Abstract
Smaller engineered analogs of angiogenic cytokines may provide translational advantages including enhanced stability and function, ease of synthesis, lower cost, and most importantly the potential for modulated delivery via engineered biomaterials. In order to create such a peptide, computational molecular modeling and design was employed to engineer a minimized, highly efficient polypeptide analog of the SDF molecule. After removal of the large, central β-sheet region, a designed diproline linker connected the native N-terminus (responsible for receptor activation and binding) and C-terminus (responsible for extracellular stabilization). This yielded energetic and conformational advantages resulting in a small, low molecular weight engineered SDF polypeptide analog (ESA) that was shown to have angiogenic activity comparable to or better than recombinant human SDF both in vitro and in a murine model of ischemic heart failure.
Introduction
Treatments for ischemic heart disease often fail because they do not address the underlying ventricular cellular pathophysiology and, most importantly, do not restore microvascular perfusion, which has been shown to be a critical, independent predictor of ventricular remodeling, reinfarction, heart failure, and death (Araszkiewicz et al. 2006; Bolognese et al. 2004). Alternatively, post-infarction patients who develop robust angiographic collateralization manifest improved regional ventricular function, suggesting an important role of the native revascularization process (Ejiri et al. 1990; Ortiz-Perez et al. 2010). Current therapeutic options for ischemic heart disease are only capable of intervening in relatively large arteries, leaving pervasive microvascular dysfunction unaddressed. This is important because microvascular integrity, specifically microvascular blood velocity and flow, predicts functional recovery of ischemic myocardium (Balcells et al. 2003; Bolognese et al. 2004; Garot et al. 2003; Rigo et al. 2004). In addition, current interventions are instituted relatively late in the overall time course of the disease process with macrorevascularization possible in only 63–80% of patients with ischemic heart disease (Boodhwani et al. 2006). Even with re-establishment of epicardial coronary artery flow through thrombolysis, percutaneous intervention, or bypass grafting, a paucity of patent, functional microvasculature remains. Angiogenic cytokine therapy is a microrevascularization strategy that can serve as a primary therapy at any point in the disease process and can be employed synergistically with traditional coronary revascularization methods (Atluri and Woo 2008). A wide variety of cytokines and microrevascularization techniques have been employed with varying degrees of success (Atluri et al. 2008; Jayasankar et al. 2003; Jayasankar et al. 2005; Kolakowski et al. 2006).
Stromal cell-derived factor-1α (SDF) is a powerful chemoattractant and considered to be one of the key regulators of hematopoietic stem cell trafficking between the peripheral circulation and bone marrow. It has been shown to effect EPC proliferation and mobilization to induce vasculogenesis, and is significantly upregulated in response to both myocardial ischemia and infarction (Pillarisetti and Gupta 2001; Yamaguchi et al. 2003). SDF, a 67 amino acid protein, is also remarkably conserved among species; a single amino acid substitution is all that differentiates the human and murine sequences (De La Luz Sierra et al. 2004). It belongs to the CXC family of chemokines and is the sole ligand for the G protein–coupled receptor, CXCR4, through which its biologic effects are mediated. Experimentally, delivery of SDF to ischemic myocardium has been shown to increase circulating endothelial progenitor cells (EPC), significantly enhance myocardial EPC density, increase vasculogenesis, and augment myocardial function by enhancing perfusion, reversing cellular ischemia, increasing cardiomyocyte viability, and preserving ventricular geometry (Atluri et al. 2006; Woo et al. 2005).
However, recombinant SDF diffuses quickly, is rapidly degraded by multiple proteases (Davis et al. 2005; De La Luz Sierra et al. 2004) that are upregulated at the time of myocardial infarction (Kai et al. 1998; McQuibban et al. 2001; Petit et al. 2002), has a large and complex tertiary structure involving multiple disulfide linkages (Crump et al. 1997), and is very expensive.
In order to maximize the angiogenic efficacy of the SDF chemokine, a number of novel and mechanistically elucidating delivery approaches have been attempted (Ghadge et al. 2011). These include cardiac adenoviral-mediated SDF overexpression (Tang et al. 2009a; Tang et al. 2009c), the genetic and pharmacological inhibition of SDF degradation (Zaruba et al. 2009), intramyocardial delivery of lentiviral engineered mesenchymal stem cells overexpressing SDF (Zhao et al. 2009), and intramyocardial transplant of nonviral SDF transfected skeletal myoblasts (Elmadbouh et al. 2007). While successfully increasing vascular density and left ventricular function, each delivery method contains serious problems in terms of clinical translatability. Gene therapy, utilizing either plasmid DNA or viral vectors targeting specific tissues, may yield prolonged and sustained expression of the protein in the target tissues, however, the short- and long-term toxicities of various vectors are incompletely understood and gene-based therapy can theoretically cause detrimental sustained expression leading to pathologic angiogenesis or inflammatory reactions (Boodhwani et al. 2006). Cell-based therapies are also fraught with translational hurdles. In addition to complex and labor intensive culture requirements, the survival and integration of transplanted cells is typically low. Many cells are lost through the vasculature when injected intramyocardially, and a low percentage of cells infused into coronary arteries ultimately engraft. The inflammatory environment of infarcted myocardium is particularly hostile for any type of transplanted cell leading to a typical 90% cell death within a week (Segers and Lee 2008).
