Abstract
The National Alzheimer's Project Act, signed into law in 2011, mandates a National Plan to Address Alzheimer's Disease that is updated annually. In the Plan, the term Alzheimer disease includes not only Alzheimer disease (AD) proper, but also several specified related dementias, namely, frontotemporal, Lewy body, vascular, and mixed dementia. In response to a specific action item in the 2012 National Plan, the National Institute of Neurological Disorders and Stroke, in collaboration with the National Institute on Aging, convened panels of experts and conducted a 2-day public conference to develop research priorities and timelines for addressing Alzheimer disease–related dementias (ADRD) in 5 topic areas: multiple etiology dementias, health disparities, Lewy body dementias including dementia with Lewy bodies and Parkinson disease dementia, frontotemporal dementia and related tauopathies, and vascular contributions to ADRD. By design, the product was up to 8 prioritized research recommendations in each topic area including estimated timelines from when work on a recommendation is started to completion or to full implementation of an ongoing activity, and recognition of shared research themes across recommendations. These included increased education and training of both researchers and health care professionals, addressing health disparities, fundamental neurobiology research, advanced diagnostics, collaborative biosample repositories, and a focus on developing effective interventions to prevent or treat ADRD by the year 2025 as targeted by the National Plan.
Dementia is a syndrome, an overlapping constellation of signs and symptoms of impaired cognition caused by multiple disorders, which often can be difficult to distinguish clinically.1 While Alzheimer disease proper, referred to as AD here, is the most common cause of dementia in older adults, in this group of individuals, AD frequently coexists with other diseases that may contribute to dementia (termed multiple etiology dementia) resulting in, for example, “AD plus Lewy body dementia (LBD)” or, most frequently, “AD plus vascular cognitive impairment (VCI)/vascular dementia (VaD),” although LBD and VCI/VaD can lead to dementia in the absence of AD.2,3 Moreover, although AD is the major cause of dementia in the elderly, this is not the case during middle age when frontotemporal degeneration (FTD) has a similar incidence and prevalence as AD.4–6 The situation is further complicated by the fact that the current evidence base for molecular and cellular mechanisms underlying some of these diseases is limited, with the existing data derived overwhelmingly from those of European ancestry.
The diseases that make up these AD-related dementias (ADRD) are typically chronic diseases. While this may seem obvious, the significance of this point is that clinical expression of chronic diseases develops over time with stages that occur before full clinical manifestation (in this case dementia) including partial clinical expression (prodrome) or even an apparent lack of signs and symptoms (latency).1,7–10 The complexity of the dementia syndrome represents a serious challenge to accurate diagnosis, often exceeds the expertise of general practitioners, and is on track to far exceed the capacity of our nation's specialist workforce unless appropriate steps are taken soon. Complementary to accurate diagnosis in individuals from disparate populations is the essential need to discover safe and effective interventions that target the full spectrum of this syndrome to prevent, delay onset, slow progression, or treat cognitive impairment.11 Knowledge of the molecular mechanisms underlying ADRD remains incomplete, especially their potential interaction, and these gaps in our knowledge about fundamental neurobiology are a major barrier to the development of experimental models that are representative of human disease and fuel therapeutic discovery.
The National Alzheimer's Project Act (NAPA) that was signed into law in 2011 by President Obama represents the culmination of a number of efforts, including the Congressional Task Force on Alzheimer's Disease (founded in 1999), the Alzheimer's Study Group (founded in 2007), and coordinated efforts from not-for-profit groups. NAPA mandates an integrated National Plan to Address Alzheimer's Disease that is updated annually.12,13 This Plan specifically addressed ADRD with the following statement in 2012: “In this plan, the term ‘Alzheimer's disease,’ or AD, refers to Alzheimer's disease and related dementias, consistent with the approach Congress used in NAPA. Related dementias include frontotemporal, Lewy body, mixed, and vascular dementia. It is often difficult to distinguish between Alzheimer's disease and other dementias in terms of clinical presentation and diagnosis. Some of the basic neurodegenerative processes have common pathways. People with dementia and their families face similar challenges in finding appropriate and necessary medical and supportive care.” The Plan, therefore, has high significance for stroke and several neurodegenerative disorders in addition to AD: dementia with Lewy bodies, Parkinson disease (PD) with dementia, VaD, and FTD.
