Abstract
BACKGROUND
Ventricular remodeling after myocardial infarction begins with massive extracellular matrix deposition and resultant fibrosis. This loss of functional tissue and the stiffening of myocardial elastic and contractile elements starts the vicious cycle of mechanical inefficiency, adverse remodeling, and eventual heart failure. We hypothesize that SDF-1α therapy to microrevascularize ischemic myocardium will rescue salvageable peri-infarct tissue and subsequently improve myocardial elasticity.
METHODS
Immediately following LAD ligation, mice were randomized to receive peri-infarct injection of either saline or SDF. After six weeks, the animals were sacrificed and samples were taken from the peri-infarct borderzone, the infarct scar, and the left ventricle of non-infarcted control mice. Determination of the tissues’ elastic moduli was carried out by mechanical testing in an atomic force microscope.
RESULTS
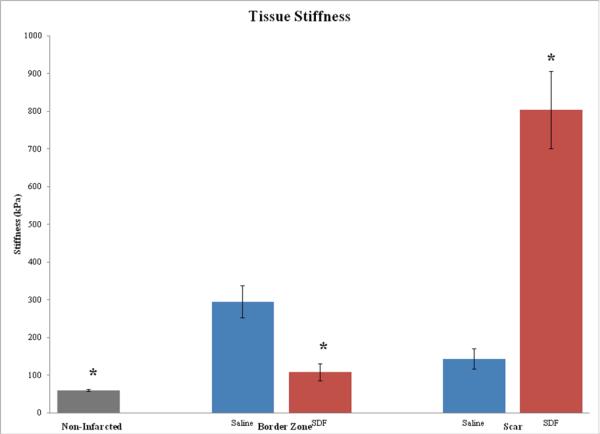
SDF treated peri-infarct tissue most closely approximated the elasticity of normal ventricle and was significantly more elastic than saline treated myocardium (109 + 22.9 kPa vs. 295 + 42.3 kPa, p < 0.0001). The myocardial scar, the strength of which depends on matrix deposition from vasculature at the peri-infarct edge, was stiffer in SDF treated animals when compared to controls (804 + 102.2 kPa vs. 144 + 27.5 kPa, p < 0.0001).
CONCLUSIONS
This study, through direct quantification of myocardial elastic properties, has demonstrated the ability of SDF to re-engineer the evolving myocardial infarct and peri-infarct tissue. By increasing the elasticity of the ischemic and dysfunctional peri-infarct borderzone and bolstering the weak aneurysm prone scar, SDF therapy may confer a mechanical advantage to resist adverse remodeling following infarction.
Background
Current therapies for ischemic heart disease consist of pharmacologic optimization and limited revascularization, reconstructive or replacement options. These modalities are effective for only a fraction of patients. Also, they address neither the significant microvascular deficiencies that persist even when an occluded artery is stented or bypassed nor the abnormal mechanical stress and loading of the infarct and perfused viable borderzone region adjacent to the infarct.
It has been shown that immediately after the onset of ischemia, abnormal ventricular loading results in thinning and stretching of the infarct as well as increased mechanical stress in the peri-infarct borderzone. In addition, it has been demonstrated experimentally that infarct expansion is associated with progressive loss of contractile function in the perfused borderzone adjacent to the infarct and that this dysfunctional region becomes more hypocontractile and begins to involve additional perfused myocardium as remodeling continues and heart failure progresses.(1, 2) Also, after an infarction, the extent of microvascular obstruction increases greatly over the first 48 hours and there is significant progressive microvascular and myocardial injury well beyond the infarct zone, even with reperfusion.(3, 4) This is important because an increased number of capillaries has been correlated with an increase in contractility and function under stress conditions.(5) Endogenous repair machinery is inadequate to correct this deficiency and tremendous resources have been devoted to developing molecular therapies that enhance both the microvascular perfusion and the function of ischemic or infarcted myocardium.
