Abstract
A method for cobalt-catalyzed, aminoquinoline-directed ortho-functionalization of sp2 C–H bonds with alkenes has been developed. Reactions proceed at room temperature in trifluoroethanol solvent, use oxygen from air as an oxidant, and require Mn(OAc)3 as a cocatalyst. Benzoic, heteroaromatic, and acrylic acid aminoquinoline amides react with ethylene as well as mono- and disubstituted alkenes affording products in good yields. Excellent functional group tolerance is observed; halogen, nitro, ether, and unprotected alcohol functionalities are compatible with the reaction conditions.
Transition-metal catalyzed coupling of sp2 C–H bonds with alkenes is perhaps one of the earliest synthetically useful C–H bond functionalization reactions.1 Pioneering reports by Fujiwara,2 Lewis,3a and Murai3b have disclosed alkene and arene hydroarylation methodology. Following these discoveries, a substantial number of arene/alkene couplings have been developed. Currently, even directed, selective meta-functionalization of arenes is possible.4 However, most of the catalytic processes employ scarce second-row transition metal catalysts.5 Relatively few papers describe use of cobalt- or nickel-catalyzed arene C–H functionalization by alkenes.6 Furthermore, the scope of the transformations is often limited, as acrylates, vinylsilanes, or styrenes are the most commonly employed coupling partners. Use of other functionalized alkenes or olefins that are prone to double bond migration,5o such as 1-butene, is rare.5a,5n,5t,5u,6b,6d
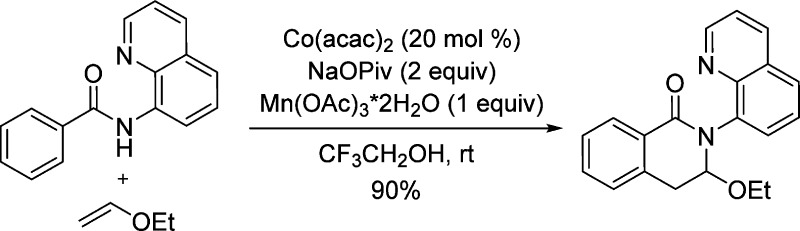
In 2005, we introduced 8-aminoquinoline, picolinamide, and 2-pyridinylmethylamine auxiliaries for palladium-catalyzed C–H bond arylation by aryl iodides.7a,7b The auxiliaries were subsequently used for copper-catalyzed sp2 C–H bond sulfenylation, amination, fluorination, and etherification.7c−7f Others have extensively used bidentate, monoanionic directing groups for palladium-, ruthenium-, iron-, and copper-catalyzed reactions.8 Furthermore, we have recently reported cobalt-catalyzed aminoquinoline- and picolinamide-directed coupling of alkynes with sp2 C–H bonds.9 Excellent functional group tolerance was observed, and both internal and terminal alkynes are competent substrates for the coupling. The reaction employs Co(OAc)2·4H2O as a catalyst, Mn(OAc)2 as a cocatalyst, and oxygen from air as a terminal oxidant (Scheme 1). It is likely that alkenes would also participate in similar coupling reactions, since both alkenes and alkynes can insert into Co(III)–C bonds.10 Relevant low-valent cobalt-catalyzed, directed hydroarylation of arenes has been described by Yoshikai.6c−6e Chatani has reported ruthenium-catalyzed ortho-alkylation of aminoquinoline amides by electron-deficient alkenes.8d We report here a method for cobalt-catalyzed, aminoquinoline-directed sp2 C–H bond coupling with alkenes.
Scheme 1. Coupling with Alkynes.
Based on previous results,9 we decided to use readily available cobalt(II) catalysts in trifluoroethanol solvent and sodium pivalate base. The reaction optimization was carried out with respect to the catalyst, reaction temperature, and cooxidant (Table 1). Cobalt(II) acetate is an inferior catalyst compared with Co(acac)2 (entry 1 vs 2). Silver nitrate (entry 2) and Mn(OAc)3·2H2O (entry 5) are competent cooxidants, while Mn(OAc)2 is somewhat inferior (entry 4). Reaction under air without a cooxidant affords low conversion (entry 3), while the presence of a Co catalyst is essential (entry 6). The reaction can be performed at room temperature, and nearly complete conversion was observed (entry 7).
