Abstract
The profiles of the interaction of antimycobacterial drugs with macrophage (MΦ) antimicrobial mechanisms have yet to be elucidated in detail. We examined the effects of various antimycobacterial drugs on the anti-Mycobacterium avium complex (MAC) antimicrobial activity of reactive oxygen intermediates (ROIs), especially of an H2O2-halogen (H2O2-Fe2+-NaI)-mediated bactericidal system, reactive nitrogen intermediates (RNIs), and free fatty acids (FFAs), which are known as central antimicrobial effectors of host MΦs against mycobacterial pathogens. We have found that certain drugs, such as rifampin (RIF), rifabutin (RFB), isoniazid (INH), clofazimine (CLO), and some fluoroquinolones, strongly or moderately reduced the anti-MAC activity of the H2O2-Fe2+-NaI system, primarily by inhibiting the generation of hypohalite ions and in part by interfering with the halogenation reaction of bacterial cell components due to the H2O2-Fe2+-NaI system. This phenomenon is specific to the H2O2-Fe2+-NaI system, since these drugs did not reduce the anti-MAC activity of RNIs and FFAs. From the perspective of the chemotherapy of MAC infections, the present findings indicate an important possibility that certain antimycobacterial drugs, such as rifamycins (RIF and RFB), INH, CLO, and also some types of fluoroquinolones, may interfere with the ROI-mediated antimicrobial mechanisms of host MΦs against intracellular MAC organisms.
Mycobacterium avium complex (MAC) infections, in particular M. avium infections, are frequently encountered among patients with AIDS in the United States and European countries, as well as in other nations (7, 14). The clinical management of MAC infections, in particular those in AIDS patients, is generally difficult, in part because of the severely depressed state of the host defense mechanisms in AIDS patients due to the suppression of T-cell-mediated immunity, accompanied by a severe reduction in the antimicrobial capacity of host macrophages (MΦs), which are central effector cells of the host defense mechanisms against MAC (6, 14). In addition, many drugs, with the exception of macrolides, are generally ineffective or weakly effective for MAC infections for the following reasons. First, MAC organisms have an intrinsic resistance to the majority of common antimycobacterial drugs due to their impermeability to these agents (13, 14, 26). Furthermore, the range of susceptibilities of clinical isolates of MAC to most antimicrobial drugs, except macrolides, is very broad (13, 26). Second, polyclonal MAC infections in AIDS patients may contribute significantly to the lack of success in treating MAC infections (7, 29).
In this context, the following situations with respect to the activity of rifamycins, particularly rifalazil (RLZ; formerly known as KRM-1648), and macrolides such as clarithromycin (CLR) may be noteworthy. First, although RLZ has a much lower MIC against MAC than other antituberculosis drugs, its therapeutic efficacy against MAC infection is not as good as expected based on its low MIC (22, 26-28). In contrast, the bacteriological response in the patients with MAC infection to treatment with CLR depends on the in vitro susceptibility (based on the CLR MIC) of individual MAC pathogens to the drug (26, 27). Second, the therapeutic efficacies of both a rifamycin (RLZ) and macrolide (CLR) against MAC infection induced in mice well correlate to their antimicrobial activities against intramacrophage MAC (22, 27). Therefore, from the viewpoint of clinical treatment of MAC infections, the elucidation of the detailed profiles of the antimicrobial effects of rifamycins and macrolides against intramacrophage MAC is of particular interest.
Despite some controversies on the subject, effectors of the antimycobacterial activity of MΦs are believed to act in the following ways. First, reactive nitrogen intermediates (RNIs) have been demonstrated to play an important role in the activity of MΦs against MAC and other mycobacteria including Mycobacterium tuberculosis (18, 23). Studies employing gamma interferon gene- or inducible nitric oxide synthase gene-disrupted mice indicated that RNIs were required for the activity of MΦs against M. tuberculosis (5, 17) but not entirely responsible for the MΦ function to cope with M. avium (8). Second, with respect to the role of reactive oxygen intermediates (ROIs), it has been reported that ROIs are insufficient in inhibiting and/or killing M. tuberculosis organisms (18, 19). However, we have previously found that an H2O2-halogen (H2O2-Fe2+-NaI)-mediated antimicrobial system (15), but not ROI molecules themselves, is potently efficacious in killing MAC and M. tuberculosis organisms (1, 2, 32, 33). It thus appears that the H2O2-Fe2+-NaI system may be involved in the activity of MΦs against MAC and M. tuberculosis, when the phagosomes of MΦs are supplied with catalytic Fe2+ by the action of divalent cation transporter proteins encoded by the Nramp-1 gene (9). In this bactericidal system, the following reactions are thought to occur, resulting in the generation of hypoiodous acid (HOI): Fe2+ + H2O2 → Fe3+ + OH− + OH·, OH· + I− → OH− + I·, I· + H2O2 → IOH + OH· (15). In cases of ROI-mediated bacterial killing mechanisms in phagocytes, extremely bactericidal hypohalite radicals (HOCl and HOI) play more important roles than more reactive but less toxic hydroxyl radicals (12).
In addition, our previous studies showed that free fatty acids (FFAs), such as arachidonic acid and linolenic acid, play important roles in the expression of MΦ antimicrobial activity against MAC and M. tuberculosis in collaboration with RNIs (1, 2). Moreover, we recently found that, in MΦs infected with mycobacterial organisms, type IV cytosolic phospholipase A2 translocates to phagosomes engulfing mycobacterial organisms and exhibits catalytic effects to release arachidonic acid from membrane phospholipids (unpublished data). This subsequently causes an attack of arachidonic acid on the mycobacterial organisms residing within phagosomal vesicles (2).
To date, the profiles of the interaction of antimycobacterial drugs with antimicrobial effector molecules of host MΦs are not well known. Moreover, the consideration of these situations leads us to speculate that rifamycins, but not macrolides, may affect the expression of the activity of MΦ antimicrobial effectors, such as RNIs, ROIs, and FFAs in MΦ phagosomes engulfing MAC organisms. In the present study, we examined the effects of various antimycobacterial drugs, including rifamycins, macrolides, aminoglycosides, isoniazid (INH), ethambutol (EMB), pyrazinamide (PZA), fluoroquinolones, and clofazimine (CLO), on the anti-MAC antimicrobial activity of ROI (H2O2-Fe2+-NaI system), RNIs, and FFAs.
MATERIALS AND METHODS
Microorganisms.
A smooth and transparent (SmT) colonial variant of MAC strain N-444 (serovar 8) was used throughout the experiments. In some experiments, the SmT and a smooth, opaque and dome-shaped (SmD) colonial variant of MAC strain N-260 (serovar 16) were used. These two MAC strains were isolated from patients in Japan with MAC infection and were identified as M. avium and Mycobacterium intracellulare, respectively, by a DNA probe test.