It is our belief that smaller engineered analogs of SDF may provide translational advantages including enhanced stability and function, ease of synthesis, lower cost, and most importantly the potential for modulated delivery via engineered biomaterials (Baumann et al. 2012; Luo et al. 1999; Prokoph et al. 2012; Segers et al. 2007). With that in mind, we set out to design and engineer a minimized, highly efficient polypeptide analog of the SDF molecule using computational molecular modeling and design. The primary design goal was to remove the large, central β-sheet region and link the native N-terminus (responsible for receptor activation and binding) and the C-terminus (responsible for extracellular stabilization) while maintaining functionality and the approximately perpendicular orientation between the two termini (Crump et al. 1997). Two disulfide bonds located in the large central β-sheet region help to maintain the conformation of native SDF. In order to recover a similar conformation after excision of this portion of the protein, proline residue linkers were considered to connect the N- and C-termini. The cyclic proline residue limits the conformational space accessible to the polypeptide. Loop modeling calculations revealed the variability of conformations explored by linkers comprising different numbers of proline residues. A designed diproline linker yielded energetic and conformational advantages resulting in a small, low molecular weight engineered SDF polypeptide analog (ESA) that was shown to have activity comparable to or better than recombinant human SDF both in vitro and in a murine model of ischemic heart failure (Hiesinger et al. 2011).
Discussion and Results
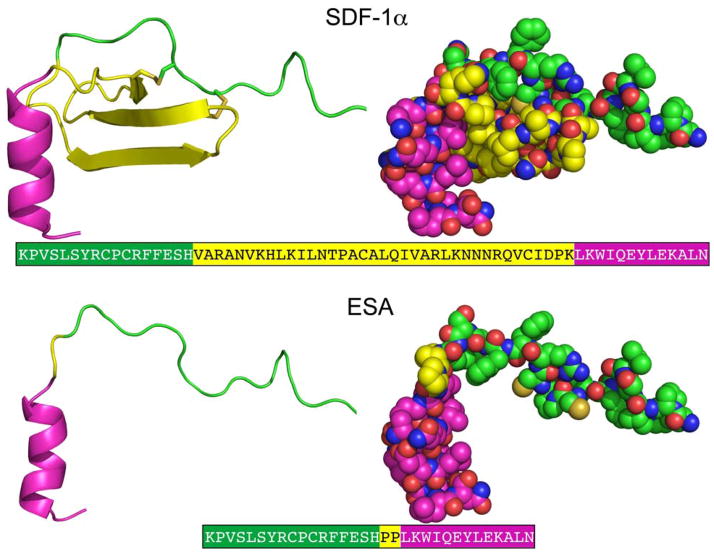
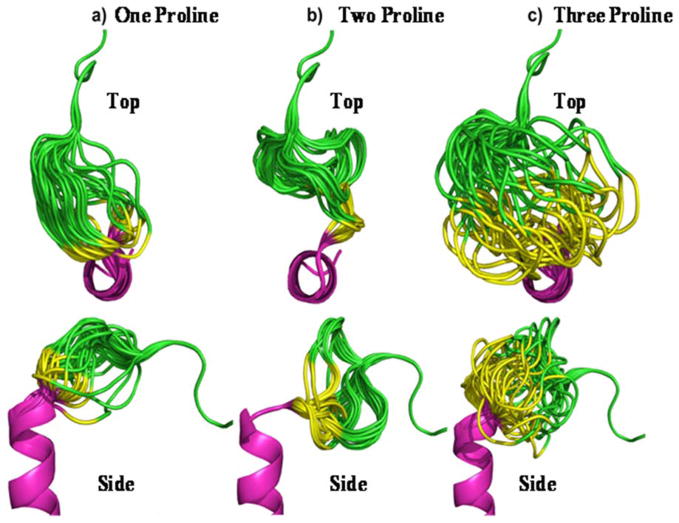
The crystallographic structure of SDF at 2.2 Å resolution was used to guide design and modeling [Figure 1] (Dealwis et al. 1998). The central beta sheet region residues (amino acids 18–54) were replaced with a two-proline linker, resulting in the 32-residue designed polypeptide ESA. The coordinates of residues 14FESHPPL20 were allowed to relax upon modeling with MODELLER (a computer program which implements a loop modeling algorithm that consists of an optimization of a defined segment of protein structure in a fixed environment, guided by a pseudo energy scoring function (Fiser et al. 2000)), while the coordinates of the remainder of the structure were constrained at their crystallographic values. Fifty different possible structures were generated for peptides with a one, two, or three proline linker. In general, the number of stable conformations increases substantially as the number of linking amino acids is increased [Figure 2]. The peptide with the two proline linker (subsequently designated ESA) yielded energetic and conformational advantages. The ESA peptide structure model with the lowest scoring function was chosen for further analysis. Importantly, the two proline residues were found to have energetically favorable backbone dihedral angles (φ and ψ) consistent with those observed for proline in natural protein structures. The introduction of a two consecutive proline linker does not produce steric clashes within the modeled polypeptide structure. In addition, based on the calculated structure, phenylalanine 14 (F14ESA) is interacting with glutamic acid 15 (E15ESA), histidine 17 (H17ESA) and leucine 20 (L20ESA or L55 in SDF). These interactions are not present in the crystallographic structure of SDF and this group of residues may form a small cluster-like structure which, when coupled with the diproline spacer, bias the peptide toward conformations similar to those found in native SDF. Further structural analysis reveals that the model does not contain any steric clashes or unusual conformations of the backbone and the amino acid side chains.
