Abstract
Objective
Thrombosis occurs at sites of vascular injury when platelets adhere to subendothelial matrix proteins and to each other. Platelets express many surface receptor proteins, the function of several of these remains poorly characterized. Cadherin 6 is expressed on the platelet surface and contains an arginine-glycine-aspartic acid motif, suggesting that it might have a supportive role in thrombus formation. The aim of this study was to characterize the role of cadherin 6 in platelet function.
Methods and Results
Platelet aggregation was inhibited by both antibodies and exogenous soluble cadherin 6. Platelet adhesion to immobilized cadherin 6 was inhibited by arginine-glycine-aspartic acid-serine tetrapeptides. Antibodies to αIIbβ3 inhibited platelet adhesion to cadherin 6. Because platelet aggregation occurs in fibrinogen and von Willebrand factor double-deficient mice, we investigated whether cadherin 6 is an alternative ligand for the integrin αIIbβ3. Platelet aggregation in fibrinogen and von Willebrand factor double-deficient mice was significantly inhibited by an antibody to cadherin 6. In flow-based assays, inhibition of cadherin 6 caused a marked reduction in thrombus formation in both human and mouse blood.
Conclusion
This study demonstrates the role of cadherin 6 as a novel ligand for αIIbβ3 and highlights its function in thrombus formation.
Keywords: cadherin 6, αIIbβ3, thrombus formation, platelet aggregation
Platelet thrombus formation is a dynamic process; the same biological process is also essential for the maintenance of hemostasis at sites of vascular damage. Platelets bind to von Willebrand factor (VWF), and through a complex signaling mechanism the integrin receptor αIIbβ3 undergoes a conformational change and binds fibrinogen cross-linking platelets. Recent evidence in mice lacking both fibrinogen and VWF shows that platelet aggregation still occurs in vivo and in vitro,1,2 suggesting the presence of other functional receptors or αIIbβ3 ligands in platelets that mediate aggregation.
Platelets express many different surface receptors whose functions are poorly understood. We have previously characterized the role of 81 platelet surface receptors in platelet activation using cell-permeable peptides. Cadherins were first identified on platelets by Elrod et al.3 Moreover, we have recently demonstrated that a peptide targeting the intracellular domain of cadherin 6 inhibited platelet aggregation, suggesting a role for this protein in platelet function.4 The cadherins are a superfamily of Ca++-dependent proteins, which mediate homophilic cell adhesion and play an important role in embryonic development. They typically consist of 5 tandem extracellular cadherin domains, a transmembrane spanning segment, and cytoplasmic region. Cadherin 6 is a type II classic cadherin. It is unusual in that it has an arginine-glycine-aspartic acid (RGD) motif in the first extracellular domain.5 The integrin-binding RGD motif is found in fibrinogen and other ligands of platelet αIIbβ3, including VWF, vitronectin, and fibronectin.6,7 Many snake venoms containing an RGD motif specifically inhibit ligand binding to integrins and are potent integrin antagonists.8–10
Because our peptide data demonstrated that targeting the intracellular region of cadherin 6 inhibited platelet aggregation and also that cadherin 6 has an RGD motif, we therefore aimed to characterize the role of cadherin 6 in platelet function. In the present study, we confirm that cadherin 6 is expressed on the platelet surface and show that blocking cadherin 6 with both monoclonal and polyclonal anti-cadherin 6 antibodies or exogenous protein inhibits platelet aggregation in both normal blood donors and fibrinogen and VWF double-deficient (Fg/VWF−/−) mice. We demonstrate that platelets adhere to cadherin 6 in an αIIbβ3-dependent manner. Moreover, we demonstrate that inhibition of cadherin 6 by antibodies or soluble cadherin 6 protein causes a significant reduction in thrombus formation in flow-based assays of both human and mouse blood.
Methods
Materials
Thrombin was purchased from Enzyme Research Laboratories Ltd (Swansea, UK). Mouse monoclonal anti-human cadherin 6 antibody, clone 2B6, was purchased from Millipore (Cork, Ireland). A polyclonal anti-human cadherin 6 antibody, derived from sheep immunized with the whole extracellular domain of cadherin 6, purified recombinant cadherin 6_IgG fusion protein (Cdh6_IgG), purified placental cadherin_IgG recombinant fusion protein (P-Cdh_IgG), and vascular endothelial cadherin_IgG fusion protein (VE-Cdh_IgG) were from R&D Systems (Abingdon, UK). PAC1 and P-selectin antibodies were from Becton Dickinson (Oxford, UK). Fibrinogen and thrombin receptor activating peptide (TRAP; SFLLRN-NH2) were purchased from Sigma-Aldrich Ireland (Dublin, Ireland). Mouse thrombin receptor activating peptide (TRAP4; AYPGKF-NH2) was purchased from Peptides International (Louisville, KY). Extended peptide corresponding to the RGD-containing segment of the first cadherin 6 ectodomain (CDH6_RGD: YVGKLHSDQDRGDGSLKYILSGD and CDH6_RGE: YVGKLHSDQDRGEGSLKYILSGD) were purchased from BioBasic (Markham, ON). DiOC6 dye was purchased from Invitrogen (Burlington, ON). Collagen type I was purchased from Nycomed Canada, Inc (Oakville, ON).
Mice
Fg/VWF−/−mice have been previously described.1,11 C57BL/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Genotypes of all animals were confirmed by polymerase chain reaction from ear tissue using the REDExtract-N-Amp Tissue polymerase chain reaction kit as per the manufacturer’s protocol (Sigma, Oakville, ON). Fg/VWF−/− mice were housed in the research vivarium at St. Michael’s Hospital in Toronto, and all experimental procedures were approved by the Animal Care Committee.
Platelet Preparation
Gel-filtered human platelets were prepared as previously described.12 Platelet count was adjusted to 3×108/mL by the addition of an appropriate volume of buffer A (130 mmol/L NaCl, 10 mmol/L trisodium citrate, 9 mmol/L NaHCO3, 6 mmol/L dextrose, 0.9 mmol/L MgCl2, 0.81 mmol/L KH2PO4, 10 mmol/L Tris, pH7.4) with 1.8 mmol/L CaCl2. Platelet-rich plasma (PRP) was prepared from human platelets anticoagulated with 3.2% sodium citrate and centrifuged for 10 minutes at 170g.
Mouse PRP was prepared from Fg/VWF−/− or C57BL/6J mice anesthetized with 15 µL/g of 2.5% tribromoethanol and bled via the retro-orbital plexus using a heparin-coated glass capillary tube into 3.2% sodium citrate. PRP was obtained by centrifuging anticoagulated whole blood at 300g for 7 minutes and removing the plasma and buffy coat to a new tube. Platelet-poor plasma was prepared by centrifugation of PRP at 10 000g for 5 minutes.
