Abstract
Helicobacter pylori is highly susceptible to bismuth, a heavy metal with antimicrobial activity linked to its effect on bacterial iron uptake. Three strains of H. pylori were analyzed for indicators of iron limitation following exposure to the MIC of colloidal bismuth subcitrate (MICCBS). Similar morphologic and outer membrane changes were observed following growth in iron-limiting medium and at the MICCBS that inhibited the growth of all three strains. These changes, which were also observed for iron-limited bacteria, were alleviated by the addition of iron to the cultures. H. pylori ATP levels, reduced in iron-limiting medium, were below the limits of detection in two of the three strains following exposure to bismuth. The addition of iron partially restored bacterial ATP levels in these two strains, although not to normal concentrations. In contrast, exposure of the same strains to the MICCBS failed to deplete intracellular levels of iron, which were significantly reduced by culturing in iron-limiting medium. Thus, the antimicrobial effect of bismuth and of iron limitation on H. pylori may be similar. However, the respective mechanisms of intracellular action would appear to be mediated by different pathways within the cell.
Prior to the discovery of the link between Helicobacter pylori and peptic ulcer disease (10), ulcers were thought to result from abnormal levels of gastric acid secretion. Bismuth compounds, commonly prescribed for ulcers, were initially thought to act as a barrier to the digestive effects of stomach acid by coating the ulcer site (31). Instead, it appears that H. pylori is highly susceptible to bismuth compounds (39), and treatment with colloidal bismuth subcitrate (CBS) is associated with a reduction in bacterial numbers and a concurrent reduction in gastritis in vivo (37). Despite these findings, bismuth monotherapy often fails to completely eradicate these bacteria (13, 43), an observation that correlates with increasing ulcer relapse rates over time (34).
The oldest regimen, an extremely effective one for clearing H. pylori infection, uses bismuth in combination with certain antibiotics (2, 51). Bismuth “triple therapy” consists of a bismuth compound (usually CBS or bismuth subsalicylate) in combination with metronidazole and tetracycline or amoxicillin (16) and reportedly cures 87.9% of patients within 1 week of treatment and 89.2% of patients within 2 weeks of treatment (49). H. pylori strains resistant to bismuth have not been reported and presumably arise at a lower frequency than strains resistant to antimicrobial agents such as nitroimidazoles, macrolides, and tetracycline (40). Bismuth compounds may reduce the development of resistance to coadministered antibiotics (27) and are also effective at treating H. pylori strains with established resistance to other antibiotics (3, 40).
How bismuth is toxic to H. pylori is not known. A number of studies have linked the antimicrobial activities of many heavy metals, including bismuth, to their effects on iron uptake by bacteria (1, 21, 23, 24). Iron is required for growth because it is a cofactor for many essential enzymatic processes (42), and many of the observed effects of bismuth on bacteria could be the result of iron limitation. These effects include a reduction in intracellular ATP levels (44), inhibition of protein and cell wall synthesis and of membrane function (32), and a reduction in capsular polysaccharide production (19, 20, 22, 28).
A reduction in outer membrane lipopolysaccharide (LPS) expression occurs when H. pylori is deprived of iron (J. Keenan, unpublished data), whereas several iron-repressible outer membrane proteins are concomitantly up-regulated (18, 29, 52). These observations led us to hypothesize that the antimicrobial action of bismuth could be due to it competitively inhibiting iron uptake. To test this hypothesis, we searched for indicators of iron limitation in bismuth-exposed H. pylori and investigated whether iron could protect these bacteria from the antimicrobial effect of bismuth.
MATERIALS AND METHODS
Bacterial strains and cultures.