Table 1. Optimization of Reaction Conditionsa.
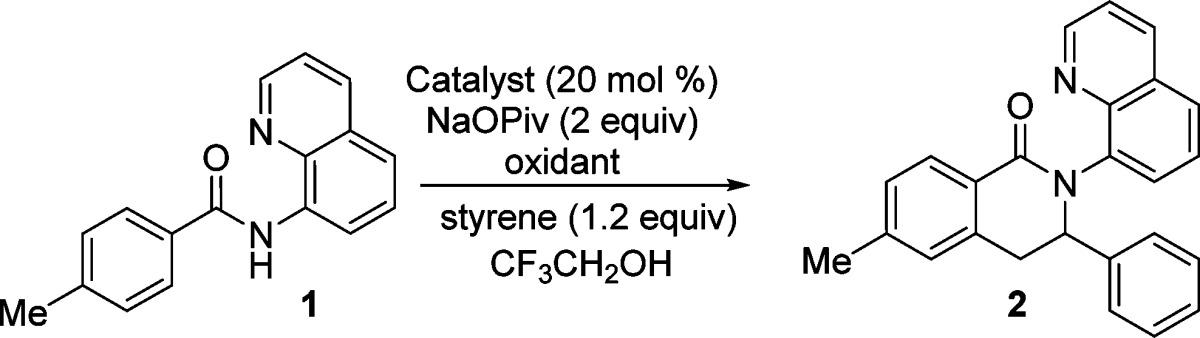
| entry | catalyst | cooxidant | t, time | yield of 2, % |
|---|---|---|---|---|
| 1b | Co(OAc)2 | AgNO3 | 80 °C, 16 h | 16 |
| 2b | Co(acac)2 | AgNO3 | 80 °C, 16 h | 66 |
| 3 | Co(acac)2 | none | 80 °C, 16 h | 15 |
| 4c | Co(acac)2 | Mn(OAc)2 | 80 °C, 16 h | 41 |
| 5c | Co(acac)2 | Mn(OAc)3·2H2O | 80 °C, 16 h | 90 |
| 6c | none | Mn(OAc)3·2H2O | 80 °C, 16 h | <1 |
| 7c,d | Co(acac)2 | Mn(OAc)3·2H2O | rt, 16 h | 93 |
Amide (0.1 mmol), styrene (0.12 mmol), catalyst (0.02 mmol), base (0.2 mmol), CF3CH2OH (1 mL), under air. Yield determined by NMR of crude reaction mixtures; 1,1,2-trichloroethane internal standard.
AgNO3, 2 equiv.
Mn(OAc)2 or Mn(OAc)3·2H2O, 0.5 equiv.
Reaction open to air.
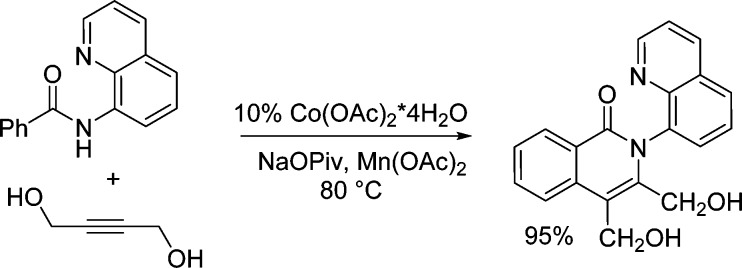
The reaction scope with respect to amides is illustrated in Table 2. Electron-poor (entries 2–5) as well as electron-rich (entries 6, 7) amides are reactive. A number of functionalities, such as a bromine, iodine (entries 3, 5), and nitro moiety (entry 4) are compatible with the reaction conditions. Furthermore, heterocyclic amides such as furane and thiophenecarboxylic acid derivatives (entries 8 and 9) are reactive affording coupling products in good yields. For electron-rich and heterocyclic amides, 1 equiv of cooxidant is necessary to obtain reasonable reaction rates.
Table 2. Reaction Scope with Respect to Amidesa.