The National Plan to Address Alzheimer's Disease is organized into 5 goals with corresponding strategies. One of these is to “identify research priorities and milestones” and includes a call to “convene a scientific workshop on other dementias in 2013.” This workshop complements the National Institute on Aging (NIA) Alzheimer's Disease Research Summit 2012: Path to Treatment and Prevention held on May 14 and 15, 2012. In response to this charge, the National Institute of Neurological Disorders and Stroke (NINDS), in collaboration with the NIA, convened panels of experts and conducted a 2-day public conference, Alzheimer's Disease–Related Dementias (ADRD): Research Challenges and Opportunities, on May 1 and 2, 2013. Herein, we communicate the outcome of that conference and its recommendations to the community of investigators, funding organizations, policy-makers, patients, caregivers, and other interested parties.
METHODS
The overall process was organized into preconference, conference, and postconference activities.
Preconference.
Preconference efforts began in the fall of 2012 when the ADRD Steering Committee (Drs. Ronald Petersen, David Holtzman, Neil Buckholtz, and Sharon Hesterlee) and NIH members of the Organizing Committee assembled 5 committees of international experts focused on the 5 topic areas: (1) multiple etiology dementias and their diagnostic challenges; (2) health disparities; (3) LBD including dementia with Lewy bodies (DLB) and PD dementia (PDD); (4) FTD and related tauopathies; and (5) vascular contributions to dementia. The decision process that led to these 5 areas, and the creation of corresponding committees for each, was driven by the 2012 National Plan's guidance on related dementias. Because it was not possible for the ADRD 2013 conference to cover the full spectrum of dementias, it was decided that those named in the Plan, FTD, LBD, and vascular, would be covered in disease-specific sessions. Mixed dementias, which we refer to as multiple etiology dementia, are also named in the Plan, affording the opportunity for a session to address cross-cutting themes that could touch on, for example, diagnostic issues related to all dementias. Health disparities, also prioritized in the National Plan, were selected for their significance across all ADRD.
Each of the 5 topic areas had a committee of 8 to 18 members (appendix e-1 on the Neurology® Web site at Neurology.org) that was tasked with developing a prioritized list of up to 8 research recommendations and an approximate timeline to completion or full implementation for an ongoing activity for each recommendation. Each committee met a number of times by teleconference between December 2012 and May 2013 to develop and revise draft recommendations for consideration at the conference. In addition to these calls, monthly executive oversight calls were held that included the Steering Committee, the Scientific Chair, cochairs of the 5 committees, and NIH members of the Organizing Committee.
The resulting format of prioritized research recommendations differed somewhat among the 5 topic areas. The decision to allow this was deliberate, and had the goal of not unnecessarily constraining optimal prioritization within each topic area. Timelines were made uniform across topic areas (1–3 years, 3–7 years, 7–10 years, or >10 years) and reflect time from the start of work to completion or achieving fully operational status of the recommendation.
The conference was broadly advertised to the scientific community, government agencies, nongovernmental organizations, and the public. The conference's abridged agenda was available online to meeting registrants in April 2013, and a paper copy of the drafted proposed recommendations was distributed to all conference attendees on the morning of May 1, 2013.
Conference.
The ADRD Conference was held on May 1 and 2, 2013, in Natcher Auditorium on the NIH campus. There were 567 registrants, of which 322 individuals joined in person; in addition, more than 200 people joined online. The goal of the conference was to solicit input and feedback on the proposed recommendations and timelines by engaging the audience in a discussion of the rationale for each recommendation, its priority level, and anticipated timeline. There were 5 sessions covering the 5 topic areas, which were led by the cochairs for that committee. The conference concluded with a public discussion section open to all attendees.
Postconference.
Postconference work to prepare the final report included several conference calls among the committees to refine the content, prioritization, and proposed timelines for the research recommendations. The final research recommendations (appendix e-2) were presented to the National Advisory Neurological Disorders and Stroke Council and approved on September 12, 2013. They were presented to the NAPA Advisory Council meeting on Alzheimer's Research, Care, and Services on December 2, 2013. The Research Subcommittee of the Advisory Council on Alzheimer's Research, Care, and Services recommended at the February 3, 2014, NAPA Council meeting that, based on the recommendations of the 2013 ADRD Research Workshop, interim milestones for achieving specific research goals for the study of AD-related disorders should be explicitly added to the National Plan.
RESULTS
The product was the prioritized research recommendations, estimated timelines, and a summary of shared themes.
Prioritized research recommendations.
Research recommendations are presented by topic area, focus areas within topic areas, and prioritization within each topic and focus area in this section, and by timeline in the next section. The language used in the recommendations represents the final consensus agreed to by each committee. Detailed discussion points for the top priority recommendations are summarized here to illustrate the scope of the discussions. Discussion points for all recommendations are provided in appendix e-2.
Multiple etiology dementias.