Figure 1.
Crystallographic structures of SDF(Dealwis et al. 1998) and designed model structure ESA. The different regions of the structure are colored as, N terminal (green), central region (yellow) and C terminal (magenta). The central β-sheet region (yellow) is replaced by a diproline linker in ESA. The corresponding amino acid sequences of SDF-1α and ESA are also depicted, where the different regions are colored accordingly.
Figure 2.
Top and side view of experimental SDF analog peptides utilizing one proline (a), two proline (b), and three proline (c) residues to link the N and C terminus. The images depict a composite of the 50 most energetically stable conformations of each peptide sequence. The peptide with the two proline linker (b) adopts a more uniform tertiary profile than the others and recovers the perpendicular orientation between the N- and C- termini found in native SDF.
The ESA peptide was engineered to link the native N-terminus (responsible for receptor activation and binding) and the C-terminus (responsible for extracellular stabilization) with a diproline amino acid spacer. Though ESA may not have a well-defined tertiary structure, the linker is designed to bias the polypeptide toward structures that present the N- and C-termini in a manner similar to that present in the native SDF protein. Based on the modeled structure, F14ESA is in proximity to E15ESA, H17ESA and L20ESA thereby forming a small cluster of side chains within ESA [Figure 3]. These interactions, coupled with the diproline linker, may provide a conformational bias sufficient to recover the activity associated with SDF. The end product, ESA, is a novel polypeptide that contains less than half of the number of amino acid residues, is less than half of the molecular weight, and has enhanced physiologic performance when compared to recombinant SDF.
Figure 3.
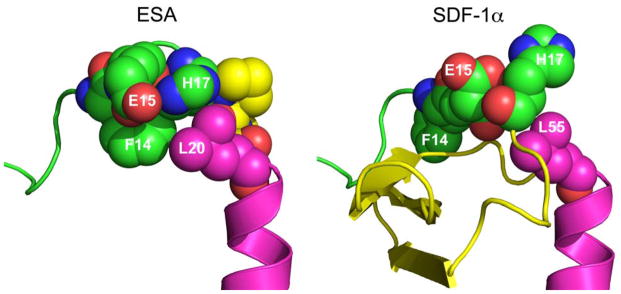
Space-filling representation of the ESA and SDF-1α structures. The bulky side chain of phenylalanine 14 (F14ESA) is interacting with glutamic acid 15 (E15ESA), histidine 17 (H17ESA) and to some extent with leucine 20 (L20ESA or residue 55 in native SDF-1α). The interactions of F14SDF, E15SDF, H17SDF and L55SDF are absent in crystallographic structure of SDF-1α. These new interactions form a small cluster-like structure which, when coupled with the diproline spacer (yellow in ESA), may help to provide the necessary conformational stability and rigidity found in native SDF.