Stromal cell-derived factor-1α (SDF) is a key regulator of physiological cell motility during both embryogenesis and after birth and is constitutively expressed in a wide variety of cells including endothelial cells, dendritic cells, and stromal cells.(6) This powerful chemoattractant is significantly upregulated in response to both myocardial ischemia and infarction and has been shown to effect endothelial progenitor cell (EPC) proliferation and mobilization to induce vasculogenesis.(7, 8) Experimentally in both a mouse and rat model, peri-infarct myocardial injection of SDF has been shown to significantly enhance myocardial EPC density, increase vasculogenesis and capillary density, and augment myocardial function by enhancing perfusion, reversing cellular ischemia, increasing cardiomyocyte viability, and ultimately preserving ventricular geometry.(8-16)
An ideal therapy to stem the tide of infarct expansion would be one that could both stiffen and reinforce the fibrotic myocardial scar to reduce the cardiac wall stress in an injured ventricle while simultaneously rescuing the dysfunctional peri-infarct borderzone by addressing the microvascular deficit and normalizing the elasticity of the viable myocardium. In this study, it is our hypothesis that angiogenic borderzone SDF therapy will yield a less stiff, more elastic, more mechanically efficient myocardium.
Methods
Animal Care and Biosafety
Male CD-1 mice (n = 18) weighing 25 - 30 g were obtained from Charles River and randomly assigned to equal groups to receive direct intramyocardial injection of either saline (30 μl) or SDF (6μg/kg in 30 μl), or to receive no infarction. Food and water were provided ad lib. This investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, revised 1996) and was approved by the Institutional Animal Use and Care Committee of the University of Pennsylvania (Protocol #709026).
Ischemic Cardiomyopathy Model
Mice were anesthetized with ketamine (100 mg/kg) and xylazine (10 mg/kg), intubated with a 22-gauge catheter, and mechanically ventilated (Hallowell EMC). With the animal supine, an anterior thoracotomy was performed in the left fourth intercostal space, and an 8-0 polypropylene suture was placed around the mid-left anterior descending coronary artery (LAD) midway between the left atrial appendage and left ventricular apex and ligated to produce a large anterolateral myocardial infarction of approximately 30% of the left ventricle. The extent of infarction is highly reproducible in our hands and progression to cardiomyopathy has been well documented.(14) Following ligation, animals were randomly assigned to receive direct intramyocardial injection into the peri-infarct borderzone of either saline (30 μl) [n = 6] or SDF (6μg/kg in 30 μl) [n=6]. Injections were given in three divided doses of 10μl to three predetermined locations, which included the peri-infarct myocardium to the right and left of the ligating suture and the left ventricular apex. The thoracotomy was then closed and the animals were extubated and recovered. Buprenorphine (0.5mg/kg) was administered for postoperative analgesia. The SDF treatment group received subcutaneous injections of 40 μg/kg liquid sargramostim (GMCSF), diluted in saline for a total volume of 100 μl immediately postoperatively and on postoperative day one. We did not include a control group that received subcutaneous injections of GMCSF because the preponderance of the literature has demonstrated no difference between control groups receiving intramyocardial saline injection only or groups receiving saline injection plus subcutaneous GMCSF.(9, 10, 12, 13)