Focus areas chosen were Differential Diagnosis and Epidemiology with 3 recommendations for each (table 1). The top priority Differential Diagnosis recommendation is to develop clinical algorithms for detection of prototypical neurodegenerative dementias and VCI in primary care, general neurology, and general psychiatry outpatient settings, and clinical algorithms for referral to specialists in appropriate cases that also might involve consultations using novel technologies. Advances have occurred in the definition of clinically important features that distinguish the dementia of AD from VCI, LBD, behavioral variant FTD, primary progressive aphasia, normal pressure hydrocephalus, and prion disease, as well as other rapidly progressive dementias and syndromes with multiple neurodegenerative and vascular elements. There is a pressing need to translate new advances in diagnoses and care to where cognitive disorders present. Detection of the prototypical presentations by first-line clinicians must be emphasized, and new approaches to diagnosis of cognitive disorders in primary care should be pursued and evaluated using rigorous criteria for effectiveness. The consensus of the panel is that there is currently a critical shortage of cognitive specialists and researchers,14 and that it is imperative to support high-quality clinical research training programs that attract physicians and nonphysician researchers in geriatrics, behavioral neurology, and geriatric psychiatry. The top priority Epidemiology recommendation is to conduct population-based studies of dementia prevalence and incidence in diverse ethnic groups and age ranges using imaging and fluid biomarkers. Almost all of the currently available estimates of incidence and prevalence of diseases that cause dementia have utilized the single diagnosis model for reporting results. Future studies should develop the capability of reporting prevalence and incidence in terms of multiple etiology, utilize currently available imaging and fluid biomarker assessments to allow more refined and complete assessments of etiology(ies), and serve as test-beds for identification and validation of new biomarkers. These studies must involve diverse ethnic groups because of potential differences in risk factors for dementia and in response to therapies.
Table 1.
Topic area 1: Research recommendations for multiple etiology dementias

Health disparities.
Focus areas chosen were Recruitment and Advancing Treatment and Prevention Strategies, with 4 recommendations for each (table 2). The top priority Recruitment recommendation is to initiate and leverage ongoing longitudinal community-based cohort studies of incident dementia in diverse populations incorporating imaging, fluid biomarkers, and autopsy. This would include the following: enrolling people without dementia at baseline; using community-based rather than clinic-based recruitment strategies; including individuals representing demographic diversity; and assessing a wide range of risk factors including via cutting-edge imaging and fluid biomarkers (both blood and CSF) and autopsy when possible. The top priority Advancing Treatment and Prevention Strategies recommendation is to enhance the design of all vascular health intervention trials to improve their application to diverse populations. Evidence exists that vascular health is critical to delaying onset of dementia, potentially not only VCI/VaD but also LBD and AD, and may be different across diverse populations. Intervention trials for cardiovascular and stroke outcomes could provide valuable secondary evidence on prevention of dementia, if high-quality standardized cognitive outcomes are included.
Table 2.
Topic area 2: Research recommendations for health disparities

LBD: DLB and PDD.
The LBD panel prioritized their focus areas as well, with 2 recommendations for each (table 3). The top priority focus, Establish Longitudinal Cohorts with Common Measures, Culminating in Autopsy Studies, prioritizes initiating clinical trials for DLB and PDD using existing and newly developed symptomatic therapies that address key symptoms affecting patient function and caregiver burden. The aim is to engage existing clinical networks and to establish new ones, including movement disorders specialists, behavioral neurologists, psychiatrists, and sleep disorder specialists, to use well-characterized cohorts of DLB and PDD for treatment trials with current Food and Drug Administration–approved drugs. Also recommended are efforts to capitalize on existing longitudinal cohorts studying late-life dementia disorders, such as Alzheimer's Disease Neuroimaging Initiative, by enriching the population with individuals with potential early manifestations of DLB. Although the majority of patients with PD will eventually develop dementia, the time from the onset of motor symptoms to dementia varies markedly. Very few prospective biomarker studies exist. Such biomarkers may provide insight into the mechanisms leading to cognitive decline in PD and thus represent future therapeutic markers. The next focus, Discover Disease Mechanisms Through Brain Mapping and Genetics, prioritizes using well-defined autopsy cohorts with DLB or PDD to systematically map disease-specific changes in the brain, spinal cord, and peripheral autonomic nervous system with state-of-the-art methods to identify underlying disease mechanisms for future biomarker and therapeutic approaches. Data generated in this mapping initiative should be incorporated into an open-access database that links clinical, biological, and autopsy data.
Table 3.
Topic area 3: Research recommendations for Lewy body dementias—DLB and PDD

FTD and related tauopathies.