Three well-characterized H. pylori strains were used in this study: H. pylori 60190 (ATCC 49503), a cag pathogenicity island (PAI)-positive toxigenic strain (5, 14); cag PAI-negative H. pylori strain Tx-30a (ATCC 51932) (14), which fails to produce detectable cytotoxin activity in vitro (35); and mouse-adapted H. pylori Sydney strain 1 (SS1) (33). Individual strains were grown in the base medium, brucella broth (BB; Difco, Detroit, Mich.) supplemented with 5% fetal bovine serum (FBS; Gibco BRL, Auckland, New Zealand), for 72 h at 37°C under microaerophilic conditions and with constant rotation (120 rpm).
Iron limitation and bismuth inhibition were achieved by growing the strains in BB-5% FBS with the addition of an iron chelator (deferoxamine mesylate; Sigma, St. Louis, Mo.) at a final concentration of 50 μM or CBS at the MIC (MICCBS) determined for each strain (see below). Iron salts (ferrous ammonium sulfate; Sigma) at final concentrations of 50 to 1,000 μM were added to the base medium containing MICCBS to determine the amount of iron required to alleviate the effects of bismuth. The deferoxamine, CBS, and iron salts solutions were prepared immediately before use. Deferoxamine and iron salts solutions were filter sterilized prior to addition to the medium.
Morphologic changes.
The morphologic changes in the bacteria were assessed by transmission electron microscopy (TEM) over the 72-h period. Washed bacteria were placed on carbon-colloidin-coated mesh grids and negatively stained with 1% aqueous phosphotungstic acid (pH 7.0). Photographs were taken by using a CM12 transmission electron microscope (Philips).
Determining MICCBS values for individual H. pylori strains.
MICCBS values were determined by using 12-well tissue culture plates inoculated with CBS stock solution to a final volume of 2 ml/well. CBS, prepared from bismuth citrate powder and ammonia (26), was combined with 2 × 107 H. pylori organisms per ml at final concentrations of 0, 1, 2, 4, 8, 16, 32, and 64 mg/liter in the base medium, and the mixture was incubated for 72 h in a microaerophilic environment with constant rotation. H. pylori growth assessed by measuring the optical density at 650 nm in each well (corrected by using uninoculated controls) was confirmed as the growth of gram-negative, urease-positive colonies on blood agar plates. Each strain was tested six times per experiment, performed on three separate occasions, and controls containing ammonia without bismuth were included to ensure that any inhibitory effects were due to bismuth alone.
OMV.
Bacteria were removed from 72-h broth cultures by centrifugation (10,000 × g, 15 min, 4°C), and the supernatants were ultracentrifuged (100,000 × g, 2 h, 4°C) to recover outer membrane vesicles (OMV) as described previously (30). The OMV pellet was washed twice with phosphate-buffered saline (PBS) and assayed for protein content (36). OMV components separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing conditions were silver stained to visualize protein and LPS profiles (30). OMV-associated protease activity was detected by zymography (29). Briefly, OMV were electrophoresed under nonreducing conditions through an acrylamide gel containing a copolymerized substrate (gelatin). After extensive washing to remove sodium dodecyl sulfate, the gel was incubated in 50 mM Tris buffer (pH 7.4) with 10 mM calcium chloride for 4 h at 37°C. OMV-associated proteolytic activity was visualized as clear bands (indicative of substrate lysis) against the blue background of the gels following Coomassie blue staining.
Collection of samples for analysis of ATP and intracellular iron levels.
H. pylori organisms were grown in BB-5% FBS for 24 h before being adjusted to the conditions described above for the remaining 48 h of culturing. At 72 h, a 1-ml aliquot was removed to determine the effects of the various culture conditions on bacterial numbers by viable colony counting. A second aliquot was snap-frozen (in liquid nitrogen) and stored at −80°C prior to analysis of ATP levels. The remaining bacteria were harvested (10,000 × g, 20 min) to determine intracellular iron levels. ATP and iron levels were determined in relation to bacterial dry weight.
Viable colony counting.
After serial dilution in PBS, bacterial titers were inferred from CFU per milliliter, determined by spreading bacterial suspensions over the surface of blood agar plates containing 5% defibrinated sheep blood. The agar plates were incubated for 5 days at 37°C under microaerophilic conditions (as described above).