For preparation of a bacterial suspension as an inoculum, test organisms were grown in 7H9 broth (Difco Laboratories, Detroit, Mich.) supplemented with albumin-dextrose-catalase enrichment and 0.05% (vol/vol) Tween 80 (vol/vol) for 5 days. Bacterial suspensions prepared with phosphate-buffered saline containing 1% (wt/vol) bovine serum albumin were gently sonicated by using a sonicator (Model UR-20P; Tomy Seiko Co., Tokyo, Japan) for 5 s and centrifuged at 150 × g for 5 min to eliminate bacterial clumps, and the upper layer (about 80% volume) was saved as an inoculum. The bacterial suspension prepared in this fashion contained no obvious bacterial clumps on microscopic observation. Drug susceptibility in terms of MICs for test organisms has been determined by the broth dilution method with 7HSF medium (22) and determined as indicated in Table 1.
TABLE 1.
MICs of test antimycobacterial drugs for three test strains of MACa
| Drug | MIC(μg/ml) for strain:
|
||
|---|---|---|---|
| N-444 SmT | N-260 SmT | N-260 SmD | |
| RIF | 8 | 8 | 0.25 |
| RFB | 1 | 1 | 0.13 |
| CLR | 8 | 4 | 0.13 |
| EMB | 16 | 16 | 16 |
| STR | 8 | 16 | 2 |
| KAN | 8 | 16 | 2 |
| INH | 64 | 16 | 4 |
| CIP | 8 | 8 | 0.5 |
| LVX | 16 | 16 | 0.5 |
| SPX | 2 | 4 | 0.25 |
| STX | 2 | 4 | 0.13 |
| GAT | 4 | 8 | 0.25 |
| CLO | 0.5 | 1 | 0.5 |
| PZAb | >128 | >128 | >128 |
| CLS | 32 | 128 | 32 |
Determined by the broth dilution method with 7HSF medium.
Among the test drugs, only PZA requires acidic pH (pH 6.0) in 7HSF medium.
Special agents.
Special agents and antimicrobial drugs used in this study were as follows: CLR (Taisho Pharmaceutical Co., Tokyo, Japan), rifampin (RIF; Wako Pure Chemical Industries, Osaka, Japan), ciprofloxacin (CIP; Bayer Yakuhin, Ltd., Osaka, Japan), levofloxacin (LVX; Daiichi Pharmaceutical Co., Tokyo, Japan), sitafloxacin (STX; Daiichi Pharmaceutical Co.), INH (Daiichi Pharmaceutical Co.), rifabutin (RFB; Farmitalia Carlo Erba Research Laboratories, Milan, Italy), gatifloxacin (GAT; Kyorin Pharmaceutical Co., Tokyo, Japan), sparfloxacin (SPX; Dainippon Pharmaceutical Co., Tokyo, Japan), PZA (Sankyo Co., Tokyo, Japan), EMB (Japan Lederle Co., Tokyo, Japan), streptomycin (STR; Meiji Seika Co., Tokyo, Japan), kanamycin (KAN; Meiji Seika Co.), CLO (Ciba-Geigy Co., Tokyo, Japan), cycloserine (CLS; Wako Pure Chemical Industries), arachidonic acid (Sigma Chemical Co., St. Louis, Mo.), monocholorodimedon (MCD; Sigma Chemical Co.), myeloperoxidase (MPO; Sigma Chemical Co.), luminol (Wako Pure Chemical Industries), phorbol myristate acetate (PMA; Sigma Chemical Co.), and Zymosan A (Sigma Chemical Co.).
Bacterial killing by antimicrobial effectors.
The antimicrobial activities of the H2O2-Fe2+-NaI system and RNIs against MAC organisms were measured as follows. First, the activity of the H2O2-Fe2+-NaI system (15) was measured by treating MAC organisms (ca. 106 CFU) in a reaction mixture (1.0 ml) consisting of 10 μM (N-260) or 20 μM (N-444) concentrations of H2O2, NaI, and FeSO4 in 100 mM sodium acetate buffer (pH 5.5) at 37°C for 1 or 2 h. In this experiment, H2O2, NaI, and FeSO4 alone at test concentrations were not toxic for the MAC organisms. Second, for measurement of the activity of RNIs, MAC organisms (ca. 106 CFU) were treated with RNIs generated in a reaction mixture (1.0 ml) containing 15 to 20 mg of NaNO2/ml in 100 mM sodium acetate buffer (pH 5.5) at 37°C for 2 h. This reaction system has been demonstrated to yield RNIs including NO radical (24). Third, for measurement of the activity of FFAs, MAC organisms (ca. 106 CFU) were treated with 20 μg of FFA (arachidonic acid) per ml, which was finely emulsified in 1.0 ml of 100 mM sodium acetate buffer (pH 5.5) at 37°C for 2 h (1). After individual treatments of test organisms, residual numbers of bacterial CFU were counted on 7H11 agar. In this study, the pH values of the antimicrobial systems were fixed at 5.5, since current evidence indicated that the phagosomes of MΦs engulfing virulent mycobacterial pathogens equilibrated to pH values of 5.5 to 5.7 after bacterial phagocytosis (21). The above three cell-free antimicrobial systems essentially mimic actual in vivo conditions, particularly the concentrations of H2O2, NaI, Fe2+, RNIs, and FFA (arachidonic acid) (1, 10, 11, 16, 24, 33).
HOI production by the H2O2-Fe2+-NaI system.
HOI production by the H2O2-Fe2+-NaI system was measured in terms of luminol-dependent chemiluminescence (CL), since hypohalite ion in combination with H2O2 produces potent luminol-dependent CL (3). The reaction mixture consisting of the H2O2-Fe2+-NaI system and 0.1 mM luminol, in a total volume of 3.0 ml, was incubated at 37°C for 30 s. Photoemission was measured in a lumiphotometer (Lumicounter ATP-237; Toyo Kagaku Industry, Tokyo, Japan).
Halogenation reaction by the H2O2-Fe2+-NaCl system.
The H2O2-Fe2+-NaCl system consisting of 20 μM concentrations of H2O2, NaCl (instead of NaI), and FeSO4, and 0.1 mM MCD as a halogen acceptor in a total volume of 3.0 ml was incubated at 25°C. Chlorination of the substrate MCD was monitored by measuring the decrease in the absorbance at 278 nm, according to the method of Morris and Hager (20).
MPO-mediated halogenation reaction.
The MPO-mediated halogenation reaction was measured according to the method of Morris and Hager (20) with slight modifications. Briefly, the assay mixture consisting of 300 μmol of potassium phosphate buffer (pH 2.75), 60 μmol of KCl, 6 μmol of H2O2, 0.3 μmol of MCD, and 0.2 U of MPO in a total volume of 3.0 ml was incubated at 25°C for 5 min. Chlorination of the substrate MCD was measured as described as above.
ROI production by MΦs.