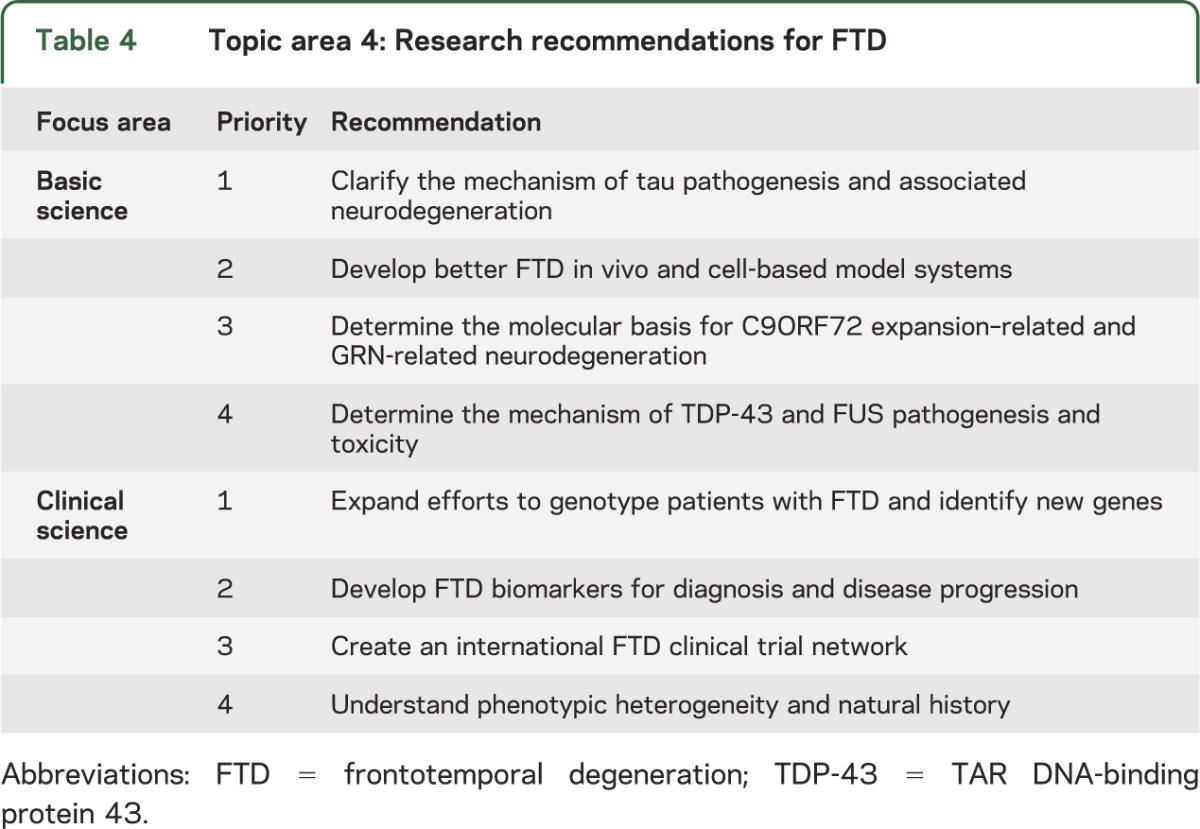
Focus areas chosen were Basic Science: Pathogenesis and Toxicity and Clinical Science: FTD Clinical Discovery, Tools, and Cohorts with 4 recommendations for each (table 4). The top priority Basic Science recommendation is to clarify the mechanism of tau pathogenesis and associated neurodegeneration. The mechanism of tau-driven neurotoxicity needs to be determined to identify optimal therapeutic approaches. Innovative cell-based, animal model, and human postmortem studies are recommended. Genetic models should be complemented with other methods that may simulate aspects of sporadic disease. The top priority Clinical Science recommendation is to expand efforts to genotype patients with FTD and identify new genes. Accelerated discovery of new familial FTD genes and genotyping support for research on patients with known genetic profiles are needed. The recommended approach is to provide increased clinical resources to identify and collect FTD families with a range of phenotypes and create core services for FTD genotyping and DNA banking. These efforts should include amyotrophic lateral sclerosis kindreds in gene discovery studies.
Table 4.
Topic area 4: Research recommendations for FTD
Vascular contributions to ADRD.