Determination of Myocardial Elastic Modulus
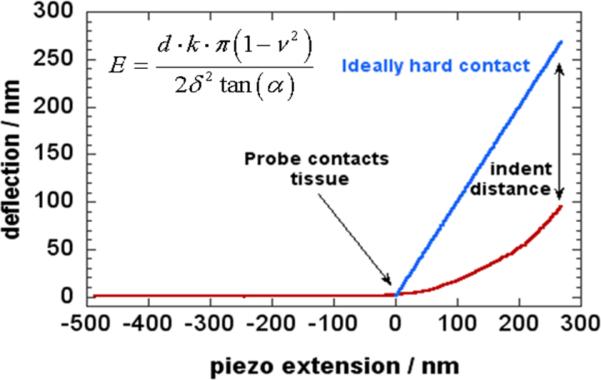
Six weeks after surgery, the animals were sacrificed and multiple myocardial samples were taken from the peri-infarct borderzone and the infarct scar of each animal. Samples were also taken from the left ventricle of non-infarcted control mice [n = 6]. Tissue specimens were cut into small, thin pieces (3 mm × 3 mm × 1 mm), fixed to glass microscope slides, and stored in DMEM buffer solution (Gibco) [Figure 1]. Determination of the tissues’ elastic moduli was carried out by mechanical testing in an atomic force microscope (Asylum MFP-3D). Force-distance measurements were performed 20-30 times at one or more locations on each specimen at the rate of 1 Hz. Each tissue sample was probed an average of 25 times (range 14 -41) for a total of 1966 data points. The measurements for each separate group were then aggregated and compared. The AFM probes’ optical sensitivities were determined first on dry glass slides to determine their spring constant, and again on glass within the buffer solution to account for absorption of the light by the buffer. Stiffness measurements were then carried out within the buffer. The elastic modulus was determined by analyzing the force curves generated by pressing the probe into the tissue and recording the cantilever deflection. The Herzian / Sneddon model of a cone indenting an elastic half-space has already been used in similar measurements(17, 18). In this analysis, the Youngs modulus of a material is , where d is the cantilever deflection, k is its bending spring constant, ν is the material Poisson ratio (assumed to be .5), δ is the indentation distance, and α is the half-angle of the cone. Matlab scripts were written to batch-process the force curves and find the slope of d vs.δ2 lines, which yielded E [Figure 2].
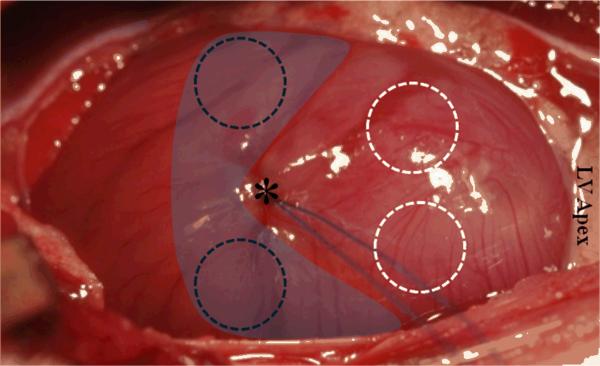
Figure 1.
Immediately following ligation, animals were randomized to receive intramyocardial injection of either SDF or saline into the peri-infarct borderzone (shaded in blue). Dashed circles (black = borderzone; white = scar) indicate tissue removed and mounted for atomic force microscopy (AFM) six weeks after surgery. The asterisk indicates LAD ligation point.
Figure 2.
In this analysis, the Youngs modulus of a material is E, where d is the cantilever deflection, k is its bending spring constant, νis the material Poisson ratio (assumed to be .5), δ is the indentation distance, and α is the half-angle of the cone. Matlab scripts were written to batch-process the force curves and find the slope of d vs. δ2 lines, which yielded E.
Statistical Analysis
The unpaired Student's t-test was used to compare groups. Values are expressed as mean ± standard error of the mean (SEM). Statistical significance was defined by P ≤ 0.05.