The SDF chemotactic homing mechanism is central to its ability to increase peri-infarct microvasculature and prevent mechanically inefficient ventricular contraction and eventually heart failure. Utilizing a Boyden Chamber assay to directly quantify the magnitude of cellular migration, we were able to demonstrate significantly enhanced migration of endothelial progenitor stem cells when placed in an ESA gradient compared to native SDF peptide (ESA 567±74 cells/HPF vs. SDF 438±46, p=0.037 cells/HPF; ESA vs. control 156±45 cells/HPF, p=0.001). In addition, a one-way analysis of variance (ANOVA) was used to provide a statistical test of whether or not the means of all three study groups were significantly different from each other, generalizing the t-test to more than two groups. EPC migration differed significantly across the three groups. A ratio of the variance between groups to the variance within groups or F (2, 6) = 41.27, and p = 0.0003. Despite ESA’s reengineered conformation, this result shows that for the same mass concentration, ESA not only retains this important chemotactic function, but surpasses the native protein. Compared to SDF, the relatively small size of ESA may enhance its diffusion potential and the speed at which the chemotactic signal is deployed. However, rapid diffusion is likely not the only factor that leads to significantly greater EPC migration. It has been shown that AKT activation is required for SDF induced cellular migration (Peng et al. 2005). To evaluate the EPC response to ESA, we incubated EPCs with culture media containing ESA, recombinant SDF, or media only (control) and quantified both phosphorylated and total AKT levels by ELISA. In concordance with the results of the EPC migration assay, we were able to demonstrate significantly increased CXCR4 receptor activation and phosphorylated AKT levels when cultured EPCs were incubated with culture media containing ESA when compared to EPCs incubated with culture media containing recombinant SDF or contol media. Also, the addition of the CXCR4 antagonist AMD3100 eliminates this difference in receptor activation between ESA, SDF, and control, thus confirming receptor specificity. It is difficult to speculate which specific receptor-peptide interactions are responsible for the enhanced activation of CXCR4 by ESA, and future computational studies will be employed to understand the surprisingly efficient activity of this novel polypeptide on a molecular level.
It is our belief that ESA, in a similar fashion to SDF, increases borderzone microvasculature which, in turn, reverses cellular ischemia, preserves cardiomyocyte viability, and increases the mechanical efficiency of peri-infarct myocardium. In support of that hypothesis, we have shown by immunoblotting in a murine model of ischemic cardiomyopathy that direct borderzone intramyocardial injections of ESA at the time of infarct significantly upregulates tissue levels of Angiopoietin-1, indicative of an ongoing angiogenic process as late as two weeks after infarction and treatment (Zeng et al. 2012). One could argue that the mechanism responsible for the upregulated angiogenic activity is an exaggerated inflammatory response resulting from the injection of ESA or even SDF. However, analysis of borderzone tissue levels of TNFα, MCP-1, SCF, NF-κB, phospho-NF-κB, phospho-p38 MAPK, phospho-Stat3, and phospho-IκB-α, revealed that levels of inflammatory markers are not increased in experimental animals compared to controls indicating that the beneficial angiogenic and functional effects are not the result of differing levels of inflammation.
An increase in microvascular perfusion should result in decreased ventricular remodeling, improved regional ventricular function, and slower progression toward heart failure. Utilizing the same murine model of ischemic cardiomyopathy, left ventricular geometry and function were evaluated at two weeks following LAD ligation using a high-resolution (30 MHz) transthoracic echocardiography system. The improved functionality of ESA in vitro was, once again, borne out in vivo. Animals treated with intramyocardial injections of the ESA peptide had better ejection fractions, cardiac output, stroke volume, and fractional area contraction than both the SDF and control groups. [TABLE 1]
Table 1.
Animals treated with intramyocardial injections of the ESA peptide had significantly improved ejection fractions, cardiac output, stroke volume, and fractional area contraction than control animals. The ESA treated mice also had a significantly increased fractional area change when compared to SDF treated mice (52 ± 3.6% vs. 42 ± 3.2%, p=0.04) and a strong trend towards improvement in all other ventricular function parameters assessed. The unpaired Student’s t-test was used to compare the individual groups found in the Table 1.
| Modality | Parameter | ESA (n=10) | Control (n=7) | SDF (n=13) |
|---|---|---|---|---|
| Echo | Ejection Fraction (%) | 57 ± 2.9 | 42 ± 1.6, p=0.002 | 51 ± 2.7, p=0.16 |
| Fractional Area Change (%) | 52 ± 3.6 | 29 ± 4.9, p=0.001 | 42 ± 3.2, p=0.04 | |
| Stroke Volume (μl) | 61 ± 3.6 | 48 ± 2.9, p=0.02 | 55 ± 2.4, p=0.14 | |
| Cardiac Output (ml/min) | 30 ± 1.8 | 23 ± 1.3, p=0.01 | 27 ± 1.3, p=0.29 |
Values are expressed as mean ± standard error of the mean (SEM). Statistical significance was defined by P ≤ 0.05. A one-way ANOVA was used to generate an overall comparison of the three study groups for each cardiac functional parameter. Values differed significantly across the three groups for ejection fraction (F (2, 27) = 5.71, p = 0.009), cardiac output (F (2, 27) = 3.98, p = 0.03), stroke volume (F (2, 27) = 3.83, p = 0.03), and fractional area change (F (2, 27) = 7.91, p = 0.002).