Cadherin 6 Expression
Cadherin 6 expression on the surface of human platelets was quantified using the Platelet Gp Screen kit from Biocytex (Marseille, France) according to the manufacturer’s instructions. Cadherin 6 in resting platelets or platelets activated with 1 U/mL thrombin was measured using a sheep polyclonal antibody against cadherin 6 (2 µg/mL). Receptor number was calculated on the basis of fluorescent intensity according to the kit protocol.
The expression of cadherin 6 on mouse platelets was measured by flow cytometry. PRP was preincubated with 40 µg/mL of polyclonal sheep anti-cadherin 6 antibody±10 µg/mL TRAP4. Samples were washed with PBS at 950g and incubated with a 1:100 dilution of fluorescein isothiocyanate–conjugated anti-sheep antibody for 1 hour at room temperature in the dark. Samples were washed, diluted with PBS, and analyzed on an FACS can flow cytometer.
Total platelet lysate (20 µg) was run on a 10% sodium dodecal sulphate-polyacrylamide gel electrophoresis gel and transferred onto a polyvinylidene fluoride membrane. Cadherin 6 expression was confirmed by Western blotting with 1 µg/mL sheep anti-cadherin polyclonal antibody.
Platelet Aggregation Assays
A Bio-Data PAP-4 platelet aggregometer was used to assess the role of cadherin 6 in platelet aggregation. Gel-filtered platelets were incubated with buffer A, polyclonal sheep anti-cadherin 6 antibody (50 µg/mL, azide free), sheep IgG isotype control antibody (50 µg/mL), purified Cdh6_IgG fusion protein (10 µg/mL), or purified P-Cdh_IgG fusion protein (10 µg/mL) for 3 minutes. Both fusion proteins were composed of the extracellular portion of the respective cadherin molecule fused to the Fc region of human IgG1 via a peptide linker (IEGRMD). TRAP was added to the samples at concentrations that gave submaximal aggregation (1–2.5 µmol/L), and platelet responses were monitored over 3 minutes. To exclude an interaction between the fusion proteins and the platelet FcγRIIa receptor, the FcγRIIa blocking antibody, IV.3 (5 µg/mL), was included in experiments where the respective Cdh6_IgG or P-Cdh_IgG fusion proteins were used. Aggregations were repeated in PRP obtained from whole blood anticoagulated with 3.2% sodium citrate. PRP was incubated for 3 minutes in the presence of buffer A, polyclonal sheep anti-cadherin 6 antibody (50 µg/mL), sheep IgG isotype control antibody (50 µg/mL), purified Cdh6_IgG fusion protein (10 µg/mL), or purified P-Cdh_IgG fusion protein (10 µg/mL), then stimulated with 2 µmol/L ADP and the platelet response measured for a further 3 minutes. The effect of the monoclonal anti-cadherin 6 antibody, clone 2B6, on platelet aggregation in response to ristocetin, collagen and TRAP was also investigated.
Platelet aggregation with Fg/VWF−/− mice was performed at 37°C as previously described.2,13 PRP was mixed with autologous platelet-poor plasma to a final concentration of 3×108 platelets per mL and aggregation induced with either 100 or 250 µmol/L TRAP4 (AYPGKF-NH2) in the presence of 1 mmol/L Ca++. Where indicated, PRP was coincubated with 50 µg/mL of polyclonal sheep anti-cadherin 6 antibody.
Platelet Adhesion Assays
Platelet adhesion to fibrinogen, cadherin 6, VE-cadherin, and P-cadherin was measured using a static adhesion assay. A 96-well plate was coated overnight with 1 µg/mL purified Cdh6_IgG fusion protein, VE-Cdh_IgG, P-Cdh_IgG, or 20 µg/mL fibrinogen. VE-cadherin is a type II cadherin, which also contains an RGD motif, albeit in the second cadherin ectodomain. P-cadherin is a type I cadherin that does not contain an RGD sequence. The plate was blocked for 2 hours with 1% BSA, washed with buffer A containing 1.8 mmol/L CaCl2, and gel-filtered platelets at a final concentration of 2×108/mL were allowed to adhere to the wells for 30 minutes at 37°C. To determine whether platelet adhesion to cadherin 6 can be inhibited by RGD peptides, platelets were incubated with either arginine-glycine-aspartic acid-serine (RGDS) peptide (100 µmol/L) or arginine-glycine-glutamate-serine peptide for 3 minutes before addition to the plate. To further determine whether there was an interaction between cadherin 6 and αIIbβ3, platelets were incubated with the anti-αIIbβ3 antibodies, SZ21 and abciximab, or an anti-GPIbα antibody, WM23, for 3 minutes and then added to the plate. The wells were gently washed to remove nonadherent platelets. Adherent platelets were determined using an acid phosphatase assay by incubating with 10 µmol/L p-nitrophenylphosphate (Pierce, Rockford, IL), 100 mmol/L sodium acetate (Sigma, Dublin), and 0.1% Triton X-100 (Sigma, Dublin) for 1 hour at 37°C. Absorbance was measured on a Wallac VICTOR2 V Multilabel Counter (Perkin Elmer, Wellesley, MA) at 405 nm.
PAC1 Binding Assay
PAC1 is an antibody that binds to αIIbβ3 when platelets are activated and the integrin has undergone a conformational change where it can now bind fibrinogen. To determine whether recombinant cadherin 6 could compete with PAC1 for αIIbβ3 binding sites, PRP was incubated for 15 minutes in the presence of fluorescein isothiocyanate-labeled PAC1 antibody with 10 µg/mL Cdh6_IgG, PECAM_IgG, monomelic IgG, or buffer and 2 µM ADP. To determine whether the protein caused platelet activation, PRP was incubated with Cdh6_IgG and then assayed for P-selectin expression, a marker for platelet activation. Samples were diluted with fresh PBS and median fluorescent intensity read on a Becton Dickinson FACSCalibur flow cytometer.
Peptide Adhesion Assays
Gel-filtered murine and human platelets (2× 108 per mL) were allowed to adhere for 90 minutes at 37°C to slides that had been coated overnight with 20 µg/mL fibrinogen, 2 µg/mL Cdh6_IgG, or 25 µg/mL custom synthetic peptides (CDH6_RGD and CDH6_RGE) followed by blocking with 4% BSA. Slides were washed with Tyrode buffer. Adherent platelets were counted using a Zeiss Axiovert 135-inverted microscope (60×W objective; Zeiss, Oberkichen, Germany) under bright field.