Results
Force-distance measurements were performed 20-30 times at one or more locations on each specimen at the rate of 1 Hz. Each tissue sample was probed an average of 25 times (range 14 -41) for a total of 1966 data points. Ventricular tissue from non-infarcted mice was significantly more elastic (60.4 + 2.6 kPa, p < 0.02 ) than any of the tissue regions measured from mice that had undergone LAD ligation, whether or not they received SDF. However, SDF treated peri-infarct borderzone most closely approximated the elasticity of normal left ventricle and was significantly more elastic than peri-infarct myocardium treated with saline (109 + 22.9 kPa vs. 295 + 42.3 kPa, p < 0.0001). Consistent with the presumed angiogenic mechanism of action of SDF, the myocardial scar proper, the strength of which is dependent of on fibroblast and collagen deposition from vasculature at the peri-infarct edge, was stiffer in SDF treated animals when compared to controls (804 + 102.2 kPa vs. 144 + 27.5 kPa, p < 0.0001) [Figure 3]. Indentation depth was less than 1 μm for nearly all measurements, meaning the base of the cantilever advanced one micron or less between making contact with the tissue and turning back around. This should not be confused with cantilever deflection or actual penetration into the tissue. All of the measurements were performed at 1 Hz, but the actual velocity depended also on the amount of retraction needed to ensure complete separation of the probe tip from the myocardial tissue sample.
Figure 3.
By increasing the elasticity of the dysfunctional peri-infarct borderzone and stiffening the infarct scar, SDF therapy may confer a mechanical advantage to resist adverse ventricular remodeling following infarction.
Discussion
Currently many groups are exploring the use of injectable or implantable materials to limit infarct expansion and to normalize the post-infarct myocardial stress distribution.(19-25) A wide range of cell and material types with varying properties that have been tested, mostly to bolster to the infarct scar proper with the ultimate goal being to reduce strain on the peri-infarct borderzone and prevent infarct expansion. In this experiment, we approached this problem from a novel direction. We know that post-infarction patients who develop robust angiographic collateralization manifest improved regional ventricular function, and that SDF has been shown repeatedly to play a critical role in the rescue of myocardial function, stem cell recruitment to the heart after myocardial infarction, and improved microrevascularization.(8-11, 14-16, 26-30) Given this information, we hypothesized that angiogenic peri-infarct SDF therapy would yield a less stiff, more elastic, more mechanically efficient myocardium. Given the preponderance of historical data, we speculate that borderzone SDF therapy which results in a vastly augmented peri-infarct microvascular bed will provide an increase in dynamic flow reserve to these areas of maximal mechanical stress. We believe that the enhanced capillarity in the peri-infarct borderzone will help to alleviate biomechanical stress, prevent ultrastructural alterations, and improve cardiomyocyte viability, yielding a less stiff, more elastic, more mechanically efficient myocardium, which could ultimately prevent remodeling and improve ventricular performance. In addition, increased vasculature at the peri-infarct edge may intensify myofibroblast accumulation in the infarct scar proper, producing large amounts of extracellular matrix proteins which will ultimately stiffen and provide mechanical support to the ventricle.(31)
The results of this experiment demonstrated that SDF treatment to the peri-infarct borderzone at the time of myocardial infarction, does in fact re-engineer the evolving myocardial infarct and peri-infarct tissue. Determination of the myocardial elastic moduli was carried out by direct mechanical testing in an atomic force microscope incorporating nearly 2000 data points, and revealed that peri-infarct borderzone tissue treated with SDF was significantly more elastic than peri-infarct borderzone treated with saline and most closely approximated normal, noninfarcted ventricle. Additionally, it was found that the infarct scar of SDF treated animals was much stiffer than the infarct scar of control animals. This is important because it has been shown that stiffer infarcts are associated with improved ventricular function and less progression toward heart failure.(32, 33) It is wholly possible that the changes seen in SDF treated borderzone actually follow from changes to the scar itself. This would still be consistent with the presumed angiogenic mechanism of action of SDF since the strength of the myocardial scar is dependent on fibroblast and collagen deposition from vasculature at the peri-infarct edge.