Angiogenic cytokine therapy for ischemic heart disease has proven to have great potential in numerous pre-clinical and clinical trials. The ability to replenish myocardial microvasculature could prove life saving to the millions of patients with myocardial ischemia. SDF, in particular, is among the most potent and specific angiogenic cytokines; its sole target, the CXCR4 cell surface antigen, is expressed in significant levels on CD34+ EPCs and expression of this receptor is related to efficient SDF induced transendothelial migration (Mohle et al. 1998). SDF has been shown repeatedly to play a critical role in the rescue of myocardial function and stem cell recruitment to the heart after myocardial infarction (Abbott et al. 2004; Askari et al. 2003; Atluri et al. 2006; Tang et al. 2008; Tang et al. 2009b; Woo et al. 2005; Zhao et al. 2009). However, significant barriers remain between the diverse experimental success of SDF angiogenic cytokine therapy and its wide-spread clinical translation. To combat this problem, we employed advanced computational protein design techniques to engineer a more efficient and translatable molecule (ESA) evolved from the native SDF protein. The result was a novel, low molecular weight polypeptide with the enhanced physiologic ability to induce EPC migration, EPC receptor activation, and improve ventricular performance when compared to native SDF. This peptide offers a more clinically translatable neovasculogenic therapy that could conceivably be deployed at any point in the time course of ischemic heart disease to address critical deficits in microvascular perfusion. However, this peptide, like the field of therapeutic angiogenesis, is in its nascent stages. To broadly paraphrase Drs. Renault and Losordo (Renault and Losordo 2007), we are still working to identify an ideal agent, an ideal form of said agent, an optimal dose, and an optimal delivery system. The ESA peptide should serve as the roots for an evolutionary tree whose branches continue to reach toward an ideal form of this potent angiogenic peptide. The design possibilities are limited by imagination alone and we have already begun to see some amazing iterations of the SDF protein, including a protease-resistant, nanofiber delivered version (Baumann et al. 2012; Segers et al. 2007). Computer modeling will continue to be a fertile ground for this type of drug discovery, sharpening broad ideas and eliminating a lot of costly bench trial and error, as well as helping elucidate biologic interactions and molecular mechanisms [Figure 4]. Stromal Cell–Derived Factor-1α functions in nature as an essential regulator of vasculogenesis and stem cell homing from embryogenesis through adulthood. It is now our charge to give nature a boost and create the next species in the C-X-C chemokine genus, rendering it capable of effecting and repairing congestive heart failure and ischemic heart disease.
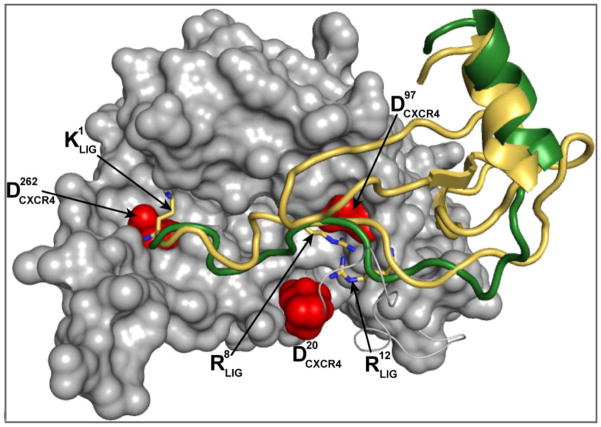
Figure 4.
Homology modeling of the CXCR4 receptor and docking of SDF and ESA yields detailed characterization of the atomic interactions of the systems CXCR4/SDF and CXCR4/ESA. This figure shows a superposition of docked SDF (gold) and ESA (green) in the receptor complex, where equivalent positions of both ligands contact the surface of CXCR4. As proposed in previous studies, the N-terminal region of both ligands is bound to the receptor in a relatively shallow groove. In the model, ESA positions the C-terminal helix in a manner that superimposes its location on SDF.