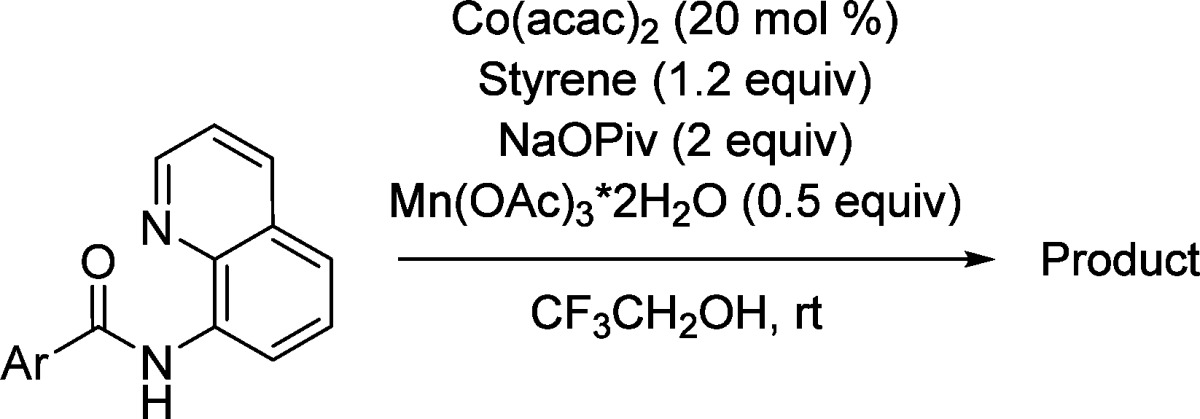
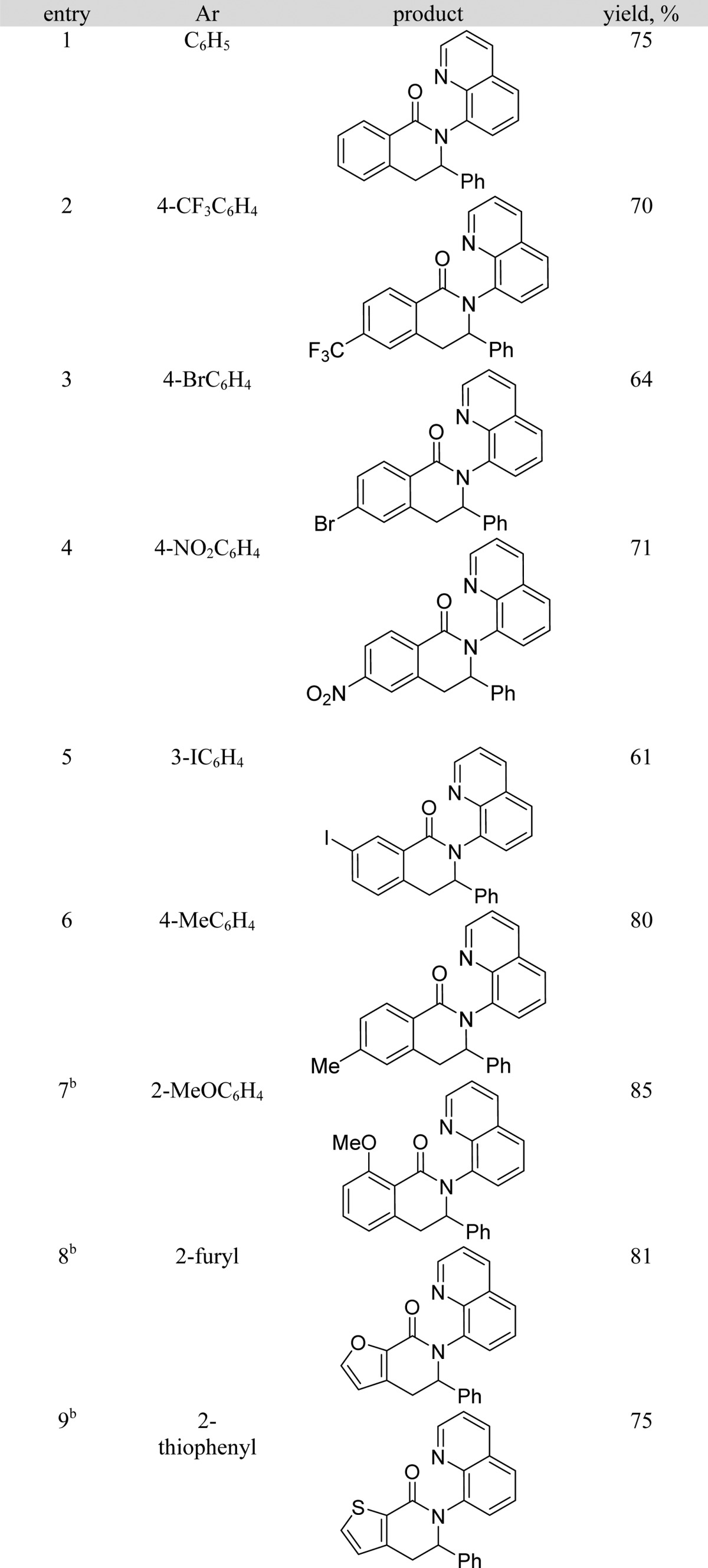
Amide (0.5 mmol), styrene (0.6 mmol), Co(acac)2 (0.1 mmol), base (1 mmol), Mn(OAc)3·2H2O (0.25 mmol), trifluoroethanol (5 mL), reaction vessel open to air. Yields are isolated yields. Please see Supporting Information for details.
Cooxidant Mn(OAc)3·2H2O, 0.5 mmol.
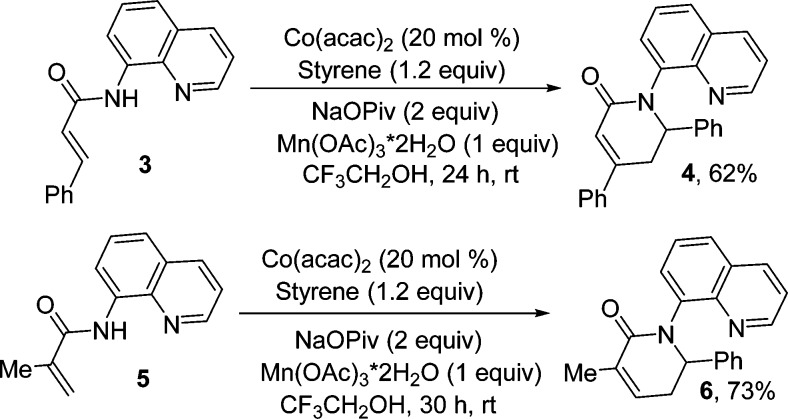
The reactions are not limited to aromatic amides. Aminoquinoline amides of cinnamic and