The application of atomic force microscopy to directly determine the myocardial elastic modulus was both a great strength of the study and a potential source of error. When utilizing AFM there are four major areas for possible error: indentation velocity, indentation distance, contact point assignment, and data sampling rate. To combat these potential pitfalls we limited indentation rates to less than 2 μm/s, which appropriately explores the elastic rather than the viscoelastic properties of cells and extracellular matrix.(34) Indentation depth was in fact less than 1 μm for most measurements and performed at a rate of 1 Hz.(35, 36) Contact points assigned through the Domke Radmacher formula (which allows for the determination of the contact point when the AFM tip first makes contact with a soft material) rely upon accurate selection of the appropriate analysis range(37) and that the data sampling rate was slowed appropriately to accommodate indentation rates, repeat measurements at each site, and spatial movement to the next site of measurement. Also, tensile strength is a uniaxial measurement that varies with tissue strip orientation, because normal myocardium and healing infarcts are anisotropic tissues. To minimize anisotropic differences in tensile strength, we very carefully tried to reproducibly dissect and measure the tissue strips in a uniform direction.(38)
In conclusion, SDF therapy may confer a mechanical advantage to resist adverse ventricular remodeling and infarct expansion by increasing the elasticity of the dysfunctional peri-infarct borderzone and bolstering the weak aneurysm prone scar. Treatment with SDF also offers a clinically translatable, potentially noninvasive or catheter based therapy that could be deployed at any point in the time course of ischemic heart disease and can address critical deficits in microvascular perfusion as well alter myocardial biomechanical material properties.
Acknowledgements
This research was partially supported by the University of Pennsylvania Nano/Bio Interface Center through the National Science Foundation NSEC DMR08-32802. Use of Nano/Bio Interface Center instrumentation is acknowledged.
Funding: This work was supported in part by NIH 1R01HL089315-01 (Y.J.W.), NIH/TSFRE K08 HL072812 (Y.J.W.), a Thoracic Surgery Foundation Research Award (W.H.), and NIH T32-HL-007843-13 (W.H.).
References
- 1.Gorman RC, Jackson BM, Burdick JA, Gorman JH. Infarct restraint to limit adverse ventricular remodeling. J Cardiovasc Transl Res. 2011 Feb;4(1):73–81. doi: 10.1007/s12265-010-9244-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jackson BM, Gorman JH, Moainie SL, Guy TS, Narula N, Narula J, et al. Extension of borderzone myocardium in postinfarction dilated cardiomyopathy. J Am Coll Cardiol. 2002 Sep 18;40(6):1160–7. doi: 10.1016/s0735-1097(02)02121-6. discussion 8-71. [DOI] [PubMed] [Google Scholar]
- 3.Rochitte CE, Lima JA, Bluemke DA, Reeder SB, McVeigh ER, Furuta T, et al. Magnitude and time course of microvascular obstruction and tissue injury after acute myocardial infarction. Circulation. 1998 Sep 8;98(10):1006–14. doi: 10.1161/01.cir.98.10.1006. [DOI] [PubMed] [Google Scholar]
- 4.Tarantini G, Razzolini R, Cacciavillani L, Bilato C, Sarais C, Corbetti F, et al. Influence of transmurality, infarct size, and severe microvascular obstruction on left ventricular remodeling and function after primary coronary angioplasty. Am J Cardiol. 2006 Oct 15;98(8):1033–40. doi: 10.1016/j.amjcard.2006.05.022. [DOI] [PubMed] [Google Scholar]