Analysis of ATP levels.
ATP levels were measured by using a luminescent ATP detection assay kit (ATPLite-Packard Bioscience) according to manufacturer directions. Briefly, frozen samples were quickly defrosted, and 100-μl portions of the samples were added to the wells of 96-well tissue culture plates in duplicate. Following the addition of 50 μl of lysis solution, the plates were shaken for 5 min (700 rpm), and then 50 μl of substrate buffer solution was added. The plates were shaken for a further 5 min and dark adapted for 10 min before luminescence was measured with a luminometer (BMG Labtechnologies). ATP standards, prepared for each of the four conditions used to culture H. pylori and ranging from 10−5 M to the blank, were used to generate a standard curve (Graphpad-Instat) from which unknown sample concentrations were calculated.
Analysis of iron levels.
PBS-washed bacterial pellets were oven dried at 80°C for approximately 18 h in 1.5-ml Eppendorf tubes and then transferred to 10-ml acid-washed flasks for the determination of dry weights. Each sample was dissolved in 1 ml of concentrated nitric acid at 55 to 65°C overnight. Dissolved samples were cooled to an ambient temperature and then brought to 10 ml with Milli-Q distilled water. Samples were examined on a Spectra-10 (Varian) atomic absorption spectrometer, and iron concentrations were determined from a standard curve that ranged from 0 to 5,000 μg/liter (25).
Statistical analyses.
The effects of each of the growth conditions on bacterial iron and ATP levels were evaluated by using a factorial analysis of variance. Significant effects indicated by this analysis were further explored by using Fisher's least-significant-difference test. The ATP measurements were natural logarithm transformed prior to analysis, so that very small values could be easily analyzed.
RESULTS
Bismuth or iron limitation induces a change in H. pylori morphology and outer membrane composition.
The MICCBS values were found to be 4 mg/liter (10 μM) for strains 60190 and SS1 and 8 mg/liter (20 μM) for Tx-30a. The addition of ammonia alone (see Materials and Methods) did not inhibit the growth of any of the strains. Thus, the inhibitory effects of CBS were due to bismuth alone (results not shown).
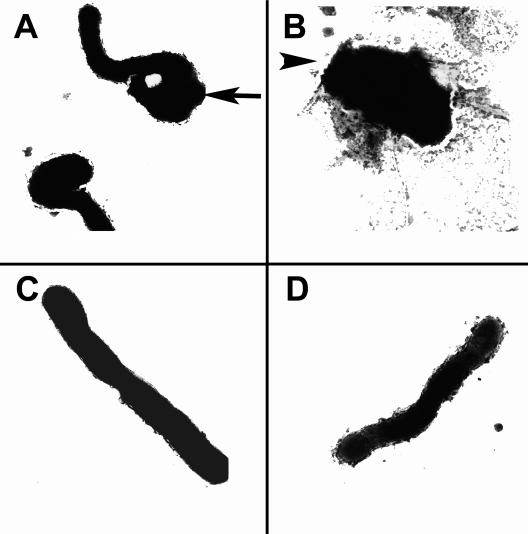
Each of the three H. pylori strains was examined by TEM following 72 h of growth in base medium (control), iron-limiting medium (containing 50 μM deferoxamine), or medium containing MICCBS (Fig. 1). Both iron limitation and exposure to MICCBS resulted in the transition of H. pylori from helical to coccoid morphology (Fig. 1A); a larger proportion of MICCBS-exposed bacteria had completed this transition by 72 h. Exposure to MICCBS resulted in some more distorted forms of H. pylori (Fig. 1B). In contrast, bacteria grown under normal culture conditions exhibited normal curved-rod morphology.
FIG. 1.
Effects of bismuth exposure and iron limitation on H. pylori morphology. TEM images (magnification, ×28,900) show strain 60190 following growth in 50 μM deferoxamine (A), MICCBS (B), MICCBS and protective iron (C), and normal culture conditions (D). The arrow in panel A indicates coccoid morphology; the arrowhead in panel B indicates distorted morphology. Similar results were obtained for strains Tx-30a and SS1 (data not shown).