Acknowledgments
Sources of Funding
NIH 1R01HL089315-01 “Angiogenic Tissue Engineering to Limit Post-Infarction Ventricular Remodeling.” (YJW)
NIH NHLBI/Thoracic Surgery Foundation for Research and Education jointly sponsored Mentored Clinical Scientist Development Award, 1K08HL072812, “Angiogenesis and Cardiac Growth as Heart Failure Therapy.” (YJW)
The Thoracic Surgery Foundation Research Award (TSFRE). (WH)
NIH Training Grant, T32-HL-007843-13. “Training Program in Cardiovascular Biology and Medicine.” (WH)
Footnotes
Disclosures
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott JD, Huang Y, Liu D, Hickey R, Krause DS, Giordano FJ. Stromal cell-derived factor-1alpha plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation. 2004;110:3300–5. doi: 10.1161/01.CIR.0000147780.30124.CF. [DOI] [PubMed] [Google Scholar]
- Araszkiewicz A, Grajek S, Lesiak M, Prech M, Pyda M, Janus M, Cieslinski A. Effect of impaired myocardial reperfusion on left ventricular remodeling in patients with anterior wall acute myocardial infarction treated with primary coronary intervention. Am J Cardiol. 2006;98:725–8. doi: 10.1016/j.amjcard.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, et al. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- Atluri P, Liao GP, Panlilio CM, Hsu VM, Leskowitz MJ, Morine KJ, Cohen JE, Berry MF, Suarez EE, Murphy DA, et al. Neovasculogenic therapy to augment perfusion and preserve viability in ischemic cardiomyopathy. Ann Thorac Surg. 2006;81:1728–1736. doi: 10.1016/j.athoracsur.2005.12.015. [DOI] [PubMed] [Google Scholar]
- Atluri P, Panlilio CM, Liao GP, Suarez EE, McCormick RC, Hiesinger W, Cohen JE, Smith MJ, Patel AB, Feng W, et al. Transmyocardial revascularization to enhance myocardial vasculogenesis and hemodynamic function. J Thorac Cardiovasc Surg. 2008;135:283–91. 291 e1. doi: 10.1016/j.jtcvs.2007.09.043. discussion 291. [DOI] [PubMed] [Google Scholar]
- Atluri P, Woo YJ. Pro-angiogenic cytokines as cardiovascular therapeutics: assessing the potential. BioDrugs. 2008;22:209–22. doi: 10.2165/00063030-200822040-00001. [DOI] [PubMed] [Google Scholar]
- Balcells E, Powers ER, Lepper W, Belcik T, Wei K, Ragosta M, Samady H, Lindner JR. Detection of myocardial viability by contrast echocardiography in acute infarction predicts recovery of resting function and contractile reserve. J Am Coll Cardiol. 2003;41:827–33. doi: 10.1016/s0735-1097(02)02962-5. [DOI] [PubMed] [Google Scholar]
- Baumann L, Prokoph S, Gabriel C, Freudenberg U, Werner C, Beck-Sickinger AG. A novel, biased-like SDF-1 derivative acts synergistically with starPEG-based heparin hydrogels and improves eEPC migration in vitro. J Control Release. 2012 doi: 10.1016/j.jconrel.2012.04.049. [DOI] [PubMed] [Google Scholar]
- Bolognese L, Carrabba N, Parodi G, Santoro GM, Buonamici P, Cerisano G, Antoniucci D. Impact of microvascular dysfunction on left ventricular remodeling and long-term clinical outcome after primary coronary angioplasty for acute myocardial infarction. Circulation. 2004;109:1121–6. doi: 10.1161/01.CIR.0000118496.44135.A7. [DOI] [PubMed] [Google Scholar]
- Boodhwani M, Sodha NR, Laham RJ, Sellke FW. The future of therapeutic myocardial angiogenesis. Shock. 2006;26:332–41. doi: 10.1097/01.shk.0000225318.08681.a7. [DOI] [PubMed] [Google Scholar]
- Crump MP, Gong JH, Loetscher P, Rajarathnam K, Amara A, Arenzana-Seisdedos F, Virelizier JL, Baggiolini M, Sykes BD, Clark-Lewis I. Solution structure and basis for functional activity of stromal cell-derived factor-1; dissociation of CXCR4 activation from binding and inhibition of HIV-1. EMBO J. 1997;16:6996–7007. doi: 10.1093/emboj/16.23.6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis DA, Singer KE, De La Luz Sierra M, Narazaki M, Yang F, Fales HM, Yarchoan R, Tosato G. Identification of carboxypeptidase N as an enzyme responsible for C-terminal cleavage of stromal cell-derived factor-1alpha in the circulation. Blood. 2005;105:4561–8. doi: 10.1182/blood-2004-12-4618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Luz Sierra M, Yang F, Narazaki M, Salvucci O, Davis D, Yarchoan R, Zhang HH, Fales H, Tosato G. Differential processing of stromal-derived factor-1alpha and stromal-derived factor-1beta explains functional diversity. Blood. 2004;103:2452–9. doi: 10.1182/blood-2003-08-2857. [DOI] [PubMed] [Google Scholar]