Production of ROIs (especially HOCl and H2O2) by MΦs was measured in terms of PMA-triggered luminol-dependent CL (1, 3). Peritoneal exudate cells were harvested from BALB/c mice (Japan Clea Co., Osaka, Japan) 4 days after intraperitoneal injection of 1.0 mg of Zymosan A. The peritoneal exudate cells (106) were preincubated in 1.0 ml of Hanks' balanced salt solution (free of phenol red) containing 10 mM HEPES, 0.2% glucose, 0.1 mM luminol, and 10 μM concentrations of the test antimicrobial drugs at 37°C for 10 min. MΦ photoemission was then measured at 37°C in a lumiphotometer for 3 min after the addition of 100 ng of PMA dissolved in 10 μl of dimethyl sulfoxide.
Statistical analysis.
Statistical analysis was performed by using Bonferroni's multiple t test.
RESULTS
Effects of antimicrobial drugs on the expression of the anti-MAC activity of ROIs.
First, we examined the effects of various antimicrobial drugs on the antimicrobial activity of the H2O2-Fe2+-NaI system against an SmT colonial variant of MAC strain N-444 (M. avium). As shown in Table 2, when test drugs were added at the same molar concentration (10 μM each, equivalent to the following amounts: CLR, 7.5 μg/ml; RIF, 8.2 μg/ml; RFB, 8.5 μg/ml; EMB, 2.8 μg/ml; STR, 7.3 μg/ml; KAN, 5.8 μg/ml; INH, 1.4 μg/ml; CLO, 4.7 μg/ml; PZA, 1.2 μg/ml; CLS, 1.0 μg/ml; CIP, 3.9 μg/ml; LVX, 3.7 μg/ml; SPX, 3.9 μg/ml; STX, 4.4 μg/ml; and GAT, 4.0 μg/ml), RIF, RFB, INH, and CLO markedly inhibited the anti-MAC activity of the H2O2-Fe2+-NaI system. Moderate to weak inhibitory effects were also observed for fluoroquinolones, including CIP, LVX, SPX, STX, and GAT. The other test drugs, including CLR, EMB, STR, KAN, PZA, and CLS, showed no such inhibitory action. Under the present experimental conditions, none of the test drugs caused significant bacterial killing of MAC organisms when each drug alone was added to the incubation buffer, since the incubation time was too short for the test drugs to exert observable bactericidal effects (data not shown).
TABLE 2.
Effects of various antimycobacterial drugs on the expression of the antimicrobial activity of H2O2-Fe2+-NaI system against M. avium strain N-444 SmTa
| Expt no. | Incubation time (h) | Drug | Log CFUb (mean ± SEM)
|
Reduction in the activity of H2O2-Fe2+-NaI system (mean ± SEM)c | |
|---|---|---|---|---|---|
| Buffer alone | H2O2-Fe2+- NaI system | ||||
| 1 | 0 | None | 6.04 ± 0.02 | ||
| 2 | None | 3.87 ± 0.01 | |||
| 2 | RIF | 5.54 ± 0.04d | 1.67 ± 0.06 | ||
| 2 | CLR | 3.75 ± 0.07 | −0.12 ± 0.07 | ||
| 2 | INH | 5.87 ± 0.05d | 2.00 ± 0.07 | ||
| 2 | STR | 4.05 ± 0.04 | 0.18 ± 0.06 | ||
| 2 | CLS | 4.11 ± 0.05 | 0.24 ± 0.07 | ||
| 2 | 0 | None | 5.97 ± 0.01 | ||
| 2 | None | 3.23 ± 0.01 | |||
| 2 | RIF | 5.20 ± 0.02d | 1.97 ± 0.01 | ||
| 2 | RFB | 5.66 ± 0.01d | 2.43 ± 0.01 | ||
| 2 | EMB | 3.33 ± 0.04 | 0.10 ± 0.02 | ||
| 2 | KAN | 3.39 ± 0.05 | 0.16 ± 0.03 | ||
| 2 | CLO | 5.04 ± 0.02d | 1.81 ± 0.01 | ||
| 2 | PZA | 3.31 ± 0.03 | 0.08 ± 0.02 | ||
| 3 | 0 | None | 5.98 ± 0.03 | ||
| 2 | None | 3.09 ± 0.14 | |||
| 2 | RIF | 5.28 ± 0.01d | 2.19 ± 0.08 | ||
| 2 | CIP | 3.41 ± 0.06 | 0.32 ± 0.09 | ||
| 2 | LVX | 3.65 ± 0.00 | 0.56 ± 0.08 | ||
| 2 | SPX | 3.77 ± 0.11 | 0.68 ± 0.10 | ||
| 2 | STX | 3.45 ± 0.10 | 0.36 ± 0.10 | ||
| 2 | GAT | 3.38 ± 0.09 | 0.29 ± 0.09 | ||
Each test drug was added at a concentration of 10 μM to the H2O2-Fe2+-NaI system. The experiments were repeated three times and yielded similar results.
Residual bacterial CFU before and after 2 h-incubation in the presence or absence of test drugs is indicated in log units (n = 3).
This parameter was calculated as follows: log CFU (H2O2-Fe2+-NaI system + drug; 2 h) − log CFU (H2O2-Fe2+-NaI system alone; 2 h).
Significantly greater than the value of the control (without drug) incubation (P < 0.01).
Next, we examined the effects of test drugs on the anti-MAC strain N-444 activity of the H2O2-Fe2+-NaI system when drugs were added at the maximum concentration in serum (Cmax) achievable after their administration at clinical dosages. As indicated in Table 3, RIF (6.2 μg/ml) and INH (7.3 μg/ml) exhibited strong inhibitory activity. Moderate to weak inhibitory effects were also noted for CLO and the fluoroquinolones. The other drugs exerted only weak inhibitory action, except for CLS and STR, which have very large Cmax values (CLS, 17 μg/ml; STR, 45 μg/ml). These results indicate that RIF, INH, and some other drugs, such as fluoroquinolones, antagonize the anti-MAC activity of the H2O2-Fe2+-NaI system, even at the in vivo concentrations achievable by administering the usual dosages. Although there was a significant difference in the inhibitory effect of the system for RIF between experiments 1 and 3, we obtained reproducible results for the profiles of the effects of individual drugs on the anti-MAC activity of the H2O2-Fe2+-NaI system in repeated experiments.
TABLE 3.