Perfusion Flow Assays
Rectangular (0.1×1mm) glass capillary microslides (VitroCom, Mountain Lakes, NJ) were coated with 100 µg/mL collagen type I at room temperature for 2 hours followed by blocking with 4% BSA at room temperature for 30 minutes before perfusion flow. Where indicated, heparinized whole blood from healthy humans or wild-type (WT) mice was preincubated with 50 µg/mL of polyclonal sheep anti-cadherin 6 antibody, Cdh6_IgG, or polyclonal sheep immunoglobulins for 10 minutes at 37°C prior to perfusion flow. Perfusion was performed as indicated using a syringe pump (Harvard Apparatus, Holliston, MA) followed by washout with Tyrode buffer. Perfusion flow was recorded and images taken using a Zeiss Axiovert 135-inverted microscope (60×W objective; Zeiss) under bright field and a Hamamatsu C4200 CCD camera (Hamamatsu Photonics, Bridgewater, NJ). Surface coverage and thrombus size were calculated from microscope images using ImageJ software. After flow, microslides were fixed with 4% paraformaldehyde and labeled with 1 mmol/l DiOC6 for 1 hour before being rinsed with PBS and imaged using a CARV confocal microscope (Atto Bioscience, Rockville, MD) and an IBM IntelliStation Z Pro computer. Thrombus volume was calculated using the Slidebook program.
Statistical Analysis
Data are presented as mean±SD. Statistical significance was assessed by either Student unpaired t test or 1-way ANOVA with Bonferroni-type correction where warranted.
Results
Cadherin 6 Is Expressed on Platelets
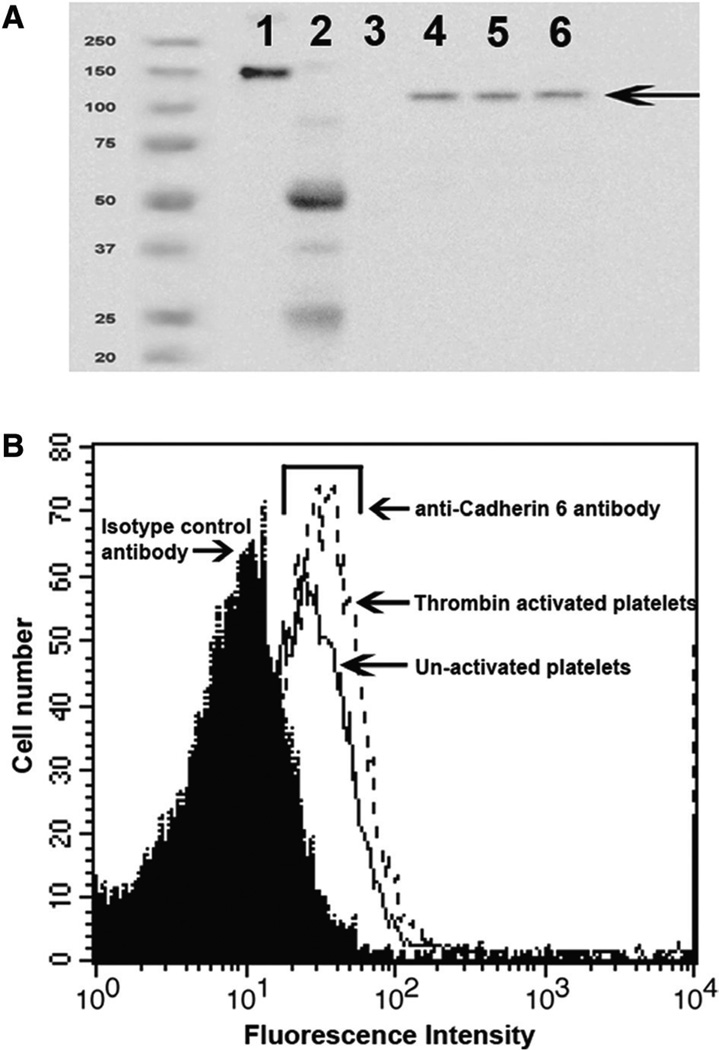
The presence of cadherin 6 was examined by Western blot analysis in total platelet lysates obtained from 3 different healthy donors (Figure 1A). We next determined the number of cadherin 6 molecules present on the platelet surface by flow cytometry.14 An average count of 1600±500 molecules per platelet was obtained in resting platelets from 5 different donors. This increased to 3200±900 molecules after thrombin stimulation (Figure 1B). Fluorescence-activated cell sorter analysis confirmed the expression of cadherin 6 on murine platelets (data not shown).
Figure 1.
Cadherin 6 is present on platelets. A, Total platelet lysate from 3 different donors was analyzed by Western blot for the presence of cadherin 6 using a sheep polyclonal antibody against cadherin 6. Lane 1, Cdh6_IgG; lane 2, sheep IgG; lane 3, human IgG; lanes 4–6, total platelet lysate. Cdh6_IgG is composed of the extracellular portion of cadherin 6 fused to the Fc domain of human IgG and therefore has a higher molecular weight than the native protein. B, Platelets were incubated with a polyclonal antibody and analyzed by flow cytometry to confirm the presence of cadherin 6 on the platelet surface. Cdh6_IgG indicates cadherin 6_IgG fusion protein.
Antibodies and Protein to Cadherin 6 Inhibit Platelet Aggregation
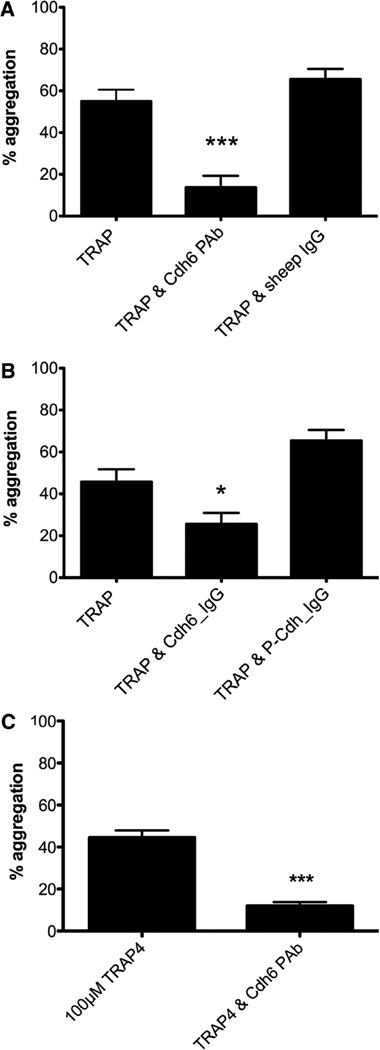
The cadherins have a role in cell adhesion. We therefore explored the role of cadherin 6 in platelet aggregation. A polyclonal antibody directed against the full-length extracellular portion of the protein inhibited low-dose TRAP-induced platelet aggregation in gel-filtered platelets (Figure 2A). The polyclonal antibody also inhibited ADP-induced aggregation in PRP (Table). A mouse monoclonal anti-human cadherin 6 antibody, clone 2B6, also inhibited platelet aggregation induced by collagen and ristocetin (Table).
Figure 2.