methacrylic acids can be coupled with styrene to afford compounds 4 and 6 in good yields (Scheme 2).
Scheme 2. Vinylamide Reactions.
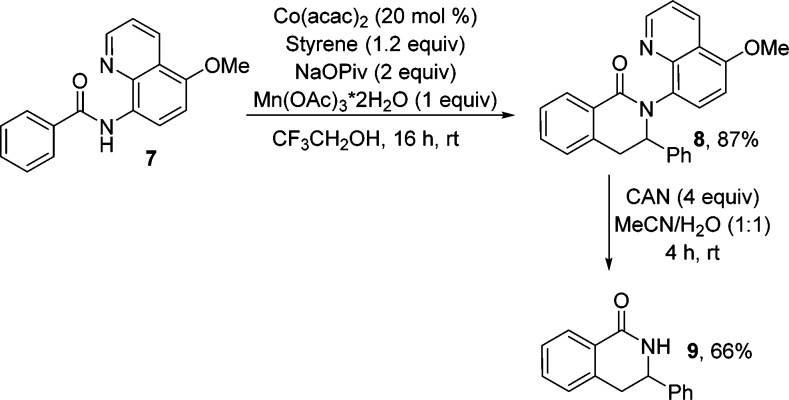
Recently, Chen has reported a modified 8-aminoquinoline auxiliary that can be removed by treatment with ceric ammonium nitrate.11 8-Amino-5-methoxyquinoline benzamide can be coupled with styrene to give product 8 in excellent yield (Scheme 3). The auxiliary can be cleaved affording heterocycle 9 in 66% yield.