Effects of various antimycobacterial drugs added at the Cmax on the expression of the antimicrobial activity of H2O2-Fe2+-NaI system against M. avium strain N-444 SmTa
| Expt no. | Incubation time (h) | Drug (μg/ml) | Log CFU (mean ± SEM)
|
Reduction in the activity of H2O2-Fe2+-NaI system (mean ± SEM)b | |
|---|---|---|---|---|---|
| Buffer alone | H2O2-Fe2+- NaI system | ||||
| 1 | 0 | None | 6.18 ± 0.02 | ||
| 2 | None | 4.91 ± 0.10 | |||
| 2 | RIF (6.2) | 5.85 ± 0.03c | 0.94 ± 0.06 | ||
| 2 | CLR (2.3) | 5.22 ± 0.06 | 0.31 ± 0.07 | ||
| 2 | LVX (2.0) | 5.58 ± 0.05d | 0.67 ± 0.06 | ||
| 2 | STX (1.0) | 5.16 ± 0.05 | 0.25 ± 0.06 | ||
| 2 | GAT (1.8) | 5.46 ± 0.10 | 0.55 ± 0.08 | ||
| 2 | 0 | None | 5.96 ± 0.03 | ||
| 2 | None | 3.53 ± 0.06 | |||
| 2 | RFB (0.38) | 2.99 ± 0.14 | −0.54 ± 0.09 | ||
| 2 | INH (7.3) | 5.82 ± 0.02c | 2.29 ± 0.04 | ||
| 2 | PZA (35) | 3.07 ± 0.12 | −0.46 ± 0.08 | ||
| 2 | CLS (17) | 4.93 ± 0.02c | 1.40 ± 0.03 | ||
| 3 | 0 | None | 5.99 ± 0.01 | ||
| 2 | None | 2.52 ± 0.23 | |||
| 2 | RIF (6.2) | 5.40 ± 0.01c | 2.88 ± 0.13 | ||
| 2 | EMB (2.1) | 2.78 ± 0.32 | 0.26 ± 0.23 | ||
| 2 | STR (45) | 3.92 ± 0.04 | 1.40 ± 0.13 | ||
| 2 | CLO (0.41) | 3.39 ± 0.24 | 0.87 ± 0.19 | ||
Each test drug was added to the H2O2-Fe2+-NaI system at the Cmax achievable in blood after administration of its clinical dosage. Other details are the same as in Table 2.
Log-unit decrease calculated as for Table 2.
Significantly greater than the value of the control (without drug) incubation (P < 0.01).
Significantly greater than the value of the control (without drug) incubation (P < 0.05).
In order to examine whether the present finding is peculiar to the SmT colonial variant of MAC strain N-444 (M. avium), we performed additional experiments with SmT and SmD colonial variants of the MAC strain N-260 (M. intracellulare), following the same protocols as for the experiment represented in Table 2. As described below in detail, essentially the same profile was observed for the inhibitory effects of test antimycobacterial drugs on the H2O2-Fe2+-NaI system against the MAC strain N-260 SmT and SmD variants as that observed when the MAC strain N-444 SmT variant was used as a target organism (Table 2). As shown in Table 4, RIF, RFB, INH, and CLO markedly inhibited the antimicrobial activity of the H2O2-Fe2+-NaI system against the MAC strain N-260 SmTvariant, and moderate inhibitory effects were observed for CLR and CIP. EMB and KAN exerted no such marked inhibitory effects. As indicated by the data in Table 5, RIF, RFB, INH, CIP, and CLO strongly inhibited the antimicrobial activity of the H2O2-Fe2+-NaI system against the MAC strain N-260 SmD variant. CLR, EMB, and KAN caused moderate to weak inhibition. These findings indicate that certain antimycobacterial drugs, especially RIF, RFB, INH, and CLO, potently interfere with the expression of the antimicrobial activity of the H2O2-Fe2+-NaI system against MAC organisms irrespective of species (M. avium or M. intracellulare) or colonial variants (SmT or SmD). It is also noted that the ROI-mediated antimicrobial activity against SmD colonial variants is considerably more susceptible to the antagonistic effects of antimycobacterial drugs than that against SmT colonial variants of MAC strains (Table 5 versus Table 4).
TABLE 4.
Effects of various antimycobacterial drugs on the expression of the antimicrobial activity of H2O2-Fe2+-NaI system against M. intracellulare strain N-260 SmTa
| Incubation time (h) | Drug | Log CFUb (mean ± SEM)
|
Reduction in the activity of H2O2-Fe2+-NaI system (mean ± SEM)c | |
|---|---|---|---|---|
| Buffer alone | H2O2-Fe2+-NaI system | |||
| 0 | None | 6.03 ± 0.02 | ||
| 1 | None | 4.79 ± 0.07 | ||
| 1 | RIF | 5.75 ± 0.02d | 0.96 ± 0.07 | |
| 1 | RFB | 5.97 ± 0.02d | 1.17 ± 0.04 | |
| 1 | CLR | 5.55 ± 0.03d | 0.75 ± 0.04 | |
| 1 | EMB | 5.18 ± 0d | 0.39 ± 0.04 | |
| 1 | KAN | 4.80 ± 0.10 | 0.01 ± 0.07 | |
| 1 | INH | 5.95 ± 0.04d | 1.15 ± 0.08 | |
| 1 | CIP | 5.64 ± 0.05d | 0.85 ± 0.09 | |
| 1 | CLO | 5.95 ± 0.02d | 1.16 ± 0.07 | |
Each test drug was added at a concentration of 10 μM to the H2O2-Fe2+-NaI system. The other details are same as in Table 2.
Residual bacterial CFU before and after 1-h incubation in the presence or absence of test drugs is indicated in log units (n = 3).
This parameter was calculated as follows: log CFU (H2O2-Fe2+-NaI system + drug; 1 h) − log CFU (H2O2-Fe2+-NaI system alone; 1 h).
Significantly greater than the value of the control (without drug) incubation (P < 0.01).
TABLE 5.
Effects of various antimycobacterial drugs on the expression of the antimicrobial activity of H2O2-Fe2+-NaI system against M. intracellulare strain N-260 SmDa
| Incubation time (h) | Drug | Log CFUb (mean ± SEM)
|
Reduction in the activity of H2O2-Fe2+-NaI system (mean ± SEM)c | |
|---|---|---|---|---|
| Buffer alone | H2O2-Fe2+-NaI system | |||
| 0 | None | 6.09 ± 0.02 | ||
| 1 | None | 3.29 ± 0.14 | ||
| 1 | RIF | 4.76 ± 0.09d | 1.47 ± 0.10 | |
| 1 | RFB | 5.44 ± 0.11d | 2.15 ± 0.12 | |
| 1 | CLR | 4.26 ± 0.10d | 0.98 ± 0.10 | |
| 1 | EMB | 3.82 ± 0.10 | 0.54 ± 0.10 | |
| 1 | KAN | 3.70 ± 0.12 | 0.42 ± 0.11 | |
| 1 | INH | 5.88 ± 0.01d | 2.57 ± 0.07 | |
| 1 | CIP | 4.40 ± 0.08d | 1.12 ± 0.09 | |
| 1 | CLO | 4.59 ± 0.05d | 1.30 ± 0.09 | |
Each test drug was added at a concentration of 10 μM to the H2O2-Fe2+-NaI system. Other details are the same as in Table 2.
Residual bacterial CFU before and after 1-h incubation in the presence or absence of test drugs is indicated in log units (n = 3).
This parameter was calculated as follows: log CFU (H2O2-Fe2+-NaI system + drug; 1 h) − log CFU (H2O2-Fe2+-NaI system alone; 1 h).