Platelet aggregation is inhibited by an antibody against cadherin 6 and an excess of cadherin 6 protein. A, A polyclonal antibody against cadherin 6 significantly inhibits thrombin receptor activating peptide (TRAP)-induced platelet aggregation in gel-filtered platelets (P<0.0001) (n=15). B, 10 µg/mL Cdh6_IgG fusion protein inhibits TRAP-induced aggregation in gel-filtered platelets (P<0.0001). The P-Cdh_IgG control fusion protein had no effect (n=5). C, 3×108 platelets per mL from Fg/VWF−/− platelet-rich protein were stimulated by either 100 or 250 mmol/L TRAP4. Coincubation of anti-cadherin 6 resulted in decreased platelet aggregation in response to 100 mmol/L TRAP4. Fg/VWF−/− indicates fibrinogen and von Willebrand factor double-deficient mice; Cdh6_IgG, cadherin 6_IgG fusion protein; and P-Cdh_IgG, placental cadherin_IgG recombinant fusion protein.
Table.
Antibodies and Recombinant Cadherin 6 protein Inhibit Platelet Aggregation
| TRAP | TRAP4 | ADP | Collagen | Risto | |
|---|---|---|---|---|---|
| Cdh6 pAb | 27% | 28% | 46% | 65% | N/D |
| Cdh6 mAb | 64% | N/D | N/D | 78% | 72% |
| Isotype control | 124% | 100% | 120% | 94% | 100% |
| Cdh6_IgG | 53% | N/D | 14% | 35% | N/D |
| P-Cdh_IgG | 150% | N/D | 175% | 133% | N/D |
Risto indicates ristocetin; N/D, not done; P-Cdh_IgG, placental cadherin_IgG fusion protein; Cdh6_IgG, cadherin 6_IgG fusion protein; TRAP, thrombin receptor activating peptide.
Aggregation is expressed as a percent of the maximum response obtained with agonist alone. TRAP=SFFLRN, TRAP4=AYPGKF.
We also investigated the effect of exogenous recombinant cadherin 6 protein on platelet aggregation. Gel-filtered platelets were incubated in the presence of buffer alone, purified Cdh6_IgG protein (10 µg/mL), or a control purified P-Cdh_ IgG protein (10 µg/mL) for 3 minutes. P-cadherin has 65% homology to cadherin 6 but, unlike cadherin 6, does not contain an RGD motif. Aggregation was induced by low-dose TRAP, and the platelet response was monitored for a further 3 minutes. Cadherin 6 fusion protein significantly inhibited platelet aggregation to TRAP whereas P-cadherin had no inhibitory effect (Figure 2B). Inclusion of the FcγRIIa blocking antibody, IV.3, had no effect on the levels of aggregation obtained (data not shown), ruling out cross-linking between the IgG fusion proteins and FcγRIIa as a confounder. ADP-induced aggregation in PRP was also significantly inhibited by Cdh6_IgG (Table).
Previous work within our group had shown that platelets from Fg/VWF−/− mice aggregate and form occlusive thrombi. We examined the effect of a cadherin 6 antibody on platelet aggregation using Fg/VWF−/− platelets. Aggregation induced by 100 µmol/L TRAP4 (AYPGKF-NH2) in PRP from Fg/VWF−/− mice was significantly inhibited by the anti-cadherin 6 antibody (P=0.0001) (Figure 2C).
Characterization of Platelet Adhesion to Immobilized Cadherin 6
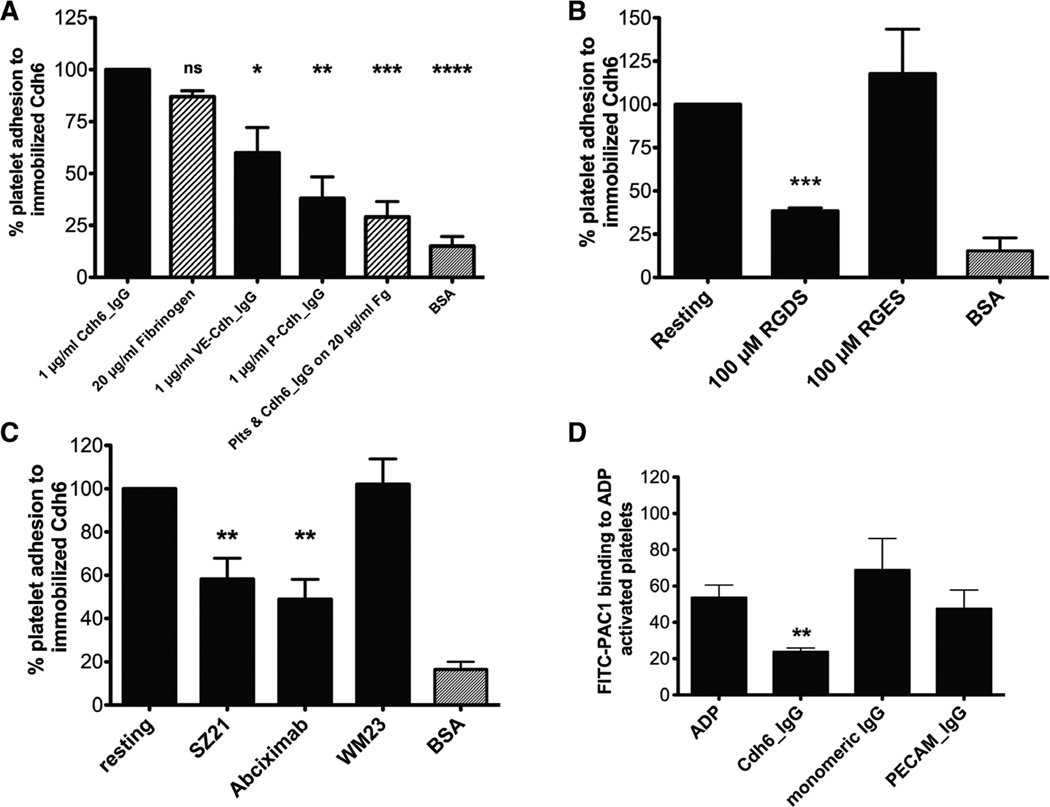
To determine whether platelets could adhere to cadherins, 96-well plates were coated with Cdh6_IgG, VE-Cdh_IgG, and P-Cdh_IgG, respectively. Platelet adhesion to wells coated with 1 µg/mL Cdh6_IgG protein was similar to platelet adhesion to wells coated with 20 µg/mL fibrinogen (adhesion was normalized by expressing it as a percentage of resting platelets adhering to cadherin 6–coated wells). There was 40% less adhesion of platelets to VE-Cdh_IgG, a type II cadherin that also contains an RGD motif. A significant decrease was observed in the number of platelets adhering to P-Cdh_IgG, a type I cadherin with no RGD motif, or to BSA-coated wells. Preincubating platelets with exogenous Cdh6_IgG abrogated adhesion to fibrinogen, indicating that Cdh6 and fibrinogen cross-compete for the same or an overlapping binding site (Figure 3A).