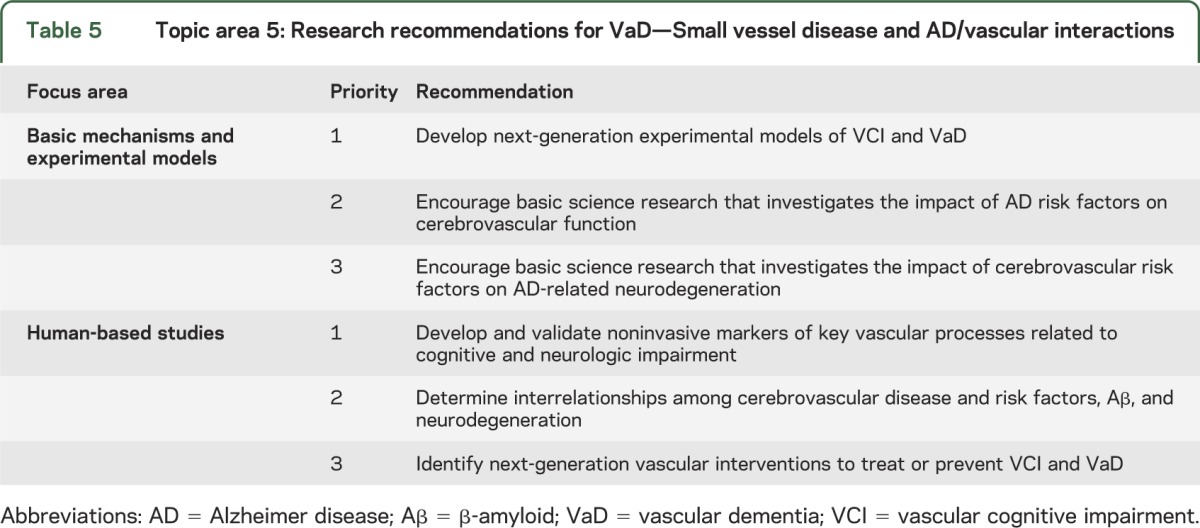
With an overall emphasis on small vessel disease and AD/vascular interactions, the selected focus areas were Basic Mechanisms and Experimental Models and Human-Based Studies, with 3 recommendations for each (table 5). The top priority Basic Mechanisms and Experimental Models recommendation is to develop next-generation experimental models of VCI and VaD. Animal models and human studies should be designed to inform each other from the cellular to the systems level. Because of the pathogenic diversity of VCI/VaD syndromes, multiple models, each recapitulating key features of a specific human disease process are needed. In particular, this means establishing animal models that reproduce small vessel disease and other key pathogenic processes thought to result in cognitive impairment. Such rodent models should also easily be applied to AD research, so VCI/VaD and AD can be studied individually and in combination, and be used to increase knowledge of lifestyle risk factors. Development of new tools for cell-specific genotyping and phenotyping is recommended, as well as testing the effect of pathogenic factors on cerebral blood vessels and how these affect brain function. The top priority Human-Based Studies recommendation is to develop and validate noninvasive markers of key vascular processes related to