Abstract
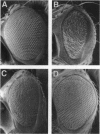
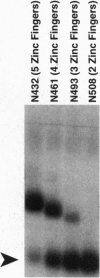
The glass gene is required for proper photo-receptor differentiation during development of the Drosophila eye glass codes for a DNA-binding protein containing five zinc fingers that we show is a transcriptional activator. A comparison of the sequences of the glass genes from two species of Drosophila and a detailed functional domain analysis of the Drosophila melanogaster glass gene reveal that both the DNA-binding domain and the transcriptional-activation domain are highly conserved between the two species. Analysis of the DNA-binding domain of glass indicates that the three carboxyl-terminal zinc fingers alone are necessary and sufficient for DNA binding. We also show that a deletion mutant of glass containing only the DNA-binding domain can behave in a dominant-negative manner both in vivo and in a cell culture assay that measures transcriptional activation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attardi L. D., Tjian R. Drosophila tissue-specific transcription factor NTF-1 contains a novel isoleucine-rich activation motif. Genes Dev. 1993 Jul;7(7B):1341–1353. doi: 10.1101/gad.7.7b.1341. [DOI] [PubMed] [Google Scholar]
- Attardi L. D., Von Seggern D., Tjian R. Ectopic expression of wild-type or a dominant-negative mutant of transcription factor NTF-1 disrupts normal Drosophila development. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10563–10567. doi: 10.1073/pnas.90.22.10563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beverley S. M., Wilson A. C. Molecular evolution in Drosophila and the higher Diptera II. A time scale for fly evolution. J Mol Evol. 1984;21(1):1–13. doi: 10.1007/BF02100622. [DOI] [PubMed] [Google Scholar]
- Colot H. V., Hall J. C., Rosbash M. Interspecific comparison of the period gene of Drosophila reveals large blocks of non-conserved coding DNA. EMBO J. 1988 Dec 1;7(12):3929–3937. doi: 10.1002/j.1460-2075.1988.tb03279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courey A. J., Tjian R. Analysis of Sp1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell. 1988 Dec 2;55(5):887–898. doi: 10.1016/0092-8674(88)90144-4. [DOI] [PubMed] [Google Scholar]
- Cress W. D., Triezenberg S. J. Critical structural elements of the VP16 transcriptional activation domain. Science. 1991 Jan 4;251(4989):87–90. doi: 10.1126/science.1846049. [DOI] [PubMed] [Google Scholar]
- Danielian P. S., White R., Lees J. A., Parker M. G. Identification of a conserved region required for hormone dependent transcriptional activation by steroid hormone receptors. EMBO J. 1992 Mar;11(3):1025–1033. doi: 10.1002/j.1460-2075.1992.tb05141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis M. C., O'Neill E. M., Rubin G. M. Expression of Drosophila glass protein and evidence for negative regulation of its activity in non-neuronal cells by another DNA-binding protein. Development. 1993 Nov;119(3):855–865. doi: 10.1242/dev.119.3.855. [DOI] [PubMed] [Google Scholar]
- Gill G., Pascal E., Tseng Z. H., Tjian R. A glutamine-rich hydrophobic patch in transcription factor Sp1 contacts the dTAFII110 component of the Drosophila TFIID complex and mediates transcriptional activation. Proc Natl Acad Sci U S A. 1994 Jan 4;91(1):192–196. doi: 10.1073/pnas.91.1.192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay B. A., Wolff T., Rubin G. M. Expression of baculovirus P35 prevents cell death in Drosophila. Development. 1994 Aug;120(8):2121–2129. doi: 10.1242/dev.120.8.2121. [DOI] [PubMed] [Google Scholar]
- Heberlein U., Rubin G. M. Structural and functional comparisons of the Drosophila virilis and Drosophila melanogaster rough genes. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5916–5920. doi: 10.1073/pnas.87.15.5916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jantzen H. M., Chow A. M., King D. S., Tjian R. Multiple domains of the RNA polymerase I activator hUBF interact with the TATA-binding protein complex hSL1 to mediate transcription. Genes Dev. 1992 Oct;6(10):1950–1963. doi: 10.1101/gad.6.10.1950. [DOI] [PubMed] [Google Scholar]
- Kassis J. A., Poole S. J., Wright D. K., O'Farrell P. H. Sequence conservation in the protein coding and intron regions of the engrailed transcription unit. EMBO J. 1986 Dec 20;5(13):3583–3589. doi: 10.1002/j.1460-2075.1986.tb04686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller A. D., Maniatis T. Only two of the five zinc fingers of the eukaryotic transcriptional repressor PRDI-BF1 are required for sequence-specific DNA binding. Mol Cell Biol. 1992 May;12(5):1940–1949. doi: 10.1128/mcb.12.5.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel B. E., Heberlein U., Rubin G. M. The homeo domain protein rough is expressed in a subset of cells in the developing Drosophila eye where it can specify photoreceptor cell subtype. Genes Dev. 1990 May;4(5):712–727. doi: 10.1101/gad.4.5.712. [DOI] [PubMed] [Google Scholar]
- Klemenz R., Weber U., Gehring W. J. The white gene as a marker in a new P-element vector for gene transfer in Drosophila. Nucleic Acids Res. 1987 May 26;15(10):3947–3959. doi: 10.1093/nar/15.10.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J., Chen J., Elenbaas B., Levine A. J. Several hydrophobic amino acids in the p53 amino-terminal domain are required for transcriptional activation, binding to mdm-2 and the adenovirus 5 E1B 55-kD protein. Genes Dev. 1994 May 15;8(10):1235–1246. doi: 10.1101/gad.8.10.1235. [DOI] [PubMed] [Google Scholar]
- Michael W. M., Bowtell D. D., Rubin G. M. Comparison of the sevenless genes of Drosophila virilis and Drosophila melanogaster. Proc Natl Acad Sci U S A. 1990 Jul;87(14):5351–5353. doi: 10.1073/pnas.87.14.5351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses K., Ellis M. C., Rubin G. M. The glass gene encodes a zinc-finger protein required by Drosophila photoreceptor cells. Nature. 1989 Aug 17;340(6234):531–536. doi: 10.1038/340531a0. [DOI] [PubMed] [Google Scholar]
- Moses K., Rubin G. M. Glass encodes a site-specific DNA-binding protein that is regulated in response to positional signals in the developing Drosophila eye. Genes Dev. 1991 Apr;5(4):583–593. doi: 10.1101/gad.5.4.583. [DOI] [PubMed] [Google Scholar]
- Pabo C. O., Sauer R. T. Transcription factors: structural families and principles of DNA recognition. Annu Rev Biochem. 1992;61:1053–1095. doi: 10.1146/annurev.bi.61.070192.005201. [DOI] [PubMed] [Google Scholar]
- Pani L., Overdier D. G., Porcella A., Qian X., Lai E., Costa R. H. Hepatocyte nuclear factor 3 beta contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila fork head protein. Mol Cell Biol. 1992 Sep;12(9):3723–3732. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland J. A., Cook A., Bannister A. J., Kouzarides T. Conserved motifs in Fos and Jun define a new class of activation domain. Genes Dev. 1992 Sep;6(9):1810–1819. doi: 10.1101/gad.6.9.1810. [DOI] [PubMed] [Google Scholar]
- Triezenberg S. J., Kingsbury R. C., McKnight S. L. Functional dissection of VP16, the trans-activator of herpes simplex virus immediate early gene expression. Genes Dev. 1988 Jun;2(6):718–729. doi: 10.1101/gad.2.6.718. [DOI] [PubMed] [Google Scholar]