- Dealwis C, Fernandez EJ, Thompson DA, Simon RJ, Siani MA, Lolis E. Crystal structure of chemically synthesized [N33A] stromal cell-derived factor 1alpha, a potent ligand for the HIV-1 “fusin” coreceptor. Proc Natl Acad Sci U S A. 1998;95:6941–6. doi: 10.1073/pnas.95.12.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ejiri M, Fujita M, Sakai O, Miwa K, Asanoi H, Sasayama S. Development of collateral circulation after acute myocardial infarction: its role in preserving left ventricular function. J Cardiol. 1990;20:31–7. [PubMed] [Google Scholar]
- Elmadbouh I, Haider HK, Jiang S, Idris NM, Lu G, Ashraf M. Ex vivo delivered stromal cell-derived factor-1alpha promotes stem cell homing and induces angiomyogenesis in the infarcted myocardium. J Mol Cell Cardiol. 2007;42:792–803. doi: 10.1016/j.yjmcc.2007.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiser A, Do RK, Sali A. Modeling of loops in protein structures. Protein Sci. 2000;9:1753–73. doi: 10.1110/ps.9.9.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garot P, Pascal O, Simon M, Monin JL, Teiger E, Garot J, Gueret P, Dubois-Rande JL. Impact of microvascular integrity and local viability on left ventricular remodelling after reperfused acute myocardial infarction. Heart. 2003;89:393–7. doi: 10.1136/heart.89.4.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghadge SK, Muhlstedt S, Ozcelik C, Bader M. SDF-1alpha as a therapeutic stem cell homing factor in myocardial infarction. Pharmacol Ther. 2011;129:97–108. doi: 10.1016/j.pharmthera.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Hiesinger W, Perez-Aguilar JM, Atluri P, Marotta NA, Frederick JR, Fitzpatrick JR, 3rd, McCormick RC, Muenzer JR, Yang EC, Levit RD, et al. Computational Protein Design to Reengineer Stromal Cell-Derived Factor-1{alpha} Generates an Effective and Translatable Angiogenic Polypeptide Analog. Circulation. 2011;124:S18–26. doi: 10.1161/CIRCULATIONAHA.110.009431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasankar V, Woo YJ, Bish LT, Pirolli TJ, Chatterjee S, Berry MF, Burdick J, Gardner TJ, Sweeney HL. Gene transfer of hepatocyte growth factor attenuates postinfarction heart failure. Circulation. 2003;108(Suppl 1):II230–6. doi: 10.1161/01.cir.0000087444.53354.66. [DOI] [PubMed] [Google Scholar]
- Jayasankar V, Woo YJ, Pirolli TJ, Bish LT, Berry MF, Burdick J, Gardner TJ, Sweeney HL. Induction of angiogenesis and inhibition of apoptosis by hepatocyte growth factor effectively treats postischemic heart failure. J Card Surg. 2005;20:93–9101. doi: 10.1111/j.0886-0440.2005.200373.x. [DOI] [PubMed] [Google Scholar]
- Kai H, Ikeda H, Yasukawa H, Kai M, Seki Y, Kuwahara F, Ueno T, Sugi K, Imaizumi T. Peripheral blood levels of matrix metalloproteases-2 and -9 are elevated in patients with acute coronary syndromes. J Am Coll Cardiol. 1998;32:368–72. doi: 10.1016/s0735-1097(98)00250-2. [DOI] [PubMed] [Google Scholar]
- Kolakowski S, Berry MF, Atluri P, Grand T, Fisher O, Moise MA, Cohen J, Hsu V, Woo YJ. Placental growth factor provides a novel local angiogenic therapy for ischemic cardiomyopathy. J Card Surg. 2006;21:559–564. doi: 10.1111/j.1540-8191.2006.00296.x. [DOI] [PubMed] [Google Scholar]
- Luo J, Luo Z, Zhou N, Hall JW, Huang Z. Attachment of C-terminus of SDF-1 enhances the biological activity of its N-terminal peptide. Biochem Biophys Res Commun. 1999;264:42–7. doi: 10.1006/bbrc.1999.1476. [DOI] [PubMed] [Google Scholar]
- McQuibban GA, Butler GS, Gong JH, Bendall L, Power C, Clark-Lewis I, Overall CM. Matrix metalloproteinase activity inactivates the CXC chemokine stromal cell-derived factor-1. J Biol Chem. 2001;276:43503–8. doi: 10.1074/jbc.M107736200. [DOI] [PubMed] [Google Scholar]
- Mohle R, Bautz F, Rafii S, Moore MA, Brugger W, Kanz L. The chemokine receptor CXCR-4 is expressed on CD34+ hematopoietic progenitors and leukemic cells and mediates transendothelial migration induced by stromal cell-derived factor-1. Blood. 1998;91:4523–4530. [PubMed] [Google Scholar]
- Ortiz-Perez JT, Lee DC, Meyers SN, Davidson CJ, Bonow RO, Wu E. Determinants of myocardial salvage during acute myocardial infarction: evaluation with a combined angiographic and CMR myocardial salvage index. JACC Cardiovasc Imaging. 2010;3:491–500. doi: 10.1016/j.jcmg.2010.02.004. [DOI] [PubMed] [Google Scholar]
- Peng SB, Peek V, Zhai Y, Paul DC, Lou Q, Xia X, Eessalu T, Kohn W, Tang S. Akt activation, but not extracellular signal-regulated kinase activation, is required for SDF-1alpha/CXCR4-mediated migration of epitheloid carcinoma cells. Mol Cancer Res. 2005;3:227–36. doi: 10.1158/1541-7786.MCR-04-0193. [DOI] [PubMed] [Google Scholar]