prediction, onset, and progression of cognitive and neurologic impairment. Identifying biomarkers of key microvascular, including biomarkers of tissue injury, vessel disease, and other vascular alterations such as blood-brain barrier dysfunction, vascular reactivity, and hypoperfusion is recommended.
Table 5.
Topic area 5: Research recommendations for VaD—Small vessel disease and AD/vascular interactions
Estimated timelines.
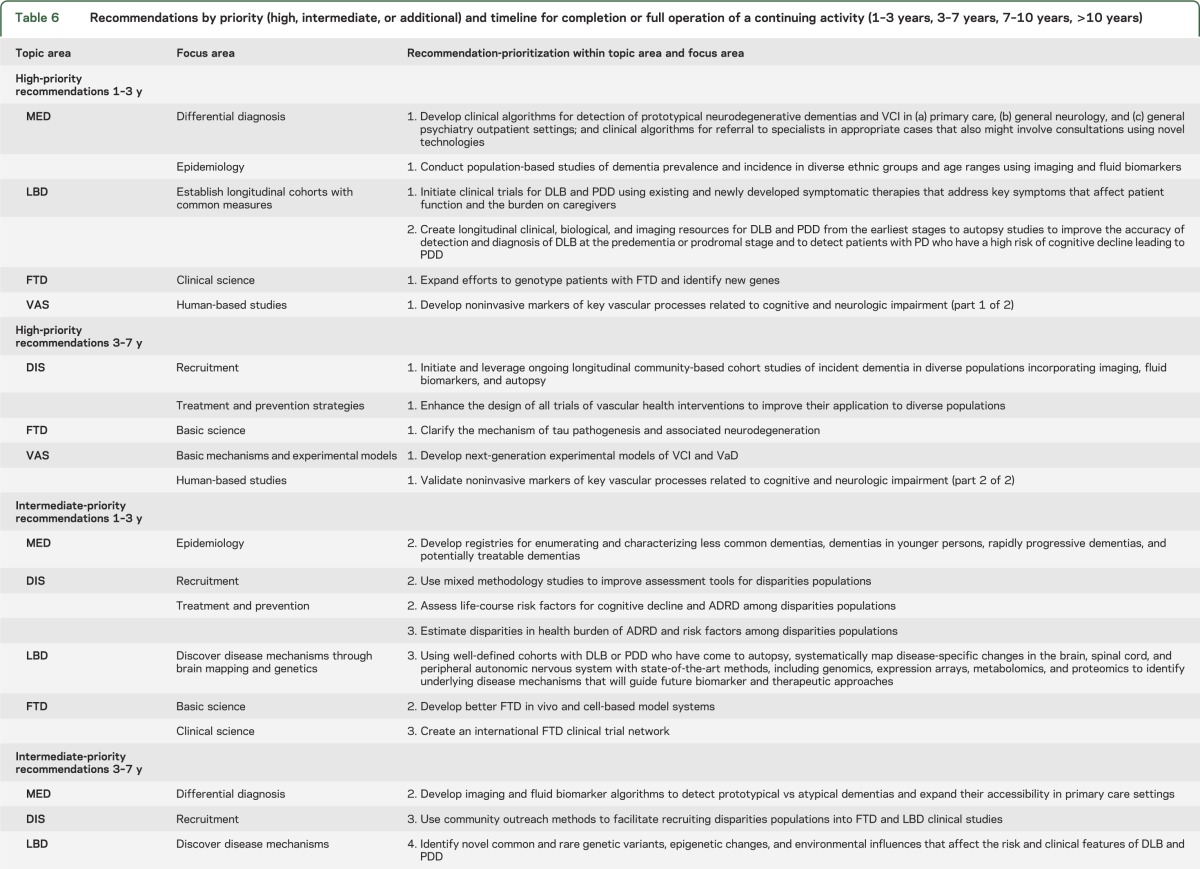
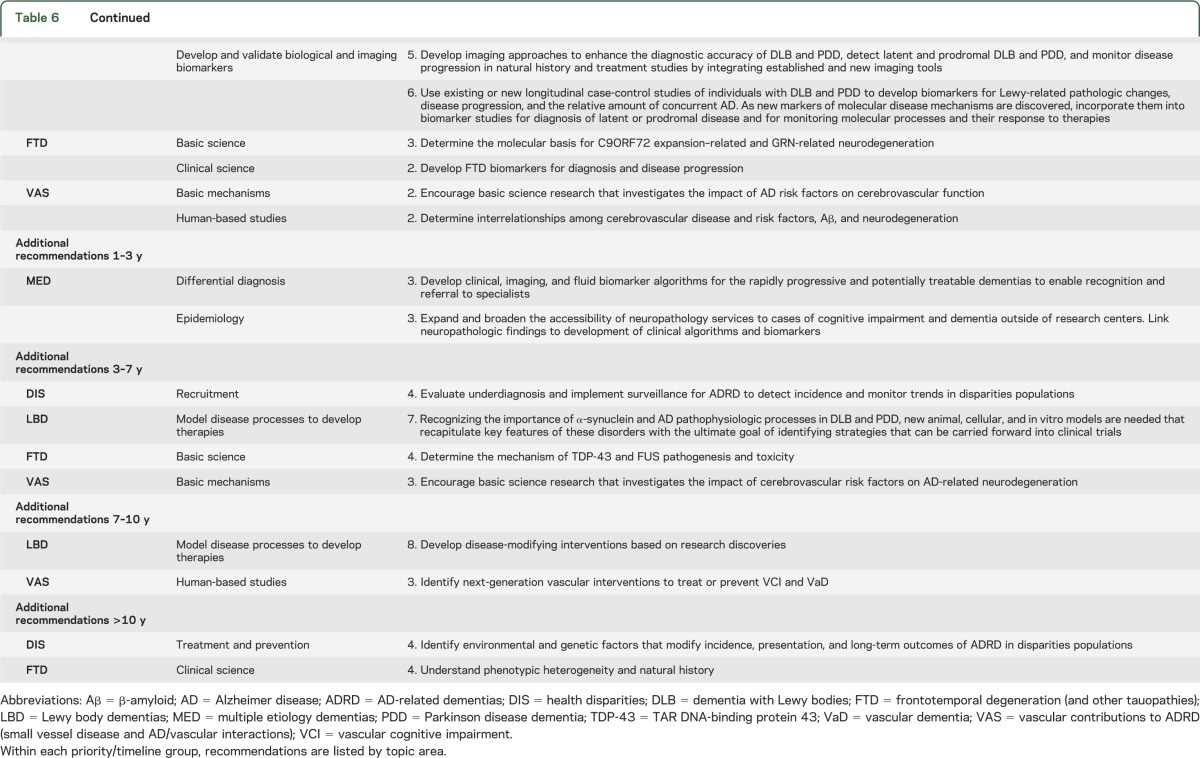
Each recommendation was given an estimated timeline for completion or full implementation of an ongoing activity. The timelines for the research recommendations are independent of priority; they are estimates of the interval from the present time to anticipated completion or full implementation of the recommendations. Estimated timelines were 1–3 years, 3–7 years, 7–10 years, or >10 years. Table 6 shows recommendations stratified by timeline for high, intermediate, and additional priority. There are several reasons why one recommendation might be expected to take longer than another, e.g., more work needs to be done, or other goals need to be accomplished before full success can be achieved. However, longer expected time to completion or full implementation did not diminish priority and should not be misconstrued as an option to delay onset of work.