Figure 3.
Platelet adhesion to immobilized cadherin 6 is αIIbβ3 dependent. A, Platelets adhere to 1 µg/mL cadherin 6, 20 µg/mL fibrinogen, and 1 µg/ml VE-Cdh. Adhesion to 1 µg/mL P-Cdh or 10 µg/mL BSA is greatly reduced in comparison with Cdh6_IgG. B, RGDS peptides inhibit platelet adhesion to immobilized cadherin 6 (P<0.005), n=3. C, Antibodies to integrin αIIbβ3 inhibit platelet adhesion to cadherin 6 (P<0.005), n=7. D, Cdh6_IgG inhibits PAC1 binding to αIIbβ3. VE-Cdh indicates vascular endothelial cadherin; P-Cdh, placental cadherin; Cdh6_IgG, cadherin 6_IgG fusion protein.
Cadherin 6 contains an RGD motif in the first cadherin domain. To examine the role of this motif in platelet adhesion to cadherin 6, gel-filtered platelets were incubated with the tetrapeptides RGDS or arginine-glycine-glutamate-serine and allowed to adhere to immobilized Cdh6_IgG. The peptide RGDS (100 µmol/L) significantly inhibited platelet adhesion to cadherin 6. In contrast, the control peptide arginine-glycine-glutamate-serine had no effect on platelet adhesion to cadherin 6 (Figure 3B). Because the RGD peptides inhibit platelet adhesion to cadherin 6, we investigated the potential of platelet cadherin 6 to interact with αIIbβ3. Platelets were incubated with antibodies (SZ21 or abciximab) that inhibit ligand binding to αIIbβ3. In the presence of αIIbβ3 blocking antibodies, platelet adhesion to cadherin 6 was significantly inhibited (P=0.0009). In contrast, WM23, an antibody to GPIbα, did not inhibit platelet adhesion to cadherin 6 (Figure 3C).
To further characterize the interaction between cadherin 6 and αIIbβ3, platelets were incubated with PAC1 alone or PAC1 with Cdh6_IgG, PECAM_IgG, or monomeric human IgG (10 µg/mL) and activated with 2 µmol/L ADP. Median fluorescent intensity of the fluorescein isothiocyanate-labeled PAC1 was measured by flow cytometry. The presence of Cdh6_IgG caused a significant reduction in median fluorescent intensity (P<0.01, 2-tailed t test) compared with PAC1 alone (Figure 3D) demonstrating that excess cadherin 6 inhibits PAC1 binding to αIIbβ3. The reduced level of PAC-1 binding was not due to inhibition of platelet activation as the presence of excess cadherin 6 protein had no effect on P-selectin binding (data not shown).
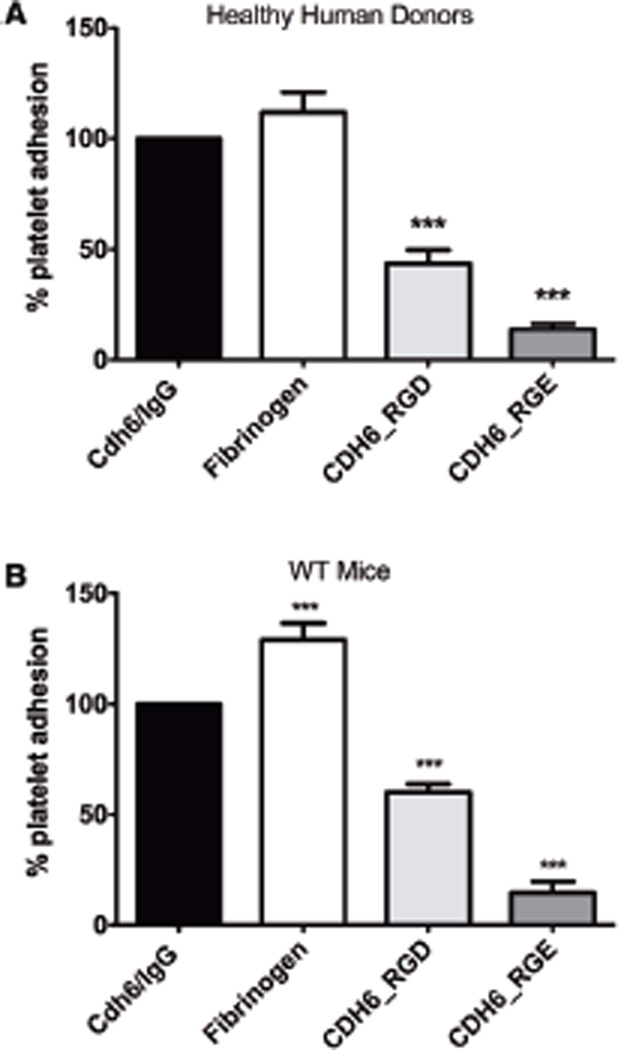
Because platelets adhere to immobilized cadherin 6 and this interaction is inhibited by both RGDS peptides (Figure 3B) and antibodies to β3 integrin (Figure 3C), we investigated whether the RGD site on cadherin 6 could directly mediate platelet adhesion. Glass slides were coated with extended peptides corresponding to the RGD-containing region of the first extracellular domain of cadherin 6 (AA 73–95), with either the RGD sequence intact (CDH6_RGD: YVGKLHSDQDRGDGSLKYILSGD) or mutated to RGE (CHD6_RGE: YVGKLHSDQDRGEGSLKYILSGD). Cdh6_ IgG and fibrinogen were used as controls. Both human and mouse platelets adhered to CDH6_RGD, although to a lesser extent than the intact cadherin 6 extracellular region. Adhesion to CDH6_RGE was significantly reduced, indicating that the RGD-containing domain of cadherin 6 could independently support platelet adhesion and that the RGD sequence was presented in such a way as to be recognized by the platelet. However, this does not exclude the possibility that additional sites within the full-length cadherin 6 molecule may contribute to the αIIbβ3/cadherin 6 interaction (Figure 4).
Figure 4.
Platelets adhere to the RGD motif presented as part of an extended cadherin 6 (Cdh6) sequence. Platelets from healthy human donors (A) and wild-type mice (B) adhered to glass slides coated with a peptide corresponding to Cdh6 amino acids 73–95, containing the Cdh6 RGD motif. Mutating the RGD to RGE abrogated platelet adhesion. RGD indicates arginine-glycine-aspartic acid.