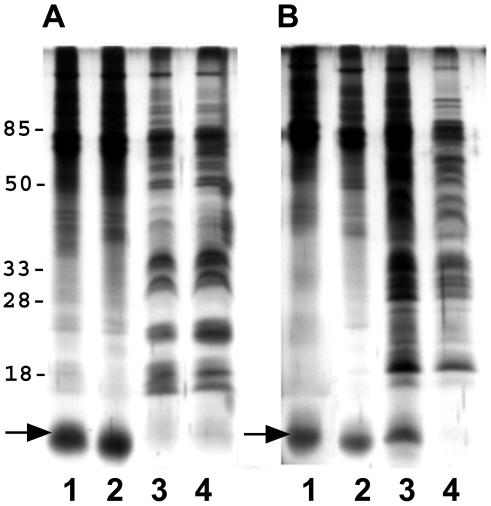
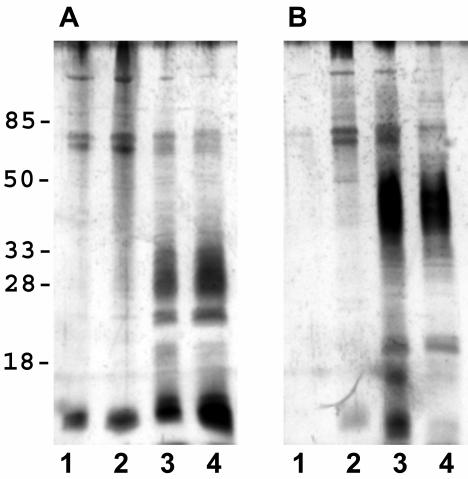
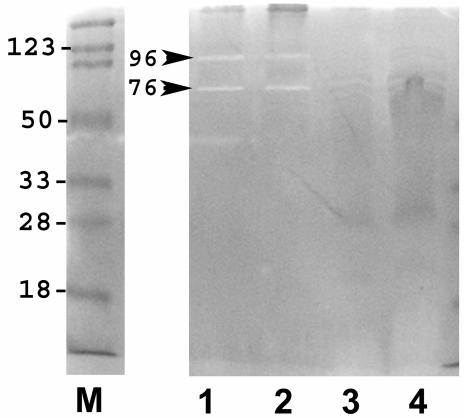
There was little observable difference in the outer membrane proteins of individual H. pylori strains following iron limitation or exposure to bismuth (Fig. 2). Specific markers for iron stress were difficult to identify, but a 12-kDa protein band was clearly enhanced in the presence of MICCBS or 50 μM deferoxamine (Fig. 2). Both iron-limited and MICCBS-exposed bacteria also had reduced levels of OMV-associated LPS (Fig. 3). In addition, zymography revealed OMV-associated protease activity in each strain following growth in the presence of MICCBS or under iron-limiting conditions (Fig. 4).
FIG. 2.
Effects of bismuth exposure and iron limitation on H. pylori OMV protein composition. Shown is silver staining of SS1 (A) and Tx-30a (B) OMV-associated proteins following growth in 50 μM deferoxamine (lanes 1), MICCBS (lanes 2), MICCBS and protective iron (lanes 3), and normal culture conditions (lanes 4). The arrows indicate a 12-kDa protein band. Results obtained for SS1 were also representative of the 60190 OMV protein composition (data not shown).
FIG. 3.
Effects of bismuth exposure and iron limitation on H. pylori OMV LPS composition. Shown is silver staining of SS1 (A) and Tx-30a (B) OMV-associated LPS following growth in 50 μM deferoxamine (lanes 1), MICCBS (lanes 2), MICCBS and protective iron (lanes 3), and normal culture conditions (lanes 4). Similar results were obtained for 60190 (data not shown).
FIG. 4.