- 5.Heilmann C, Kostic C, Giannone B, Grawitz AB, Armbruster W, Lutter G, et al. Improvement of contractility accompanies angiogenesis rather than arteriogenesis in chronic myocardial ischemia. Vascul Pharmacol. 2006 May;44(5):326–32. doi: 10.1016/j.vph.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 6.De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, et al. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004 Apr 1;103(7):2452–9. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- 7.Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25(5):293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- 8.Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003 Mar 11;107(9):1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 9.Atluri P, Liao GP, Panlilio CM, Hsu VM, Leskowitz MJ, Morine KJ, et al. Neovasculogenic therapy to augment perfusion and preserve viability in ischemic cardiomyopathy. Ann Thorac Surg. 2006;81(5):1728–36. doi: 10.1016/j.athoracsur.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 10.Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, et al. Stromal cell-derived factor and granulocyte-monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130(2):321–9. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Hiesinger W, Vinogradov SA, Atluri P, Fitzpatrick JR, 3rd, Frederick JR, Levit RD, et al. Oxygen-dependent quenching of phosphorescence used to characterize improved myocardial oxygenation resulting from vasculogenic cytokine therapy. J Appl Physiol. 2011 May;110(5):1460–5. doi: 10.1152/japplphysiol.01138.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Atluri P, Panlilio CM, Liao GP, Hiesinger W, Harris DA, McCormick RC, et al. Acute myocardial rescue with endogenous endothelial progenitor cell therapy. Heart Lung Circ. 2010 Nov;19(11):644–54. doi: 10.1016/j.hlc.2010.06.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiesinger W, Frederick JR, Atluri P, McCormick RC, Marotta N, Muenzer JR, et al. Spliced stromal cell-derived factor-1alpha analog stimulates endothelial progenitor cell migration and improves cardiac function in a dose-dependent manner after myocardial infarction. J Thorac Cardiovasc Surg. 2010 Nov;140(5):1174–80. doi: 10.1016/j.jtcvs.2010.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hiesinger W, Perez-Aguilar JM, Atluri P, Marotta NA, Frederick JR, Fitzpatrick JR, 3rd, et al. Computational Protein Design to Reengineer Stromal Cell-Derived Factor-1{alpha} Generates an Effective and Translatable Angiogenic Polypeptide Analog. Circulation. 2011 Sep 13;124(11 Suppl):S18–26. doi: 10.1161/CIRCULATIONAHA.110.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saxena A, Fish JE, White MD, Yu S, Smyth JW, Shaw RM, et al. Stromal cell-derived factor-1alpha is cardioprotective after myocardial infarction. Circulation. 2008 Apr 29;117(17):2224–31. doi: 10.1161/CIRCULATIONAHA.107.694992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang G, Nakamura Y, Wang X, Hu Q, Suggs LJ, Zhang J. Controlled release of stromal cell-derived factor-1 alpha in situ increases c-kit+ cell homing to the infarcted heart. Tissue Eng. 2007 Aug;13(8):2063–71. doi: 10.1089/ten.2006.0013. [DOI] [PubMed] [Google Scholar]
- 17.Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, et al. Mesenchymal stem cell injection after myocardial infarction improves myocardial compliance. Am J Physiol Heart Circ Physiol. 2006 Jun;290(6):H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- 18.Domke J, Radmacher M. Measuring the Elastic Properties of Thin Polymer Films with the Atomic Force Microscope. Langmuir. 1998;14:3320–5. [Google Scholar]
- 19.Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. A synthetic non-degradable polyethylene glycol hydrogel retards adverse post-infarct left ventricular remodeling. J Card Fail. 2009 Sep;15(7):629–36. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Ifkovits JL, Tous E, Minakawa M, Morita M, Robb JD, Koomalsingh KJ, et al. Injectable hydrogel properties influence infarct expansion and extent of postinfarction left ventricular remodeling in an ovine model. Proc Natl Acad Sci U S A. 2010 Jun 22;107(25):11507–12. doi: 10.1073/pnas.1004097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, et al. Effect of injectable alginate implant on cardiac remodeling and function after recent and old infarcts in rat. Circulation. 2008 Mar 18;117(11):1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- 22.Ryan LP, Matsuzaki K, Noma M, Jackson BM, Eperjesi TJ, Plappert TJ, et al. Dermal filler injection: a novel approach for limiting infarct expansion. Ann Thorac Surg. 2009 Jan;87(1):148–55. doi: 10.1016/j.athoracsur.2008.