Scheme 3. Removable Directing Group.
The reaction scope with respect to alkenes is presented in Table 3. Ethylene is reactive giving a dihydroisoquinolinone derivative in 72% yield (entry 1). Interestingly, 1-butene affords the product as a 10/1 regioisomer mixture (entry 2) representing a relatively rare example of isomerizable alkene use in C–H/alkene coupling. In all other cases, only a single isomer of product was observed. The reaction tolerates a free hydroxyl group (entries 3, 7, and 8). Ether is also compatible with the reaction conditions (entry 4). Cyclic disubstituted alkenes such as cyclopentene and cyclooctene can be coupled in good yields (entries 5 and 6). E-Cinnamyl alcohol affords the trans-product in 53% yield. Interestingly, Z-cinnamyl alcohol also gives the trans-product in nearly identical yield (entry 8). Unreacted cinnamyl alcohol from entry 8 was recovered as a mixture of cis and trans isomers.
Table 3. Reaction Scope with Respect to Alkenes.
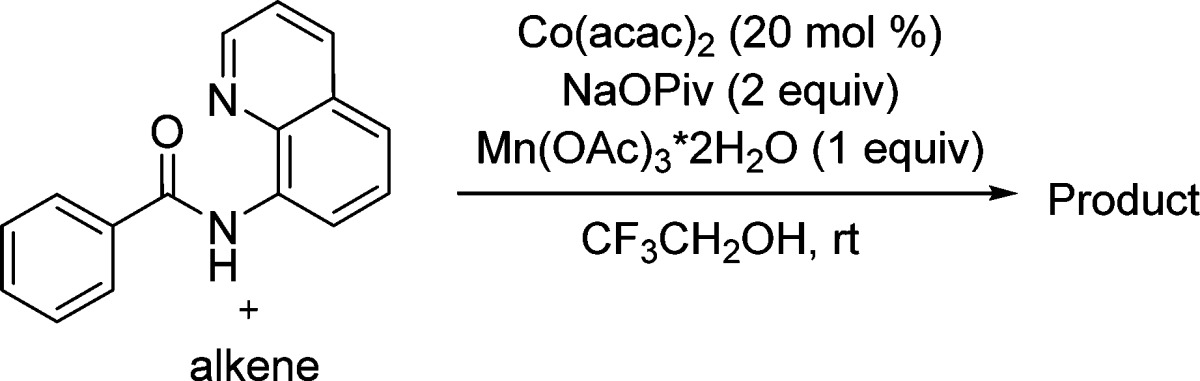