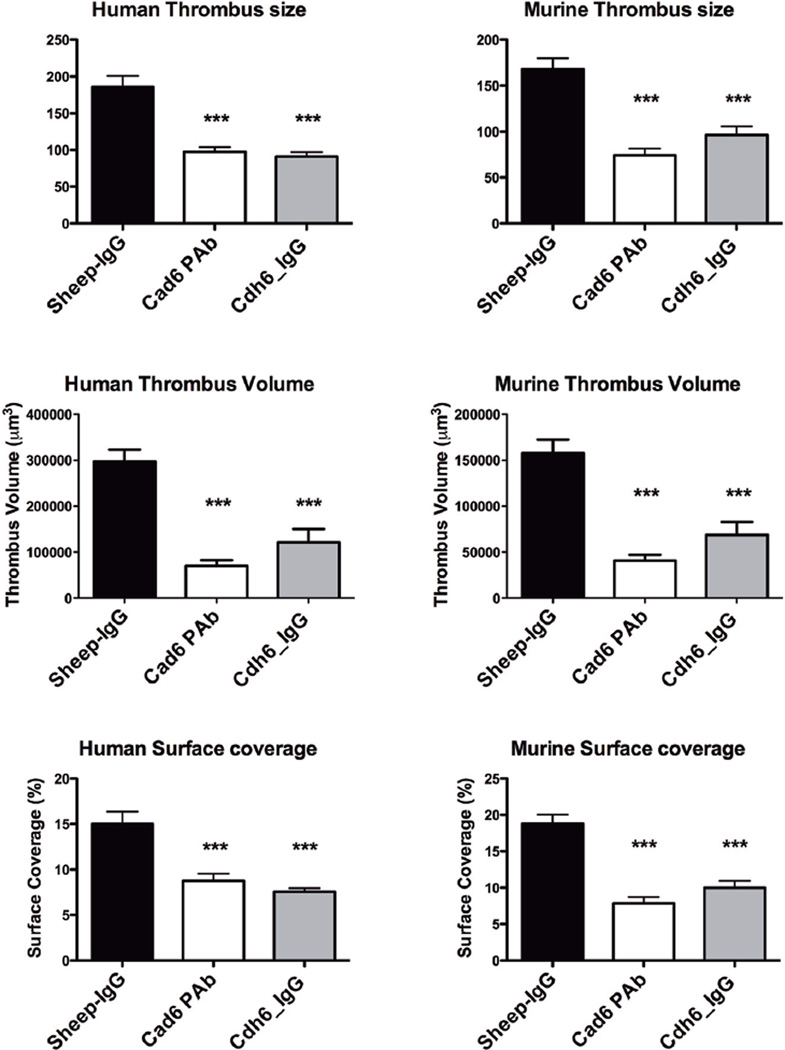
Cadherin 6 Is Required for Stable Thrombus Formation Under Flow
We investigated the role of cadherin 6 in thrombus formation. Platelets from healthy human donors and WT mice were assayed for their ability to form thrombi on collagen-coated surfaces in a whole blood perfusion system at a shear rate of 600 per inverse second. In both human and WT murine samples, treatment of anticoagulated whole blood with an anti-cadherin 6 antibody or with exogenous Cdh6_IgG fusion protein resulted in significantly smaller thrombi compared with control platelets treated with sheep IgG. The antibody caused an 86% reduction in thrombus volume in human blood and an 84% decrease in thrombus volume in WT mice (P<0.0001). The Cdh6_IgG fusion protein also significantly inhibited thrombus volume compared with control samples (P<0.0001). Thus, blockade of surface cadherin 6 with a polyclonal antibody or competition via soluble Cdh6_IgG fusion protein resulted in decreased thrombus formation, which is consistent with a supportive role of cadherin 6 in normal platelet function and aggregation (Figure 5). The ability of cadherin 6 to inhibit thrombus formation in Fg/VWF−/− mice was confirmed by intravital microscopy of mice treated with the CDH6_RGD peptide (online-only Data Supplement).
Figure 5.
Cadherin 6 contributes to platelet-rich thrombus formation at moderate shear. Whole blood from healthy human donors or wild-type mice treated with an anti-cadherin 6 antibody or Cdh6_IgG and perfused at 600 per inverse second over collagen. Cadherin 6 treatment caused a significant reduction in thrombus size, thrombus volume, and surface area covered by thrombi as assessed by confocal microscopy (P<0.0001). Cdh6_IgG indicates cadherin 6_IgG fusion protein.
Discussion
In the present study, we have shown that cadherin 6 is present in platelets and has a supportive role in platelet adhesion aggregation and thrombus formation. Our results demonstrate that cadherin 6 interacts with the platelet integrin αIIbβ3, as blockade of αIIbβ3 with the antibodies SZ21 or abciximab inhibited platelet adhesion to cadherin 6. Previously published data from mice lacking both fibrinogen and VWF has suggested the presence of other unkown ligands on platelets that could interact with αIIbβ3 2,15 Our results demonstrate an interaction between cadherin 6 and αIIbβ3. We have shown that an anti-cadherin 6 antibody inhibits TRAP4-induced aggregation in the Fg/VWF−/− mice. A peptide corresponding to the first extracellular domain of cadherin 6 caused a significant increase in the time to vessel occlusion in vivo in the double knock-out mouse model. The cadherin 6 antibody or an excess of the extracellular domain of the protein caused a significant reduction in thrombus size and volume when blood from healthy human donors or WT mice was perfused over collagen.
Our study has many limitations. We have shown that the number of cadherin 6 receptors on the platelet surface increases with thrombin activation; however, we cannot state definitively whether this is due to an internal store of the protein or not. Cell surface expression of many platelet plasma membrane glycoproteins increases 30% to 40% upon platelet activation due to externalization of the open canalicular system, which is inaccessible to antibody staining by fluorescence-activated cell sorter.16 Similarly, the results of our aggregation studies with a range of diverse platelet agonists suggest that cadherin 6 is involved in several different platelet signaling pathways. Whether the mechanisms of action of cadherin 6 on platelet signaling pathways is similar to β3 phosphorylation by CD40L,17 or whether cadherin 6 is directly involved in the formation of platelet-to-platelet bridges remains to be seen and warrants further investigation.
In addition to their homophilic binding properties, cadherins are involved in heterophilic adhesion with other cadherin family members, inhibitory receptors, and integrins. E-, N-, and R-cadherin are known to be ligands for the inhibitory receptor KLRG1.18–20 E-cadherin binds to 2 members of the integrin family, αEβ7 and α2β1.21–25 In the protocadherins, an RGD motif has been identified in the first extracellular domain of the mammalian protocadherin-α family members. Heterophilic cell adhesion has been observed between protocadherin-α4 and the β1 integrin subunit, mediated by the RGD motif.26, 27 Our results confirm the ability of the cadherins to interact in a heterophilic manner with the platelet integrin αIIbβ3. An RGDS peptide inhibited platelet adhesion to cadherin 6, suggesting a role for the RGD motif on the first extracellular domain in platelet adhesion to cadherin 6. The peptide caused 1.5-fold more inhibition than the αIIbβ3 blocking antibody SZ21. Platelet adhesion to cadherin 6 most likely occurs via both homophilic cadherin/cadherin and heterophilic cadherin 6/αIIbβ3 interactions. SZ21 can block this heterophilic interaction, with RGDS potentially able to prevent both homophilic and heterophilic adhesion. This is supported by the ability of platelets to adhere to the extended CHD6_RGD peptide but not CDH6_RGE. Neither the antibodies against αIIbβ3 nor the RGDS peptide were able to completely inhibit platelet adhesion to immobilized Cdh6_IgG. This may in part be explained by cadherin/cadherin homophilic adhesion. Both Harrison et al28 and Katsamba et al29 have previously described the strong affinity of cadherin 6 homophilic interactions, with a KD of 3.13 µmol/L in comparison with 25.8 µmol/L and 96.5 µmol/L for N-cadherin and E-cadherin, respectively.