Table 6.
Recommendations by priority (high, intermediate, or additional) and timeline for completion or full operation of a continuing activity (1–3 years, 3–7 years, 7–10 years, >10 years)
Shared themes.
It became clear early in our work that there are shared themes across ADRD, as well as with dementias more generally, including AD itself. We addressed the issue of shared themes in part through 2 cross-cutting topic areas: Multiple Etiology Dementias and Health Disparities Research. Recommendations for these topic areas are fundamental not only to ADRD, but to all diseases that cause dementia. Training and education of researchers and health care providers in these different neurodegenerative disorders are critically needed to meet the coming challenge to our health care systems nationally. Indeed, a shared priority that can be initiated immediately is improved health care professional education at all levels and training of providers of all types including behavioral neurologists, counselors, genetic counselors, general practitioners, nurses, geriatricians, neuropathologists, psychiatrists, neuroimagers, and psychologists. This shared theme, captured as the top priority recommendation presented in table 6 (Multiple Etiologies/Differential Diagnosis) and discussed above, is essential to improved diagnostics, and is linked with improved caregiver support to enhance quality of life and to fuel patient-based research. Similarly, each recommendation developed for Health Disparities research can be applied more generally across all diseases that cause dementia.
Our preconference work highlighted several other shared themes. Although we discussed these at length and coordinated our concepts, we deliberately eschewed developing overarching or cross-disorder recommendations during our preconference work because it was not clear that each would receive the same priority ranking within each topic area. However, at the conference, a consensus emerged to stress the additional major shared themes among topic areas. They include the following:
Fundamental research to determine the mechanisms of ADRD and the interactions among genetic factors, environment, and aging.
Improved diagnostics, including imaging and biomarkers, to fuel translational and clinical research in ADRD. Special focus needs to be given to development of validated diagnostics for the earliest stages of disease and for disease progression.
Optimized repositories of tissue, cells, biofluids, and molecules, both in scale and governance, as collaborative resources for fundamental and translational research. This topic is being addressed in other forums, e.g., the 2013 NINDS Repository Scientific Liaison Meeting and NIA Biospecimen Best Practices review (in progress).
All of these efforts ultimately are directed at producing effective rational interventions for ADRD to be evaluated in clinical trials.
DISCUSSION
Each recommendation in the report is an important research goal. Committees were charged with assigning ranked research priorities within each topic area, a difficult task that required balancing among differing expertise, public comments, areas of focus, and opinions about the current state and possible future directions. Each topic area's prioritized research recommendations reflect that group's consideration of what priorities and timelines chart the best route to prevent, stop, or cure ADRD. Research priorities for AD, including molecular and disease mechanisms, diagnostics, and treatments already had been established in the Alzheimer's Disease Research Summit 2012, and thus were not a focus here unless interacting with an ADRD.
We note that relative ranking should not be interpreted as indicating that any recommendation is unimportant. Indeed, inclusion in this report means that research goal is among the top priority items in its respective field. These recommendations were presented to the NINDS Council as consensus among the 5 committees convened to draft and prioritize them. During this process, it was clear that individually there were many different priorities, including different opinions about ranking and timelines. Despite these honest differences of opinion, the report reflects a carefully considered consensus with input from a large group of physicians, scientists, government officials, advocacy groups, caregivers, patients, and the public.
It is critically important to continuously explore new promising areas of research as they emerge. In this context, the ADRD 2013 recommendations are not meant to be exclusive, because it is hazardous to predict where the next scientific breakthroughs will appear. The recommendations do, however, serve as guideposts for future research supported by government, academic, not-for-profit, for-profit, and international partners designed to decrease the burden of illness due to ADRD, as well as the tragic consequences for those affected and for their loved ones. Inclusion of the related dementias in the National Plan effectively formalizes the Plan's recognition of the clinical and scientific reality that commonalities in the underlying biology of the ADRD and AD suggest that a fundamental discovery in one may affect multiple diseases. Finally, this conference made clear that much remains to be discovered and developed, and that there is an enthusiastic community to execute this research. The rate of progress seems limited only by available resources and the difficulty of the problem. Our hope is that the ADRD 2013 Conference recommendations will help guide research in ADRD to deeper knowledge and effective interventions as quickly and efficiently as possible.