- Petit I, Szyper-Kravitz M, Nagler A, Lahav M, Peled A, Habler L, Ponomaryov T, Taichman RS, Arenzana-Seisdedos F, Fujii N, et al. G-CSF induces stem cell mobilization by decreasing bone marrow SDF-1 and up-regulating CXCR4. Nat Immunol. 2002;3:687–94. doi: 10.1038/ni813. [DOI] [PubMed] [Google Scholar]
- Pillarisetti K, Gupta SK. Cloning and relative expression analysis of rat stromal cell derived factor-1 (SDF-1)1: SDF-1 alpha mRNA is selectively induced in rat model of myocardial infarction. Inflammation. 2001;25:293–300. doi: 10.1023/a:1012808525370. [DOI] [PubMed] [Google Scholar]
- Prokoph S, Chavakis E, Levental KR, Zieris A, Freudenberg U, Dimmeler S, Werner C. Sustained delivery of SDF-1alpha from heparin-based hydrogels to attract circulating pro-angiogenic cells. Biomaterials. 2012;33:4792–800. doi: 10.1016/j.biomaterials.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Renault MA, Losordo DW. Therapeutic myocardial angiogenesis. Microvasc Res. 2007;74:159–71. doi: 10.1016/j.mvr.2007.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigo F, Varga Z, Di Pede F, Grassi G, Turiano G, Zuin G, Coli U, Raviele A, Picano E. Early assessment of coronary flow reserve by transthoracic Doppler echocardiography predicts late remodeling in reperfused anterior myocardial infarction. J Am Soc Echocardiogr. 2004;17:750–5. doi: 10.1016/j.echo.2004.04.023. [DOI] [PubMed] [Google Scholar]
- Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–42. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- Segers VF, Tokunou T, Higgins LJ, MacGillivray C, Gannon J, Lee RT. Local delivery of protease-resistant stromal cell derived factor-1 for stem cell recruitment after myocardial infarction. Circulation. 2007;116:1683–92. doi: 10.1161/CIRCULATIONAHA.107.718718. [DOI] [PubMed] [Google Scholar]
- Tang J, Wang J, Song H, Huang Y, Yang J, Kong X, Guo L, Zheng F, Zhang L. Adenovirus-mediated stromal cell-derived factor-1 alpha gene transfer improves cardiac structure and function after experimental myocardial infarction through angiogenic and antifibrotic actions. Mol Biol Rep. 2009a;37:1957–69. doi: 10.1007/s11033-009-9642-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, Wang J, Yang J, Kong X. Adenovirus-mediated stromal cell-derived- factor-1alpha gene transfer induces cardiac preservation after infarction via angiogenesis of CD133+ stem cells and anti-apoptosis. Interact Cardiovasc Thorac Surg. 2008;7:767–70. doi: 10.1510/icvts.2007.169896. [DOI] [PubMed] [Google Scholar]
- Tang J, Wang J, Yang J, Kong X, Zheng F, Guo L, Zhang L, Huang Y. Mesenchymal stem cells over-expressing SDF-1 promote angiogenesis and improve heart function in experimental myocardial infarction in rats. Eur J Cardiothorac Surg. 2009b;36:644–50. doi: 10.1016/j.ejcts.2009.04.052. [DOI] [PubMed] [Google Scholar]
- Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res. 2009c;104:1209–16. doi: 10.1161/CIRCRESAHA.109.197723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo YJ, Grand TJ, Berry MF, Atluri P, Moise MA, Hsu VM, Cohen J, Fisher O, Burdick J, Taylor M, et al. Stromal cell-derived factor and granulocyte-monocyte colony-stimulating factor form a combined neovasculogenic therapy for ischemic cardiomyopathy. J Thorac Cardiovasc Surg. 2005;130:321–329. doi: 10.1016/j.jtcvs.2004.11.041. [DOI] [PubMed] [Google Scholar]
- Yamaguchi J, Kusano KF, Masuo O, Kawamoto A, Silver M, Murasawa S, Bosch-Marce M, Masuda H, Losordo DW, Isner JM, et al. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–8. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- Zaruba MM, Theiss HD, Vallaster M, Mehl U, Brunner S, David R, Fischer R, Krieg L, Hirsch E, Huber B, et al. Synergy between CD26/DPP-IV inhibition and G-CSF improves cardiac function after acute myocardial infarction. Cell Stem Cell. 2009;4:313–23. doi: 10.1016/j.stem.2009.02.013. [DOI] [PubMed] [Google Scholar]
- Zeng H, Li L, Chen JX. Overexpression of Angiopoietin-1 Increases CD133+/c-kit+ Cells and Reduces Myocardial Apoptosis in db/db Mouse Infarcted Hearts. PLoS One. 2012;7:e35905. doi: 10.1371/journal.pone.0035905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao T, Zhang D, Millard RW, Ashraf M, Wang Y. Stem cell homing and angiomyogenesis in transplanted hearts are enhanced by combined intramyocardial SDF-1alpha delivery and endogenous cytokine signaling. Am J Physiol Heart Circ Physiol. 2009;296:H976–86. doi: 10.1152/ajpheart.01134.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]