Effects of bismuth exposure and iron limitation on H. pylori OMV protease activity. Zymography was performed on OMV harvested from SS1 following growth in 50 μM deferoxamine (lane 1), MICCBS (lane 2), MICCBS and protective iron (lane 3), and normal culture conditions (lane 4). Protease activity is indicated (arrowheads). Similar results were obtained for 60190 and Tx-30a (data not shown). Lane M, markers.
Iron protects H. pylori from the inhibitory effects of bismuth.
The addition of 250 μM iron protected strains 60190 and SS1 from the inhibitory effects of 10 μM CBS. H. pylori Tx-30a, which required twofold more bismuth for growth inhibition (20 μM), also required more iron (500 μM) to achieve any protective effect. However, this amount of iron only reduced the bactericidal effect of bismuth on Tx-30a, as indicated by growth after culturing on blood agar. It did not completely reverse growth inhibition, as demonstrated by a slight increase in absorbance readings after 72 h of culturing. H. pylori grown in medium containing MICCBS and protective iron maintained the normal curved-rod morphology typical of these bacteria (Fig. 1D). Furthermore, this treatment of H. pylori 60190 (results not shown) and SS1 (Fig. 2) resulted in outer membrane protein profiles similar to those of bacteria grown in base medium. The provision of protective iron to H. pylori Tx-30a apparently failed to decrease the expression of the 12-kDa protein band (Fig. 2B). We speculate that this result may have been due to the visible overloading of the corresponding lane. However, it is possible that this protein is still expressed at a higher level in this strain under these conditions. Protease activity was no longer detectable in bacteria of all three strains grown in medium containing MICCBS and protective iron (Fig. 4)
ATP but not iron levels are reduced following exposure of H. pylori to bismuth.
Intracellular iron levels of H. pylori exposed to MICCBS were compared with those of bacteria cultured under normal and iron-limiting conditions to assess whether bismuth inhibited iron uptake. To achieve the biomass required for this analysis, cultures were grown overnight before being exposed to bismuth or limiting iron for 48 h. Colony counts confirmed that the established MICCBS values were sufficient to prevent growth even at these much higher concentrations of bacteria, and inhibition was still overcome by the addition of iron at protective concentrations (results not shown). PBS used to wash the bacterial pellet was assayed for iron by atomic absorption spectrometry. After three washes, the amount of iron present in the PBS supernatant was below the detectable limits of the assay, indicating this number of washes to be sufficient for removing excess unbound iron (results not shown).
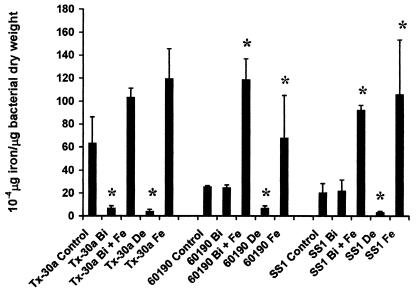
H. pylori strains 60190 and SS1 grown in the presence of MICCBS had intracellular levels of iron similar to those of bacteria cultured under normal conditions, as measured by atomic absorption spectrometry. In contrast, iron levels were significantly reduced following culturing in 50 μM deferoxamine (Fig. 5). However, MICCBS had an effect similar to that of iron limitation on the intracellular iron levels of H. pylori Tx-30a. All three strains cultured in the presence of MICCBS and protective iron had levels of intracellular iron that were higher than those of controls but not significantly different from those of bacteria cultured with protective iron alone.
FIG. 5.
Effects of bismuth exposure and iron limitation on H. pylori intracellular iron levels. Iron levels per microgram of bacterial dry weight were determined for H. pylori 60190, SS1, and Tx-30a cultured in the presence of normal growth conditions (control), iron-limiting conditions (De), MICCBS (Bi), MICCBS plus protective iron (Bi + Fe), and protective iron alone (Fe). Data are the means and standard errors of two independent experiments performed in duplicate. An asterisk indicates that the result is statistically significantly different (P < 0.05) from that for control (untreated) bacteria.