Supplementary Material
ACKNOWLEDGMENT
The authors thank Dr. Kathleen S. Montine for assistance in responding to reviewer comments, and for substantive and technical editing.
GLOSSARY
- AD
Alzheimer disease
- ADRD
Alzheimer disease–related dementias
- DLB
dementia with Lewy bodies
- FTD
frontotemporal degeneration
- LBD
Lewy body dementia
- NAPA
National Alzheimer's Project Act
- NIA
National Institute on Aging
- NINDS
National Institute of Neurological Disorders and Stroke
- PD
Parkinson disease
- PDD
Parkinson disease dementia
- VaD
vascular dementia
- VCI
vascular cognitive impairment
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Dr. Montine: manuscript preparation and critical revision of the manuscript for important intellectual content. Dr. Koroshetz, Dr. Babcock, Dr. Dickson, Dr. Galpern, Dr. Glymour, Dr. Greenberg, Dr. Hutton, Dr. Knopman, Dr. Kuzmichev, Dr. Manly, Dr. Marder, Dr. Miller, Dr. Phelps, Dr. Seeley, Dr. Sieber, Dr. Silverberg, Dr. Sutherland, Dr. Torborg, Dr. Waddy, and Dr. Zlokovic: critical revision of the manuscript for important intellectual content. Dr. Corriveau: manuscript preparation and critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This conference was supported by the NINDS, organized in collaboration with the NIA, with assistance from the FNIH, and with additional support from the Alliance for Aging Research, the Alzheimer's Association, the Association for Frontotemporal Degeneration and USAgainstAlzheimer's.
DISCLOSURE
T. Montine, W. Koroshetz, D. Babcock, D. Dickson, W. Galpern, M. Glymour, and S. Greenberg report no disclosures relevant to the manuscript. M. Hutton is an employee of Eli Lilly and Company. D. Knopman, A. Kuzmichev, J. Manly, K. Marder, B. Miller, C. Phelps, W. Seeley, B. Sieber, N. Silverberg, M. Sutherland, C. Torborg, S. Waddy, B. Zlokovic, and R. Corriveau report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:263–269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hyman BT, Phelps CH, Beach TG, et al. National Institute on Aging–Alzheimer's Association guidelines for the neuropathologic assessment of Alzheimer's disease. Alzheimers Dement 2012;8:1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer's disease and other disorders. Nat Rev Neurosci 2011;12:723–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ratnavalli E, Brayne C, Dawson K, Hodges JR. The prevalence of frontotemporal dementia. Neurology 2002;58:1615–1621 [DOI] [PubMed] [Google Scholar]
- 5.Knopman DS, Petersen RC, Edland SD, Cha RH, Rocca WA. The incidence of frontotemporal lobar degeneration in Rochester, Minnesota, 1990 through 1994. Neurology 2004;62:506–508 [DOI] [PubMed] [Google Scholar]
- 6.Mercy L, Hodges JR, Dawson K, Barker RA, Brayne C. Incidence of early-onset dementias in Cambridgeshire, United Kingdom. Neurology 2008;71:1496–1499 [DOI] [PubMed] [Google Scholar]
- 7.Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:270–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging–Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement 2011;7:280–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Litvan I, Goldman JG, Troster AI, et al. Diagnostic criteria for mild cognitive impairment in Parkinson's disease: Movement Disorder Society Task Force guidelines. Mov Disord 2012;27:349–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iadecola C. The pathobiology of vascular dementia. Neuron 2013;80:844–866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larson EB, Yaffe K, Langa KM. New insights into the dementia epidemic. N Engl J Med 2013;369:2275–2277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assistant Secretary for Planning and Evaluation. National Plan to address Alzheimer's Disease. U.S. Department of Health and Human Services, 2012. Available at: http://aspe.hhs.gov/daltcp/napa/natlplan.shtml. Accessed March 20, 2014
- 13.Assistant Secretary for Planning and Evaluation. National Plan to address Alzheimer's Disease: 2013 update. U.S. Department of Health and Human Services, 2013. Available at: http://aspe.hhs.gov/daltcp/napa/NatlPlan2013.shtml. Accessed March 20, 2014
- 14.Institute of Medicine. Retooling for an Aging America: Building the Health Care Workforce. Washington, DC: Institute of Medicine of the National Academies; 2008 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