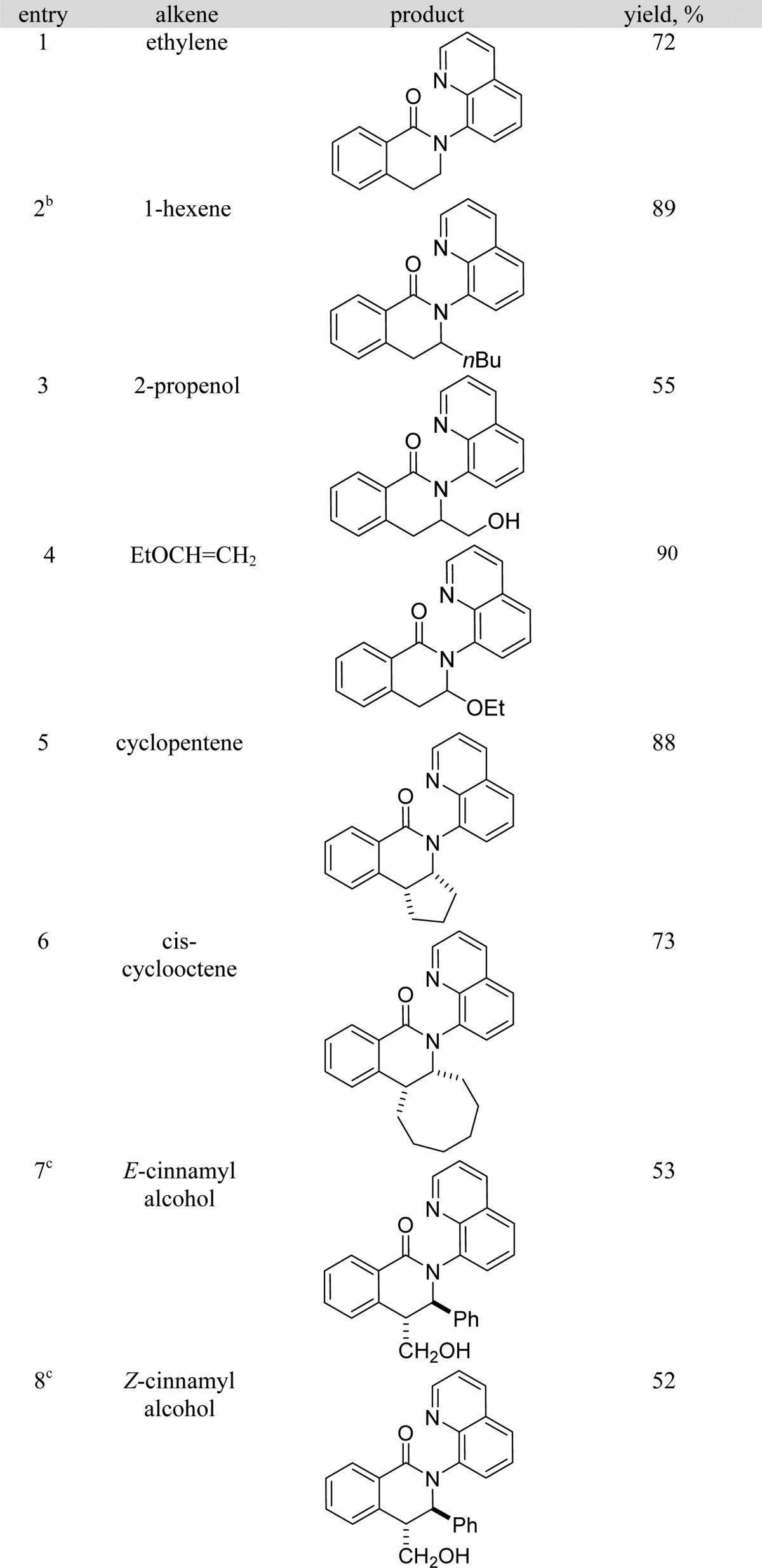
Amide (0.5 mmol), alkene (0.6 mmol), Co(acac)2 (0.1 mmol), base (1 mmol), Mn(OAc)3·2H2O (0.5 mmol), trifluoroethanol (5 mL), reaction vessel open to air. Yields are isolated yields. Please see Supporting Information for details.
Regioisomer ratio 10/1.
Cobalt(II) acetylacetonate (0.25 mmol), 80 °C.
In conclusion, we have developed a simple method for cobalt-catalyzed, aminoquinoline-directed coupling of sp2 C–H bonds with alkenes. Reactions proceed at room temperature in trifluoroethanol solvent, use oxygen from air as an oxidant, and require Mn(OAc)3 as a cocatalyst. Benzoic, heteroaromatic, and acrylic acid derivatives can be reacted with ethylene as well as mono- and disubstituted alkenes affording products in good yields. Excellent functional group tolerance is observed.
Acknowledgments
We thank the Welch Foundation (Grant No. E-1571), NIGMS (Grant No. R01GM077635), and Camille and Henry Dreyfus Foundation for supporting this research.