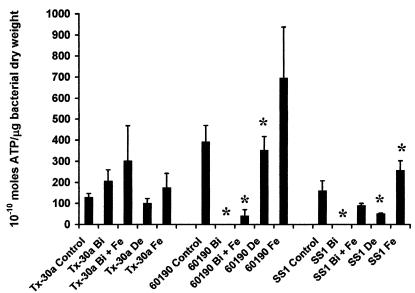
Bacterial ATP levels were only significantly reduced in strains SS1 and 60190 following growth in iron-limiting medium (Fig. 6). Exposure to MICCBS resulted in a reduction in ATP production to below the detectable limits of the assay for these two strains, whereas ATP levels in H. pylori Tx-30a were not significantly different from those in controls. The addition of iron to bacterial cultures containing MICCBS increased bacterial ATP concentrations in all three strains. However, protective iron failed to restore H. pylori 60190 ATP levels to those observed following culturing under normal or even iron-limiting conditions.
FIG. 6.
Effects of bismuth exposure and iron limitation on H. pylori intracellular ATP levels. ATP levels per microgram of bacterial dry weight were determined for H. pylori 60190, SS1, and Tx-30a following growth in the presence of normal growth conditions (control), iron-limiting conditions (De), MICCBS (Bi), MICCBS plus protective iron (Bi + Fe), and protective iron alone (Fe). Data are the means and standard errors of two independent experiments performed in duplicate. An asterisk indicates that the result is statistically significantly different (P < 0.05) from that for control (untreated) bacteria.
DISCUSSION
In this study, we have shown that exposure to MICCBS has the same effect on H. pylori as iron limitation during growth. H. pylori cultured in the presence of MICCBS underwent a morphologic conversion from the bacillary to the coccoid form that was associated with changes in outer membrane composition. These changes, which were also observed in iron-limited bacteria, were prevented by supplementation of the medium with iron. However, our hypothesis that the antimicrobial action of bismuth was due to the inhibition of iron uptake was challenged by unchanged intracellular iron levels in two of the three H. pylori strains following culturing in the presence of this bismuth compound.
Bismuth-induced changes in H. pylori morphology have been seen before (11, 45, 47), but our observations of accompanying changes in outer membrane protein and LPS profiles are new. The bismuth-induced changes included the up-regulation of a 12-kDa protein and two proteases and diminished LPS expression and are identical to those observed in other studies in which H. pylori was grown under iron-limiting conditions (9, 29). Furthermore, the addition of iron during growth mostly prevented these bismuth-induced changes. One possible exception was the continued expression of a 12-kDa protein by H. pylori Tx-30a grown in the presence of MICCBS and protective iron, even though TEM revealed helical forms of bacteria under these conditions. This observation may be linked to the incomplete reversal of bismuth-induced growth inhibition in the presence of protective iron. However, the apparent overloading of the corresponding lane may also be a contributing factor.
The change from helical to coccoid morphology was more rapid in the bismuth-exposed bacteria and was associated with a significant decrease in viability. ATP levels were below the limits of detection in H. pylori strains 60190 and SS1 following exposure to bismuth, whereas strain Tx-30a had ATP levels not significantly different from those seen under normal culture conditions, despite a rapid conversion to the coccoid form that was likewise associated with decreased viability. Strain Tx-30a was also associated, again in contrast to the other two strains, with a significant reduction in intracellular iron levels following exposure to MICCBS. Both of these results would be consistent with a loss of membrane integrity (44), leading to an accumulation of nonutilizable ATP in the extracellular medium and a concurrent loss of iron from the cell. We speculate that this effect may reflect the higher bismuth concentration needed to inhibit this strain and may also be linked to the incomplete reversal of bismuth-induced growth inhibition in the presence of protective iron.