There are many inbuilt compensatory mechanisms that allow platelets to maintain hemostasis. Glycoprotein VI, α2β1, VWF, and GPIb-XI-V act synergistically with both collagen and laminin to promote platelet adhesion at high shear flow.30 This redundant mechanism of platelet adhesion may increase the probability of stopping bleeding at sites of vascular damage, where either of the 2 extracellular matrix proteins might be exposed. Recent work within our own group has shown that platelet aggregation can occur independently of fibrinogen and VWF,1,2 pointing to intrinsic compensatory mechanisms in hemostasis and thrombosis. Subsequently, analysis of platelets from a triple knock-out mouse lacking fibrinogen, VWF, and plasma fibronectin showed enhanced aggregation in PRP and gel-filtered platelets.31,32 Aggregation was inhibited by the addition of antisera against the integrin β3 subunit, indicating that β3 is essential for platelet aggregation and that there must be additional ligands for αIIbβ3. Our results suggest that cadherin 6 is 1 ligand that can partially explain these results. In Fg/VWF−/− mice, we have been able to inhibit aggregation induced by low-dose TRAP4 by blocking cadherin 6 with an anti-cadherin 6–specific antibody. At higher concentrations of agonist, we were able to overcome the effect of blocking cadherin 6. This may be due to the presence of other as yet unidentified ligands for αIIbβ3.
In summary, we have demonstrated that the platelet-expressed receptor cadherin 6 has a functional role in platelet aggregation and thrombus formation.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by a grant from the Health Research Board, Ireland and a grant from the Heart and Stroke Foundation of Canada. MCB and PJN were recipients of Science Foundation Ireland E.T.S Walton Visitor Awards and WJ was a recipient of a Canadian Blood Services fellowship award and a fellowship award from Heart and Stroke Foundation of Canada.
Footnotes
The online-only Data Supplement is available with this article at http://atvb.ahajournals.org/lookup/suppl/doi:10.1161/ATVBAHA.112.250464/-/DCl.
Disclosures
None.
References
- 1.Ni H, Denis CV, Subbarao S, Degen JL, Sato TN, Hynes RO, Wagner DD. Persistence of platelet thrombus formation in arterioles of mice lacking both von Willebrand factor and fibrinogen. J Clin Invest. 2000;106:385–392. doi: 10.1172/JCI9896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang H, Reheman A, Chen P, Zhu G, Hynes RO, Freedman J, Wagner DD, Ni H. Fibrinogen and von Willebrand factor-independent platelet aggregation in vitro and in vivo. J Thromb Haemost. 2006;4:2230–2237. doi: 10.1111/j.1538-7836.2006.02116.x. [DOI] [PubMed] [Google Scholar]
- 3.Elrod JW, Park JH, Oshima T, Sharp CD, Minagar A, Alexander JS. Expression of junctional proteins in human platelets. Platelets. 2003;14:247–251. doi: 10.1080/0953710031000118894. [DOI] [PubMed] [Google Scholar]
- 4.Edwards RJ, Moran N, Devocelle M, Kiernan A, Meade G, Signac W, Foy M, Park SD, Dunne E, Kenny D, Shields DC. Bioinformatic discovery of novel bioactive peptides. Nat Chem Biol. 2007;3:108–112. doi: 10.1038/nchembio854. [DOI] [PubMed] [Google Scholar]
- 5.Shimoyama Y, Gotoh M, Terasaki T, Kitajima M, Hirohashi S. Isolation and sequence analysis of human cadherin-6 complementary DNA for the full coding sequence and its expression in human carcinoma cells. Cancer Res. 1995;55:2206–2211. [PubMed] [Google Scholar]
- 6.Lawrence JB, Kramer WS, McKeown LP, Williams SB, Gralnick HR. Arginine-glycine-aspartic acid- fibrinogen gamma-chain carboxy-terminal peptides inhibit platelet adherence to arterial subendothelium at high wall shear rates. An effect dissociable from interference with adhesive protein binding. J Clin Invest. 1990;86:1715–1722. doi: 10.1172/JCI114896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pareti FI, Mazzucato M, Bottini E, Mannucci PM. Interaction of porcine von Willebrand factor with the platelet glycoproteins Ib and IIb/IIIa complex. Br J Haematol. 1992;82:81–86. doi: 10.1111/j.1365-2141.1992.tb04597.x. [DOI] [PubMed] [Google Scholar]
- 8.Lu X, Lu D, Scully MF, Kakkar VV. Modulation of integrin-binding selectivity by mutation within the RGD-loop of snake venom proteins: a novel drug development approach. Curr Med Chem Cardiovasc Hematol Agents. 2003;1:189–196. doi: 10.2174/1568016033477522. [DOI] [PubMed] [Google Scholar]
- 9.Lu X, Williams JA, Deadman JJ, Salmon GP, Kakkar VV, Wilkinson JM, Baruch D, Authi KS, Rahman S. Preferential antagonism of the interactions of the integrin alpha IIb beta 3 with immobilized glycoprotein ligands by snake-venom RGD (Arg-Gly-Asp) proteins. Evidence supporting a functional role for the amino acid residues flanking the tripeptide RGD in determining the inhibitory properties of snake-venom RGD proteins. Biochem J. 1994;304(Pt 3):929–936. doi: 10.1042/bj3040929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rahman S, Flynn G, Aitken A, Patel Y, Hussain F, Lu X, Loftus JC, French D, Wijelath E, Strand K, Savidge GF. Differential recognition of snake venom proteins expressing specific Arg-Gly-Asp (RGD) sequence motifs by wild-type and variant integrin alphaIIbbeta3: further evidence for distinct sites of RGD ligand recognition exhibiting negative allostery. Biochem J. 2000;345(Pt 3):701–709. [PMC free article] [PubMed] [Google Scholar]
- 11.Inoue T, Tanaka T, Takeichi M, Chisaka O, Nakamura S, Osumi N. Role of cadherins in maintaining the compartment boundary between the cortex and striatum during development. Development. 2001;128:561–569. doi: 10.1242/dev.128.4.561. [DOI] [PubMed] [Google Scholar]