Significantly greater than the value of the control (without drug) incubation (P < 0.01).
Effects of antimicrobial drugs on the expression of the anti-MAC effects of RNIs and FFAs.
In order to know whether or not the inhibitory effects of rifamycins, INH, CLO, and quinolones against the H2O2-Fe2+-NaI system are specific to the ROI-mediated microbicidal system, we examined the effects of test drugs on the expression of the anti-MAC activity of RNIs, which are generated from acidified NaNO2 and an FFA (arachidonic acid). As shown in Table 6, none of the test drugs affected the RNI-mediated killing of MAC organisms (strain N-444 SmT variant), except for CLO, which caused a weak to moderate level of inhibition. Similarly, as indicated in Table 7, none of the test drugs significantly affected the FFA-mediated killing of MAC strain N-444 organisms. These findings indicate that rifamycins, INH, quinolones, and also CLO specifically inhibit the H2O2-Fe2+-NaI system.
TABLE 6.
Effects of various antimycobacterial drugs on the expression of the antimicrobial activity of RNIs against M. avium strain N-444 SmTa
| Experi- ment no. | Incubation time (h) | Drug | Log CFU (mean ± SEM)
|
Reduction in the activity of RNI- mediated system (mean ± SEM)b | |
|---|---|---|---|---|---|
| Buffer alone | RNI-mediated antimicrobial system | ||||
| 1 | 0 | None | 6.01 ± 0.02 | ||
| 2 | None | 4.18 ± 0.02 | |||
| 2 | RIF | 3.55 ± 0.04 | −0.63 ± 0.02 | ||
| 2 | RFB | 3.60 ± 0.03 | −0.58 ± 0.02 | ||
| 2 | EMB | 3.83 ± 0.05 | −0.35 ± 0.03 | ||
| 2 | KAN | 4.01 ± 0.03 | −0.17 ± 0.02 | ||
| 2 | CLO | 4.53 ± 0.06c | 0.35 ± 0.04 | ||
| 2 | PZA | 4.00 ± 0.04 | −0.18 ± 0.02 | ||
| 2 | 0 | None | 6.00 ± 0.02 | ||
| 2 | None | 3.70 ± 0.06 | |||
| 2 | CLR | 3.41 ± 0.02 | −0.29 ± 0.04 | ||
| 2 | INH | 3.70 ± 0.08 | 0.00 ± 0.06 | ||
| 2 | STR | 3.66 ± 0.08 | −0.04 ± 0.06 | ||
| 2 | CLO | 4.20 ± 0.02c | 0.50 ± 0.04 | ||
| 2 | CLS | 3.67 ± 0.07 | −0.03 ± 0.05 | ||
| 3 | 0 | None | 5.93 ± 0.02 | ||
| 2 | None | 3.33 ± 0.10 | |||
| 2 | RIF | 3.06 ± 0.06 | −0.27 ± 0.07 | ||
| 2 | CIP | 3.27 ± 0.08 | −0.06 ± 0.07 | ||
| 2 | LVX | 3.31 ± 0.02 | −0.02 ± 0.06 | ||
| 2 | SPX | 3.10 ± 0.13 | −0.23 ± 0.10 | ||
| 2 | STX | 3.15 ± 0.04 | −0.18 ± 0.06 | ||
| 2 | GAT | 3.16 ± 0.08 | −0.17 ± 0.07 | ||
Each test drug was added at a concentration of 10 μM to acidified NaNO2. The other details are the same as in Table 2.
This parameter was calculated as follows: log CFU (acidified NaNO2 + drug; 2 h) − log CFU (acidified NaNO2 alone; 2 h).
Significantly greater than the value of the control (without drug) incubation (P < 0.05).
TABLE 7.
Effects of various antimycobacterial drugs on the expression of the antimicrobial activity of FFA against M. avium strain N-444 SmTa
| Incubation time (h) | Drug | Log CFU (mean ± SEM)
|
Reduction in the activity of FFA- mediated system (mean ± SEM)b | |
|---|---|---|---|---|
| Buffer alone | FFA-mediated antimicrobial system | |||
| 0 | None | 5.87 ± 0.02 | ||
| 2 | None | 3.15 ± 0.27 | ||
| 2 | RIF | 3.20 ± 0.29 | 0.05 ± 0.23 | |
| 2 | RFB | 2.71 ± 0.14 | −0.44 ± 0.18 | |
| 2 | CLR | 3.38 ± 0.22 | 0.23 ± 0.20 | |
| 2 | EMB | 3.32 ± 0.07 | 0.17 ± 0.16 | |
| 2 | STR | 3.01 ± 0.09 | −0.14 ± 0.17 | |
| 2 | KAN | 3.30 ± 0.06 | 0.15 ± 0.16 | |
| 2 | INH | 3.30 ± 0.01 | 0.15 ± 0.16 | |
| 2 | CIP | 2.74 ± 0.11 | −0.41 ± 0.17 | |
| 2 | LVX | 3.03 ± 0.30 | −0.12 ± 0.24 | |
| 2 | SPX | 3.47 ± 0.09 | 0.32 ± 0.17 | |
| 2 | CLO | 3.16 ± 0.11 | 0.01 ± 0.17 | |
Each test drug was added at a concentration of 10 μM to the FFA (arachidonic acid)-mediated system. The other details are the same as in Table 2.
This parameter was calculated as log CFU (arachidonic acid + drug; 2 h) − log CFU (arachidonic acid alone; 2 h).
Mechanisms of the inhibitory action of rifamycins, INH, CLO, and quinolones against the bactericidal activity of the H2O2-Fe2+-NaI system.
