strength throughout life. BMC Med. 2010;8:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dusdal K, Grundmanis J, Luttin K, et al. Effects of therapeutic exercise for persons with osteoporotic vertebral fractures: a systematic review. Osteoporos Int. 2011;22:755–769 [DOI] [PubMed] [Google Scholar]
- 60. Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, et al. American College of Sports Medicine position stand: exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–1530 [DOI] [PubMed] [Google Scholar]
- 61. Sackett DL. Commentary: measuring the success of blinding in RCTs: don't, must, can't or needn't? Int J Epidemiol. 2007;36:664–665 [DOI] [PubMed] [Google Scholar]
- 62. Ferrucci L, Guralnik JM, Studenski S, et al. Designing randomized, controlled trials aimed at preventing or delaying functional decline and disability in frail, older persons: a consensus report. J Am Geriatr Soc. 2004;52:625–634 [DOI] [PubMed] [Google Scholar]
- 63. Lamb SE, Jorstad-Stein EC, Hauer K, et al. Development of a common outcome data set for fall injury prevention trials: the Prevention of Falls Network Europe consensus. J Am Geriatr Soc. 2005;53:1618–1622 [DOI] [PubMed] [Google Scholar]
- 64. Lach HW, Reed AT, Arfken CL, et al. Falls in the elderly: reliability of a classification system. J Am Geriatr Soc. 1991;39:197–202 [DOI] [PubMed] [Google Scholar]
- 65. Ganz DA, Higashi T, Rubenstein LZ. Monitoring falls in cohort studies of community-dwelling older people: effect of the recall interval. J Am Geriatr Soc. 2005;53:2190–2194 [DOI] [PubMed] [Google Scholar]
- 66. Hannan MT, Gagnon MM, Aneja J, et al. Optimizing the tracking of falls in studies of older participants: comparison of quarterly telephone recall with monthly falls calendars in the MOBILIZE Boston study. Am J Epidemiol. 2010;171:1031–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Meretta BM, Whitney SL, Marchetti GF, et al. The five times sit to stand test: responsiveness to change and concurrent validity in adults undergoing vestibular rehabilitation. J Vestib Res. 2006;16:233–243 [PubMed] [Google Scholar]
- 68. Whitney SL, Wrisley DM, Marchetti GF, et al. Clinical measurement of sit-to-stand performance in people with balance disorders: validity of data for the Five-Times-Sit-to-Stand Test. Phys Ther. 2005;85:1034–1045 [PubMed] [Google Scholar]
- 69. Guralnik JM, Simonsick EM, Ferrucci L, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. 1994;49:M85–M94 [DOI] [PubMed] [Google Scholar]
- 70. Haines T, Kuys SS, Morrison G, et al. Development and validation of the balance outcome measure for elder rehabilitation. Arch Phys Med Rehabil. 2007;88:1614–1621 [DOI] [PubMed] [Google Scholar]
- 71. Kuys SS, Morrison G, Bew PG, et al. Further validation of the balance outcome measure for elder rehabilitation. Arch Phys Med Rehabil. 2011;92:101–105 [DOI] [PubMed] [Google Scholar]
- 72. Shipp KM, Purse JL, Gold DT, et al. Timed loaded standing: a measure of combined trunk and arm endurance suitable for people with vertebral osteoporosis. Osteoporos Int. 2000;11:914–922 [DOI] [PubMed] [Google Scholar]
- 73. Cook DJ, Guyatt GH, Adachi JD, et al. Development and validation of the mini-osteoporosis quality of life questionnaire (OQLQ) in osteoporotic women with back pain due to vertebral fractures: Osteoporosis Quality of Life Study Group. Osteoporos Int. 1999;10:207–213 [DOI] [PubMed] [Google Scholar]
- 74.About EQ-5D Available at: http://www.euroqol.org/about-eq-5d.html.
- 75. Bank of Canada. Available at: http://www.bankofcanada.ca/
- 76. Schwarzer R, Lippke S, Luszczynska A. Mechanisms of health behavior change in persons with chronic illness or disability: the health action process approach (HAPA). Rehabil Psychol. 2011;56:161–170 [DOI] [PubMed] [Google Scholar]
- 77. Hauer KA, Kempen GI, Schwenk M, et al. Validity and sensitivity to change of the falls efficacy scales international to assess fear of falling in older adults with and without cognitive impairment. Gerontology. 2011;57:462–472 [DOI] [PubMed] [Google Scholar]
- 78. Kempen GI, Yardley L, van Haastregt JC, et al. The short FES-I: a shortened version of the falls efficacy scale-international to assess fear of falling. Age Ageing. 2008;37:45–50 [DOI] [PubMed] [Google Scholar]
- 79. Hung A, Hamidi M, Riazantseva E, et al. Validation of a calcium assessment tool in postmenopausal Canadian women. Maturitas. 2011;69:168–172 [DOI] [PubMed] [Google Scholar]
- 80. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures: Fracture Intervention Trial Research Group. Lancet. 1996;348:1535–1541 [DOI] [PubMed] [Google Scholar]
- 81. SPIRIT Statement website. Available at: http://www.spirit-statement.org/
- 82. CONSORT website. Available at: http://www.consort-statement.org/
- 83. Allison PD. Missing Data. Thousand Oaks, CA: Sage Publications Inc; 2011. Series: Quantitative Applications in the Social Sciences [Google Scholar]
- 84. Tri-Council Policy Statement. Available at: http://www.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/Default/
- 85. Giangregorio LM, Macintyre NJ, Heinonen A, et al. Too fit to fracture: a consensus on future research priorities in osteoporosis and exercise. Osteoporos Int. 2014;25:1465–1472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Canada's Strategy for Patient-Oriented Research. Available at: http://www.cihr-irsc.gc.ca/e/44000.html
- 87. Giangregorio LM, Ashe MC, Shipp K, et al. “Is this exercise safe?” Building consensus around responses to common questions about physical activity posed by people with osteoporosis. J Bone Miner Res. 2013;28(suppl 1). Available at: http://www.asbmr.org/education/Abstract Detail?aid=ccf88652-3d98-4a0d-843f-ba44e6593d5f [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