- 12.Lawler K, Meade G, O’Sullivan G, Kenny D. Shear stress modulates the interaction of platelet-secreted matrix proteins with tumor cells through the integrin alphavbeta3. Am J Physiol, Cell Physiol. 2004;287:C1320–C1327. doi: 10.1152/ajpcell.00159.2004. [DOI] [PubMed] [Google Scholar]
- 13.Reheman A, Gross P, Yang H, Chen P, Allen D, Leytin V, Freedman J, Ni H. Vitronectin stabilizes thrombi and vessel occlusion but plays a dual role in platelet aggregation. J Thromb Haemost. 2005;3:875–883. doi: 10.1111/j.1538-7836.2005.01217.x. [DOI] [PubMed] [Google Scholar]
- 14.Martin K, Meade G, Moran N, Shields DC, Kenny D. A palmitylated peptide derived from the glycoprotein Ib beta cytoplasmic tail inhibits platelet activation. J Thromb Haemost. 2003;1:2643–2652. doi: 10.1046/j.1538-7836.2003.00478.x. [DOI] [PubMed] [Google Scholar]
- 15.Zhai Z, Wu J, Xu X, Ding K, Ni R, Hu W, Sun Z, Ni H. Fibrinogen controls human platelet fibronectin internalization and cell-surface retention. J Thromb Haemost. 2007;5:1740–1746. doi: 10.1111/j.1538-7836.2007.02625.x. [DOI] [PubMed] [Google Scholar]
- 16.Escoloa G, White JG. Distribution of GPlb on the in resting platelets. Blood. 1998;92:4874–4877. [PubMed] [Google Scholar]
- 17.Prasad KS, Andre P, He M, Bao M, Manganello J, Phillips DR. Soluble CD40 ligand induces beta3 integrin tyrosine phosphorylation and triggers platelet activation by outside-in signaling. Proc Natl Acad Sci USA. 2003;100:12367–12371. doi: 10.1073/pnas.2032886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Banh C, Fugère C, Brossay L. Immunoregulatory functions of KLRG1 cadherin interactions are dependent on forward and reverse signaling. Blood. 2009;114:5299–5306. doi: 10.1182/blood-2009-06-228353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y, Hofmann M, Wang Q, Teng L, Chlewicki LK, Pircher H, Mariuzza RA. Structure of natural killer cell receptor KLRG1 bound to E-cadherin reveals basis for MHC-independent missing self recognition. Immunity. 2009;31:35–46. doi: 10.1016/j.immuni.2009.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nakamura S, Kuroki K, Ohki I, Sasaki K, Kajikawa M, Maruyama T, Ito M, Kameda Y, Ikura M, Yamamoto K, Matsumoto N, Maenaka K. Molecular basis for E-cadherin recognition by killer cell lectin-like receptor G1 (KLRG1) J Biol Chem. 2009;284:27327–27335. doi: 10.1074/jbc.M109.038802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Uchida Y, Kawai K, Ibusuki A, Kanekura T. Role for E-cadherin as an inhibitory receptor on epidermal gammadelta T cells. J Immunol. 2011;186:6945–6954. doi: 10.4049/jimmunol.1003853. [DOI] [PubMed] [Google Scholar]
- 22.Corps E, Carter C, Karecla P, Ahrens T, Evans P, Kilshaw P. Recognition of E-cadherin by integrin alpha(E)beta(7): requirement for cadherin dimerization and implications for cadherin and integrin function. J Biol Chem. 2001;276:30862–30870. doi: 10.1074/jbc.M101712200. [DOI] [PubMed] [Google Scholar]
- 23.Higgins JM, Mandlebrot DA, Shaw SK, Russell GJ, Murphy EA, Chen YT, Nelson WJ, Parker CM, Brenner MB. Direct and regulated interaction of integrin alphaEbeta7 with E-cadherin. J Cell Biol. 1998;140:197–210. doi: 10.1083/jcb.140.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Karecla PI, Green SJ, Bowden SJ, Coadwell J, Kilshaw PJ. Identification of a binding site for integrin alphaEbeta7 in the N-terminal domain of E-cadherin. J Biol Chem. 1996;271:30909–30915. doi: 10.1074/jbc.271.48.30909. [DOI] [PubMed] [Google Scholar]
- 25.Whittard JD, Craig SE, Mould AP, Koch A, Pertz O, Engel J, Humphries MJ. E-cadherin is a ligand for integrin alpha2beta1. Matrix Biol. 2002;21:525–532. doi: 10.1016/s0945-053x(02)00037-9. [DOI] [PubMed] [Google Scholar]
- 26.Morishita H, Umitsu M, Murata Y, Shibata N, Udaka K, Higuchi Y, Akutsu H, Yamaguchi T, Yagi T, Ikegami T. Structure of the cadherin-related neuronal receptor/protocadherin-alpha first extracellular cadherin domain reveals diversity across cadherin families. J Biol Chem. 2006;281:33650–33663. doi: 10.1074/jbc.M603298200. [DOI] [PubMed] [Google Scholar]
- 27.Yagi T. Clustered protocadherin family. Dev Growth Differ. 2008;50(Suppl 1):S131–S140. doi: 10.1111/j.1440-169X.2008.00991.x. [DOI] [PubMed] [Google Scholar]
- 28.Harrison OJ, Bahna F, Katsamba PS, Jin X, Brasch J, Vendome J, Ahlsen G, Carroll KJ, Price SR, Honig B, Shapiro L. Two-step adhesive binding by classical cadherins. Nat Struct Mol Biol. 2010;17:348–357. doi: 10.1038/nsmb.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katsamba P, Carroll K, Ahlsen G, Bahna F, Vendome J, Posy S, Rajebhosale M, Price S, Jessell TM, Ben-Shaul A, Shapiro L, Honig BH. Linking molecular affinity and cellular specificity in cadherin-mediated adhesion. Proc Natl Acad Sci USA. 2009;106:11594–11599. doi: 10.1073/pnas.0905349106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Inoue O, Suzuki-Inoue K, Ozaki Y. Redundant mechanism of platelet adhesion to laminin and collagen under flow: involvement of von Willebrand factor and glycoprotein Ib-IX-V. J Biol Chem. 2008;283:16279–16282. doi: 10.1074/jbc.C700241200. [DOI] [PubMed] [Google Scholar]
- 31.Reheman A, Yang H, Bai X, Spring CM, Wagner DD, Fassler R, Gross P, Freedman J, Ni H. Role of plasma fibronectin in fibrinogen/vwf-independent thrombus formation. J Thromb Haemost. 2007;5(Suppl 2) O-T-069. [Google Scholar]
- 32.Reheman A, Yang H, Zhu G, Jin W, He F, Spring CM, Bai X, Gross PL, Freedman J, Ni H. Plasma fibronectin depletion enhances platelet aggregation and thrombus formation in mice lacking fibrinogen and von Willebrand factor. Blood. 2009;113:1809–1817. doi: 10.1182/blood-2008-04-148361. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.