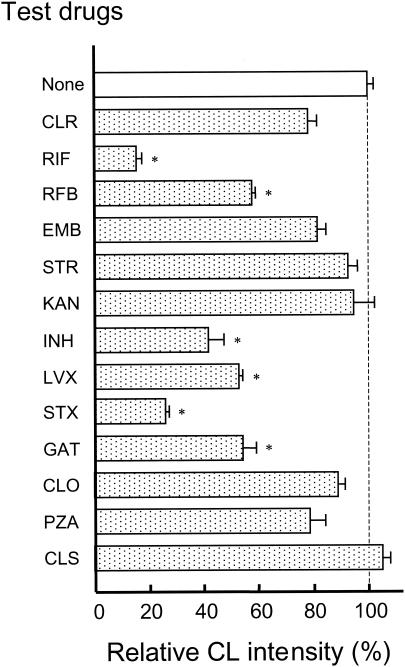
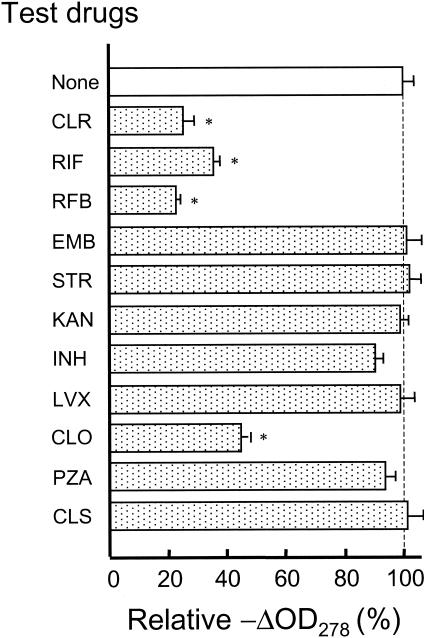
Next, we investigated the mechanisms of the inhibitory effects of rifamycins, INH, CLO, and quinolones against the H2O2-Fe2+-NaI-mediated bacterial killing of MAC organisms. First, we examined the effects of test drugs on the generation of hypohalite ions by the H2O2-Fe2+-NaI system. In this experiment, HOI generation was monitored by measuring luminol-dependent CL, which is produced by luminol oxidation due to H2O2 in combination with HOI generated by the H2O2-Fe2+-NaI system (3). As shown in Fig. 1, the H2O2-Fe2+-NaI system produced significant levels of luminol-dependent CL. Notably, rifamycins (RIF and RFB), INH, and quinolones (LVX, STX, and GAT) strongly reduced the CL, causing 42 to 85% inhibition. On the other hand, CLR, EMB, and PZA exerted weak inhibitory activity, causing 19 to 23% inhibition, while the aminoglycosides (STR and KAN), CLO, and CLS did not exhibit any significant inhibitory activity. The extent of the antimicrobial drug-mediated reduction of the CL (HOI) generation by the H2O2-Fe2+-NaI system essentially paralleled the drug-mediated inhibition of the anti-MAC activity of the same system except in the case of CLO (compare Fig. 1 and Table 2). Next, we examined the effects of test drugs on the halogenation reaction caused by the H2O2-Fe2+-NaCl system. As shown in Fig. 2, chlorination of a substrate MCD by this system was potently inhibited by CLR, RIF, RFB, and CLO, while neither EMB, STR, KAN, INH, LVX, PZA, nor CLS exhibited such inhibitory action. Although the inhibitory effects of rifamycins (RIF and RFB) and CLO against MCD chlorination paralleled their inhibitory action against the anti-MAC activity of the H2O2-Fe2+-NaI system, such parallelism was not observed for CLR, INH, and LVX (compare Fig. 2 and Table 2). The MPO-mediated halogenation reaction, measured in terms of the chlorination of MCD used as a halogen acceptor, was weakly inhibited by RIF and CLR (23.8% ± 6.6% and 16.8% ± 4.8% inhibition, respectively), but such inhibitory action was not noted for INH and EMB (−3.7% ± 4.8% and 4.0% ± 2.2% inhibition, respectively). In any case, it appears that the MPO-mediated halogenation reaction is essentially insensitive to these antimycobacterial drugs.
FIG. 1.
Effects of various antimicrobial drugs on the generation of hypohalite ions in the H2O2-Fe2+-NaI system, measured in terms of H2O2-HOI-mediated CL. Each test drug was added to the incubation mixture at a concentration of 10 μM. Each bar indicates the mean ± standard error of the mean (n = 3) of the relative CL intensity, when the value of the control incubation (without drug; 17,778 ± 371 cpm) was fixed to be 100%. *, value significantly smaller than the value of the control incubation (P < 0.01).
FIG. 2.
Effects of various antimicrobial drugs on the halogenation reaction caused by the H2O2-Fe2+-NaCl system. Chlorination of the substrate MCD added as a halogen acceptor was monitored by measuring the decrease in the absorbance at 278 nm (−ΔOD278). Each test drug was added to the reaction mixture at a concentration of 10 μM. Each bar indicates the mean ± standard error of the mean (n = 3) of the relative value of −ΔOD278, when the value of the control incubation (without drug; ca. −0.038/10 min.) was fixed to be 100%. The other details are the same as described in the legend of Fig. 1.
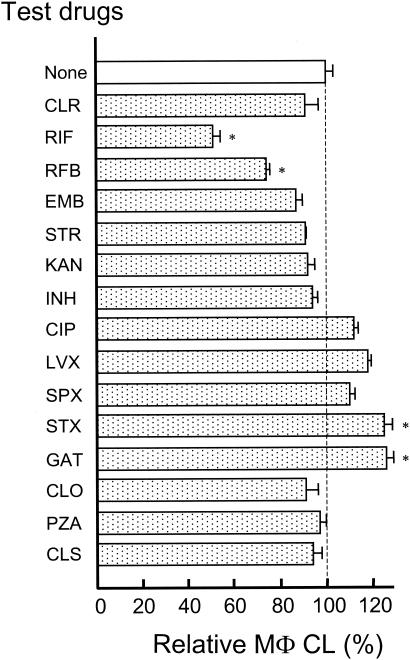
As shown in Fig. 3, although RIF and RFB significantly inhibited the PMA-induced luminol-dependent CL of MΦs, the other drugs failed to show such inhibitory action. Some fluoroquinolones (CIP, LVX, SPX, STX, and GAT) even caused a slight increase of the MΦ CL. Thus, none of the test drugs except for the rifamycins seem to affect the MΦ ability to produce ROIs in a marked fashion. Notably, the effects of test drugs on the ROI-producing ability of MΦs do not correlate with their efficacies in inhibiting the anti-MAC activity of the H2O2-Fe2+-NaI system.
FIG. 3.
Effects of various antimicrobial drugs on the ROI-producing ability of MΦs, measured in terms of PMA-triggered luminol-dependent CL. Each test drug was added to the incubation mixture at a concentration of 10 μM. Each bar indicates the mean ± standard error of the mean (n = 3) of the relative MΦ CL, when the value of the control incubation (without drug; 22,264.7 ± 680.6 cpm) was fixed to be 100%. The other details are the same as described in the legend of Fig. 1.
DISCUSSION
We have previously found that the H2O2-Fe2+-NaI system plays important roles in the antimycobacterial activity of host MΦs (1, 2). The present findings indicate that certain types of antimycobacterial drugs, including rifamycins (RIF and RFB), INH, and CLO, have potent inhibitory activity against the anti-MAC antimicrobial effects of the H2O2-Fe2+-NaI system. Moreover, some quinolones, such as LVX and SPX, also have moderate inhibitory activity against the same bactericidal system. This study, to our knowledge, is the first observation of the antagonistic effects of certain antimycobacterial drugs against the ROI-mediated antimicrobial mechanisms of MΦs. Since the above phenomenon was not observed in the case of the RNI- or FFA-mediated killing of MAC organisms, it appears that these drugs specifically inhibited the ROI-mediated bactericidal activity of the H2O2-Fe2+-NaI system.