Supporting Information Available
Detailed experimental procedures and characterization data for new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Reviews:; a Messaoudi S.; Brion J.-D.; Alami M. Eur. J. Org. Chem. 2010, 6495. [Google Scholar]; b Colby D. A.; Bergman R. G.; Ellman J. A. Chem. Rev. 2010, 110, 624. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Ritleng V.; Sirlin C.; Pfeffer M. Chem. Rev. 2002, 102, 1731. [DOI] [PubMed] [Google Scholar]; d Bras J. L.; Muzart J. Chem. Rev. 2011, 111, 1170. [DOI] [PubMed] [Google Scholar]; e Gao K.; Yoshikai N. Acc. Chem. Res. 2014, 47, 1208. [DOI] [PubMed] [Google Scholar]
- Moritani I.; Fujiwara Y. Tetrahedron Lett. 1967, 1119. [Google Scholar]
- a Lewis L. N.; Smith J. F. J. Am. Chem. Soc. 1986, 108, 2728. [Google Scholar]; b Murai S.; Kakiuchi F.; Sekine S.; Tanaka Y.; Kamatani A.; Sonoda M.; Chatani N. Nature 1993, 366, 529. [Google Scholar]
- Leow D.; Li G.; Mei T.-S.; Yu J.-Q. Nature 2012, 486, 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected examples: Ru:; a Schinkel M.; Marek I.; Ackermann L. Angew. Chem., Int. Ed. 2013, 52, 3977. [DOI] [PubMed] [Google Scholar]; b Lail M.; Arrowood B. N.; Gunnoe T. B. J. Am. Chem. Soc. 2003, 125, 7506. [DOI] [PubMed] [Google Scholar]; c Martinez R.; Chevalier R.; Darses S.; Genet J.-P. Angew. Chem., Int. Ed. 2006, 45, 8232. [DOI] [PubMed] [Google Scholar]; d Ackermann L.; Wang L.; Wolfram R.; Lygin A. V. Org. Lett. 2012, 14, 728. [DOI] [PubMed] [Google Scholar]; Pd:; e Jia C.; Piao D.; Oyamada J.; Lu W.; Kitamura T.; Fujiwara Y. Science 2000, 287, 1992. [DOI] [PubMed] [Google Scholar]; f Zhang Y.-H.; Shi B.-F.; Yu J.-Q. J. Am. Chem. Soc. 2009, 131, 5072. [DOI] [PMC free article] [PubMed] [Google Scholar]; g Wang D.-H.; Yu J.-Q. J. Am. Chem. Soc. 2011, 133, 5767. [DOI] [PMC free article] [PubMed] [Google Scholar]; h Miura M.; Tsuda T.; Satoh T.; Pivsa-Art S.; Nomura M. J. Org. Chem. 1998, 63, 5211. [Google Scholar]; i Tani M.; Sakaguchi S.; Ishii Y. J. Org. Chem. 2004, 69, 1221. [DOI] [PubMed] [Google Scholar]; j García-Rubia A.; Fernández-Ibáñez M. À.; Arrayás R. G.; Carretero J. C. Chem.—Eur. J. 2011, 17, 3567. [DOI] [PubMed] [Google Scholar]; k Boele M. D. K.; van Strijdonck G. P. F.; de Vries A. H. M.; Kamer P. C. J.; de Vries J. G.; van Leeuwen P. W. N. M. J. Am. Chem. Soc. 2002, 124, 1586. [DOI] [PubMed] [Google Scholar]; l Dams M.; De Vos D. E.; Celen S.; Jacobs P. A. Angew. Chem., Int. Ed. 2003, 42, 3512. [DOI] [PubMed] [Google Scholar]; m Li Y.; Cai G.; Fu Y.; Wan X.; Shi Z. J. Am. Chem. Soc. 2007, 129, 7666. [DOI] [PubMed] [Google Scholar]; Rh:; n Colby D. A.; Bergman R. G.; Ellman J. A. J. Am. Chem. Soc. 2006, 128, 5604. [DOI] [PMC free article] [PubMed] [Google Scholar]; o Lenges C. P.; Brookhart M. J. Am. Chem. Soc. 1999, 121, 6616. [Google Scholar]; p Neely J. M.; Rovis T. J. Am. Chem. Soc. 2013, 135, 66. [DOI] [PMC free article] [PubMed] [Google Scholar]; q Patureau F.; Glorius F. J. Am. Chem. Soc. 2010, 132, 9982. [DOI] [PubMed] [Google Scholar]; r Rakshit S.; Grohmann C.; Besset T.; Glorius F. J. Am. Chem. Soc. 2011, 133, 2350. [DOI] [PubMed] [Google Scholar]; s Tsai A. S.; Brasse M.; Bergman R. G.; Ellman J. A. Org. Lett. 2011, 13, 540. [DOI] [PMC free article] [PubMed] [Google Scholar]; t Li X.; Gong X.; Zhao M.; Song G.; Deng J.; Li X. Org. Lett. 2011, 13, 5808. [DOI] [PubMed] [Google Scholar]; u Wang D.