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Singelyn JM, DeQuach JA, Seif-Naraghi SB, Littlefield RB, Schup-Magoffin PJ, Christman KL. Naturally derived myocardial matrix as an injectable scaffold for cardiac tissue engineering. Biomaterials. 2009 Oct;30(29):5409–16. doi: 10.1016/j.biomaterials.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzpatrick JR, 3rd, Frederick JR, McCormick RC, Harris DA, Kim AY, Muenzer JR, et al. Tissue-engineered pro-angiogenic fibroblast scaffold improves myocardial perfusion and function and limits ventricular remodeling after infarction. J Thorac Cardiovasc Surg. Sep;140(3):667–76. doi: 10.1016/j.jtcvs.2009.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Angoulvant D, Fazel S, Weisel RD, Lai TY, Fedak PW, Chen L, et al. Cell-based gene therapy modifies matrix remodeling after a myocardial infarction in tissue inhibitor of matrix metalloproteinase-3-deficient mice. J Thorac Cardiovasc Surg. 2009 Feb;137(2):471–80. doi: 10.1016/j.jtcvs.2008.08.031. [DOI] [PubMed] [Google Scholar]
- 26.Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004 Nov 23;110(21):3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- 27.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003 Aug 30;362(9385):697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 28.Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, et al. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009 Oct;36(4):644–50. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- 29.Ejiri M, Fujita M, Sakai O, Miwa K, Asanoi H, Sasayama S. Development of collateral circulation after acute myocardial infarction: its role in preserving left ventricular function. J Cardiol. 1990;20(1):31–7. [PubMed] [Google Scholar]
- 30.Bonaros N, Sondermeijer H, Wiedemann D, Schlechta B, Schachner T, Schuster M, et al. Downregulation of the CXC chemokine receptor 4/stromal cell-derived factor 1 pathway enhances myocardial neovascularization, cardiomyocyte survival, and functional recovery after myocardial infarction. J Thorac Cardiovasc Surg. 2011 Mar 7; doi: 10.1016/j.jtcvs.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 31.Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG. The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol. Mar;48(3):504–11. doi: 10.1016/j.yjmcc.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pilla JJ, Gorman JH, 3rd, Gorman RC. Theoretic impact of infarct compliance on left ventricular function. Ann Thorac Surg. 2009 Mar;87(3):803–10. doi: 10.1016/j.athoracsur.2008.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Theoretical impact of the injection of material into the myocardium: a finite element model simulation. Circulation. 2006 Dec 12;114(24):2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- 34.Mahaffy RE, Shih CK, MacKintosh FC, Kas J. Scanning probe-based frequency-dependent microrheology of polymer gels and biological cells. Phys Rev Lett. 2000 Jul 24;85(4):880–3. doi: 10.1103/PhysRevLett.85.880. [DOI] [PubMed] [Google Scholar]
- 35.Buxboim A, Ivanovska IL, Discher DE. Matrix elasticity, cytoskeletal forces and physics of the nucleus: how deeply do cells ‘feel’ outside and in? J Cell Sci. Feb 1;123(Pt 3):297–308. doi: 10.1242/jcs.041186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Buxboim A, Rajagopal K, Brown AE, Discher DE. How deeply cells feel: methods for thin gels. J Phys Condens Matter. May 19;22(19):194116. doi: 10.1088/0953-8984/22/19/194116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rotsch C, Jacobson K, Radmacher M. Dimensional and mechanical dynamics of active and stable edges in motile fibroblasts investigated by using atomic force microscopy. Proc Natl Acad Sci U S A. 1999 Feb 2;96(3):921–6. doi: 10.1073/pnas.96.3.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Connelly CM, Ngoy S, Schoen FJ, Apstein CS. Biomechanical properties of reperfused transmural myocardial infarcts in rabbits during the first week after infarction. Implications for left ventricular rupture. Circ Res. 1992 Aug;71(2):401–13. doi: 10.1161/01.res.71.2.401. [DOI] [PubMed] [Google Scholar]