Careful consideration of the chemical structures of test drugs revealed an interesting relationship between the chemical structure of a given drug and its ability to inhibit the anti-MAC activity of the H2O2-Fe2+-NaI system. Without exception, rifamycins, INH, CLO, and quinolones, which exert significant inhibitory action against the H2O2-Fe2+-NaI system, possess conjugated double bonds in their chemical structures. On the other hand, CLR, EMB, the aminoglycosides, and CLS, which are inefficacious in inhibiting the H2O2-Fe2+-NaI anti-MAC system, are lacking in such conjugated double bonds. The only exception is PZA, since it exhibits no inhibitory action against the H2O2-Fe2+-NaI system in spite of having conjugated double bonds. Thus, there may be a tendency that drugs that are more sensitive to ROI-mediated oxidation have more potent activity to inhibit the H2O2-Fe2+-NaI bactericidal system.
In addition, these drugs strongly inhibited HOI generation by the H2O2-Fe2+-NaI system. The inhibitory activity of a drug against hypohalite ion production essentially paralleled the drug's ability in reducing the anti-MAC activity of the H2O2-Fe2+-NaI system except for CLO. Therefore, the inhibition of the H2O2-Fe2+-NaI system-mediated killing of MAC organisms due to these drugs is attributable to their ability to inhibit HOI generation. On the other hand, considerably different profiles were seen for the effects of test drugs on the H2O2-Fe2+-NaCl system-mediated chlorination of the halogen acceptor MCD, in accordance with the following explanation. First, RIF, RFB, and CLO strongly inhibited MCD chlorination. Therefore, it is likely that both rifamycins and CLO inhibit the H2O2-Fe2+-NaI system-mediated halogenation reaction. In particular, it appears that the inhibitory action of CLO against ROI (H2O2-Fe2+-NaI system)-mediated anti-MAC activity is mainly attributable to this effect. Second, neither INH nor the quinolones exhibited such inhibitory action, although they are efficacious in inhibiting the H2O2-Fe2+-NaI system-mediated anti-MAC action. Third, CLR displayed a potent inhibitory effect against MCD chlorination, despite the fact that it caused no reduction in the anti-MAC activity of the H2O2-Fe2+-NaI system. Thus, the inhibitory effects of RIF, RFB, and CLO against the H2O2-Fe2+-NaI-mediated bacterial killing of MAC organisms are at least in part attributable to the reduction of the halogenation reaction in the bactericidal system due to these rifamycins. Particularly, it appears that the inhibitory action of CLO against the anti-MAC activity of the H2O2-Fe2+-NaI system is mainly attributable to this effect. However, there may be no such parallelism for the other drugs, especially INH, the quinolones, and CLR.
In the cases of ROI-mediated bacterial killing mechanisms in phagocytes, extremely bactericidal hypohalite radicals (HOCl and HOI) play important roles (12). It has been reported that in the myeloperoxidase-mediated halogenation of bacterial components, hypohalite radicals play the roles of halogenating intermediates (4). However, it is also known that 50 to 70% of HOCl is consumed in peptide bond cleavage or the oxidation of bacterial components, including iron-sulfur proteins, membrane transport proteins, and the ATP generating system, and only 30 to 50% of HOCl is consumed in the chlorination of bacterial components (12, 25). Therefore, it is thought that hypohalite radicals exhibit bactericidal activity by inactivating bacterial cell components due not only to a halogenation reaction but also to other types of radical reactions. This may explain the discrepancy between the effects of the test drugs on MCD halogenation by the H2O2-Fe2+-NaCl system and their efficacies in inhibiting the anti-MAC activity of the H2O2-Fe2+-NaI system.
The result shown in Fig. 3 indicates that luminol-dependent CL in PMA-triggered MΦs was not inhibited by INH, CLO, and the quinolones, all of which exerted significant inhibitory activity against the production of the hypohalite ion in the H2O2-Fe2+-NaI system, although rifamycins (RIF and RFB) exerted strong inhibitory effects against the luminol-dependent CL in MΦs. This finding is somewhat enigmatic, because it has generally been known that luminol-dependent MΦ CL is mainly produced due to the oxidation of luminol molecules by H2O2 and hypohalite ions. However, in this context, the previous findings by Wang et al. (30, 31) may be significant. They found that PMA-induced luminol-dependent CL in MΦs is largely dependent on l-arginine metabolism by inducible nitric oxide synthase of PMA-triggered MΦs, requiring the concurrent formation of NO and O2−/H2O2, whereas the hypohalite ion is mainly responsible for luminol-dependent CL in granulocytes (30, 31). Therefore, it appears that rifamycins, but not other drugs, display inhibitory effects against NO and/or O2−/H2O2 production.
In any case, the present study strongly suggested the possibility that certain antimycobacterial drugs, such as rifamycins (RIF and RFB), INH, CLO, and some fluoroquinolones, antagonize the ROI (H2O2-Fe2+-NaI system)-mediated antimicrobial mechanisms of host MΦs against MAC organisms, primarily by inhibiting the generation of HOI by the H2O2-Fe2+-NaI system and in part by inhibiting its iodination of bacterial cell components. This is an important finding from the viewpoint of the chemotherapy of MAC-infected patients and regimens involving these drugs. A great discrepancy is known for the in vitro and in vivo anti-MAC activities of rifamycins, especially in the case of RLZ. That is, the therapeutic efficacy of rifamycins against MAC infections is much weaker than that expected from their MICs for MAC isolates (22, 26-28). This enigmatic situation may be in part explained by using the present finding that rifamycins antagonize the ROI-mediated antimicrobial mechanisms of host MΦs. A similar situation may more or less account for the obscure therapeutic outcomes of INH and fluoroquinolones in MAC-infected patients, in addition to their relatively high MICs for MAC isolates.
Acknowledgments
This study was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan and the Ministry of Health, Labor and Welfare of Japan. We thank Taisho Pharmaceutical Co., Bayer Yakuhin, Ltd., Daiichi Pharmaceutical Co., Kyorin Pharmaceutical Co., Farmitalia Carlo Erba Research Laboratories, Dainippon Pharmaceutical Co., and Ciba-Geigy Co. for providing the antimycobacterial drugs used in this study.