-H.; Engle K. M.; Shi B.-F.; Yu J.-Q. Science 2010, 327, 315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni:; a Nakao Y.; Kashihara N.; Kanyiva K. S.; Hiyama T. Angew. Chem., Int. Ed. 2010, 49, 4451. [DOI] [PubMed] [Google Scholar]; Co:; b Ilies L.; Chen Q.; Zeng X.; Nakamura E. J. Am. Chem. Soc. 2011, 133, 5221. [DOI] [PubMed] [Google Scholar]; c Gao K.; Yoshikai N. J. Am. Chem. Soc. 2011, 133, 400. [DOI] [PubMed] [Google Scholar]; d Gao K.; Yoshikai N. Angew. Chem., Int. Ed. 2011, 50, 6888. [DOI] [PubMed] [Google Scholar]; e Lee P.-S.; Yoshikai N. Angew. Chem., Int. Ed. 2013, 52, 1240. [DOI] [PubMed] [Google Scholar]
- a Zaitsev V. G.; Shabashov D.; Daugulis O. J. Am. Chem. Soc. 2005, 127, 13154. [DOI] [PubMed] [Google Scholar]; b Shabashov D.; Daugulis O. J. Am. Chem. Soc. 2010, 132, 3965. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Tran L. D.; Popov I.; Daugulis O. J. Am. Chem. Soc. 2012, 134, 18237. [DOI] [PMC free article] [PubMed] [Google Scholar]; d Tran L. D.; Roane J.; Daugulis O. Angew. Chem., Int. Ed. 2013, 52, 6043. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Truong T.; Klimovica K.; Daugulis O. J. Am. Chem. Soc. 2013, 135, 9342. [DOI] [PMC free article] [PubMed] [Google Scholar]; f Roane J.; Daugulis O. Org. Lett. 2013, 15, 5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selected examples: Ni:; a Aihara Y.; Chatani N. J. Am. Chem. Soc. 2014, 136, 898. [DOI] [PubMed] [Google Scholar]; b Song W.; Lackner S.; Ackermann L. Angew. Chem., Int. Ed. 2014, 53, 2477. [DOI] [PubMed] [Google Scholar]; Fe:; c Asako S.; Ilies L.; Nakamura E. J. Am. Chem. Soc. 2013, 135, 17755. [DOI] [PubMed] [Google Scholar]; Ru:; d Rouquet G.; Chatani N. Chem. Sci. 2013, 4, 2201. [DOI] [PubMed] [Google Scholar]; Pd:; e Zhang S.-Y.; He G.; Nack W. A.; Zhao Y.; Li Q.; Chen G. J. Am. Chem. Soc. 2013, 135, 2124. [DOI] [PubMed] [Google Scholar]; f He G.; Chen G. Angew. Chem., Int. Ed. 2011, 50, 5192. [DOI] [PubMed] [Google Scholar]; Cu:; g Nishino M.; Hirano K.; Satoh T.; Miura M. Angew. Chem., Int. Ed. 2013, 52, 4457. [DOI] [PubMed] [Google Scholar]
- Grigorjeva L.; Daugulis O.. Angew. Chem., Int. Ed.DOI: 10.1002/anie.201404579. [DOI] [PMC free article] [PubMed]
- a Brookhart M.; Volpe A. F. Jr.; Lincoln D. M.; Horvath I. T.; Millar J. M. J. Am. Chem. Soc. 1990, 112, 5634. [Google Scholar]; b Schmidt G. F.; Brookhart M. J. Am. Chem. Soc. 1985, 107, 1443. [Google Scholar]
- He G.; Zhang S.-Y.; Nack W. A.; Li Q.; Chen G. Angew. Chem., Int. Ed. 2013, 52, 11124. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.