REFERENCES
- 1.Akaki, T., K. Sato, T. Shimizu, C. Sano, H. Kajitani, S. Dekio, and H. Tomioka. 1997. Effector molecules in expression of the antimicrobial activity of macrophage against Mycobacterium avium complex: roles of reactive nitrogen intermediates, reactive oxygen intermediates, and free fatty acids. J. Leukoc. Biol. 62:795-804. [DOI] [PubMed] [Google Scholar]
- 2.Akaki, T., H. Tomioka, T. Shimizu, S. Dekio, and K. Sato. 2000. Comparative roles of free fatty acids with reactive nitrogen intermediates and reactive oxygen intermediates in expression of the anti-microbial activity of macrophages against Mycobacterium tuberculosis. Clin. Exp. Immunol. 121:302-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brestel, E. P. 1985. Co-oxidation of luminol by hypochlorite and hydrogen peroxide implications for neutrophil chemiluminescence. Biochem. Biophys. Res. Commun. 126:482-488. [DOI] [PubMed] [Google Scholar]
- 4.Chapman, A. L., M. B. Hampton, R. Senthimohan, C. C. Winterbourn, and A. J. Kettle. 2002. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of Staphylococcus aureus. J. Biol. Chem. 277:9757-9762. [DOI] [PubMed] [Google Scholar]
- 5.Cooper, A. M., D. K. Dalton, T. Stewart, J. P. Griffin, D. G. Russell, and I. M. Orme. 1993. Disseminated tuberculosis in interferon gamma gene-disrupted mice. J. Exp. Med. 178:2243-2247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellner, J. J., M. J. Goldberger, and D. M. Parenti. 1991. Mycobacterium avium infection and AIDS: a therapeutic dilemma in rapid evolution. J. Infect. Dis. 163:1326-1335. [DOI] [PubMed] [Google Scholar]
- 7.Falkinham, J. O. 1996. Epidemiology of infection by nontuberculous mycobacteria. Clin. Microbiol. Rev. 9:177-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gomes, M. S., M. Florido, T. F. Pais, and R. Appelberg. 1999. Improved clearance of Mycobacterium avium upon disruption of the inducible nitric oxide synthase gene. J. Immunol. 162:6734-6739. [PubMed] [Google Scholar]
- 9.Gunshin, H., B. Mackenzie, and U. Berger. 1997. Cloning and characterization of a mammalian proton-coupled metal-ion transporter. Nature 388:482-483. [DOI] [PubMed] [Google Scholar]
- 10.Gutterridge, J. M. C., D. A., Rowley, and B. Halliwell. 1982. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Biochem. J. 206:605-609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halliwell, B., and J. M. C. Gutterridge. 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219:1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hampton, M. B., A. J. Kettle, and C. C. Winterbourn. 1998. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood 92:3007-3017. [PubMed] [Google Scholar]
- 13.Heifets, L. 1996. Susceptibility testing of Mycobacterium avium complex isolates. Antimicrob. Agents Chemother. 40:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inderlied, C. B., C. A. Kemper, and L. E. M. Bermudez. 1993. The Mycobacterium avium complex. Clin. Microbiol. Rev. 6:266-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klebanoff, S. J. 1982. The iron-H2O2-iodide cytotoxic system. J. Exp. Med. 156:1262-1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levitz, S. M., and R. D. Diamond. 1984. Killing of Aspergillus fumigatus spores and Candida albicans yeast phase by the iron-hydrogen peroxide-iodide cytotoxic system: comparison with the myeloperoxidase-hydrogen peroxide-halide system. Infect. Immun. 43:1100-1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacMicking, J. D., R. J. North, R. LaCourse, J. S. Mudgett, S. K. Shah, and C. F. Nathan. 1997. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl. Acad. Sci. USA 94:5243-5248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacMicking, J., Q. W. Xie, and C. Nathan. 1997. Nitric oxide and macrophage function. Annu. Rev. Immunol. 15:323-350. [DOI] [PubMed] [Google Scholar]
- 19.Manca, C., S. Paul, C. E. Barry III, V. H. Freedman, and G. Kaplan. 1999. Mycobacterium tuberculosis catalase and peroxidase activities and resistance to oxidative killing in human monocyte in vitro. Infect. Immun. 67:74-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morris, D. R., and L. P. Hager. 1966. Chloroperoxidase. I. Isolation and properties of the crystalline glycoprotein. J. Biol. Chem. 241:1763-1768. [PubMed] [Google Scholar]
- 21.Oh, Y. K., and R. M. Straubringer. 1996. Intracellular fate of Mycobacterium avium: use of dual-label spectrofluorometry to investigate the influence of bacterial viability and opsonization of phagosomal pH and phagosome-lysosome interaction. Infect. Immun. 64:319-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sato, K., T. Akaki, and H. Tomioka. 1998. Antimicrobial activities of benzoxazinorifamycin KRM-1648 and levofloxacin against intracellular Mycobacterium avium complex phagocytosed by murine peritoneal macrophages. J. Antimicrob. Chemother. 41:77-83. [DOI] [PubMed] [Google Scholar]
- 23.Sato, K., T. Akaki, and H. Tomioka. 1998. Differential potentiation of anti-mycobacterial activity and reactive nitrogen intermediate-producing ability of murine peritoneal macrophages activated by interferon-gamma (IFN-γ) and tumour necrosis factor-alpha (TNF-α). Clin. Exp. Immunol. 112:63-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stuehr, D. J., and C. F. Nathan. 1989. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J. Exp. Med. 169:1543-1555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thomas, E. L. 1979. Myeloperoxidase, hydrogen peroxide, chloride antimicrobial system: nitrogen-chloride derivatives of bacterial components in bactericidal action against Escherichia coli. Infect. Immun. 23:522-531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tomioka, H. 2000. Prospects for development of new antimycobacterial drugs. J. Infect. Chemother. 6:8-20. [DOI] [PubMed] [Google Scholar]
- 27.Tomioka, H. 2003. Type II pneumocytes in the evaluation of drug antimycobacterial activity. Expert Opin. Pharmacother. 4:127-139. [DOI] [PubMed] [Google Scholar]
- 28.Tomioka, H., H. Saito, K. Sato, T. Yamane, K. Yamashita, K. Hosoe, K. Fujii, and T. Hidaka. 1992. Chemotherapeutic efficacy of a newly synthesized benzoxazinorifamycin, KRM-1648, against Mycobacterium avium complex infection induced in mice. Antimicrob. Agents Chemother. 36:387-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.von Reyen, C. F., M. Pastel, and R. D. Arbeit. 1996. Clinical and epidemiologic implications of polyclonal infection due to Mycobacterium avium complex. Res. Microbiol. 147:24-30. [DOI] [PubMed] [Google Scholar]
- 30.Wang, J. F., P. Komarov, H. Sies, and H. de Groot. 1991. Contribution of nitric oxide synthase to luminol-dependent chemiluminescence generated by phorbol-ester-activated Kupffer cells. J. Biochem. 279:311-314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang, J. F., P. Komarov, and H. de Groot. 1993. Luminol chemiluminescence in rat macrophages and granulocytes: the role of NO, O2−/H2O2, and HOCl. Arch. Biochem. Biophys. 304:189-196. [DOI] [PubMed] [Google Scholar]
- 32.Yamada, Y., H. Saito, H. Tomioka, and J. Jidoi. 1987. Susceptibility of micro-organisms to active oxygen species: sensitivity to the xanthine-oxidase-mediated antimicrobial system. J. Gen. Microbiol. 133:2007-2014. [DOI] [PubMed] [Google Scholar]
- 33.Yamada, Y., H. Saito, H. Tomioka, and J. Jidoi. 1987. Relationship between the susceptibility of various bacteria to active oxygen species and to intracellular killing by macrophages. J. Gen. Microbiol. 133:2015-2021. [DOI] [PubMed] [Google Scholar]