H. pylori 60190 and SS1 cultured with MICCBS and protective iron had ATP levels higher than those found in the presence of bismuth alone but significantly lower than those found under normal culture conditions. The use of protective iron and MICCBS was also associated with an increase in intracellular iron levels and partial protection from bismuth-induced growth inhibition. These observations, which would suggest that bismuth and iron were competing for uptake via the same pathway in these bacteria, were further supported by our observation that iron limitation had a similar (but less severe) effect on H. pylori ATP levels and viability. Unexpectedly, however, intracellular iron levels remained constant in these two strains following exposure to bismuth, whereas iron limitation over the same growth period resulted in the depletion of iron. These results would suggest that the mechanism for the antimicrobial effect of bismuth on these and other bacteria is not simply iron starvation, as suggested previously (21). Instead, the effect of bismuth only mimics the effect of iron starvation in H. pylori.
ATP synthesis in bacteria occurs at the cytoplasmic membrane through the action of multisubunit enzymes (F1F0-ATPases) that utilize the electrochemical gradient generated by respiration (48). In H. pylori, inhibition of these enzymes is thought to lead to ATP depletion and the associated inhibition of other pathways that are important for H. pylori survival (38). Other potential targets for the inhibition of ATP synthesis include the iron-sulfur clusters and cysteine-containing heme groups that act as electron carriers in the respiratory chain. The observation that bismuth complexes localize in the periplasmic space (between the cytoplasmic and outer membranes) in H. pylori (4, 32) would be consistent with a mechanism of action of bismuth that may involve the inhibition of ATP synthesis via one or more of these pathways.
The accumulation of bismuth at the cytoplasmic membrane might have prevented iron incorporation into iron-utilizing proteins, thereby creating a functional iron limitation within the respiratory chain. Thus, the bismuth-induced production of iron-repressible outer membrane proteins could be a response to perceived iron stress, despite no change in intracellular iron levels, an effect similar to that caused by other heavy metals through competition for incorporation into iron binding proteins (8). However, bismuth is also able to inhibit the electron transport chain through binding to the sulfhydryl groups within the enzyme complex, and this mechanism might instead account for the rapid reduction in intracellular ATP levels (6, 7). Interestingly, McGowan et al. showed that F1F0-ATPase activity is required for the survival of H. pylori when the external pH is nearly neutral (38). This finding leads us to speculate that the increased efficacy of quadruple therapy, in which bismuth and a proton pump inhibitor are given in combination with two antibiotics (17), is likely to be linked to this F1F0-ATPase-dependent survival at a neutral pH.
Despite these subtle differences in the mechanisms for shutting down ATP synthesis, the effects are likely to be the same. Moreover, the structural and compositional changes that we observed in the H. pylori outer membrane are likely to have direct consequences on the survival of these bacteria in the gastric mucosa. Reduced LPS synthesis could affect the stability of the bacterial glycocalyx (46) as well as increase the exposure of surface antigens, thereby rendering H. pylori more susceptible to host defenses and/or hydrophilic antimicrobial agents (50).
Interestingly, considerable strain-dependent variations in the ability to preserve ATP levels were observed following culturing of the H. pylori strains in the presence of MICCBS and protective iron. H. pylori 60190 ATP levels were considerably lower than those observed under both normal and iron-limiting culture conditions, and one explanation might be an increased requirement for energy by this strain. H. pylori 60190 carries the complete cag PAI, a locus of about 37 kb containing up to 31 genes (12). Several of these genes code for a type IV secretion system (41) that includes an ATP-regulated inner membrane pore (53). Supporting this hypothesis is the observation of higher levels of ATP in the other two strains grown under the same conditions. These strains do not possess the cag PAI (Tx-30a) or carry only a partial cag PAI (SS1) (14, 15).
In summary, our results suggest that whereas the antimicrobial effects of bismuth and iron deprivation on H. pylori may be similar, their respective mechanisms of intracellular action would appear to be mediated by different pathways within the cell.
Acknowledgments
This study was supported by a summer studentship grant to M.V.B. from the Canterbury Medical Research Foundation.
We thank Stephanie Neal and Trevor Walmsley for assistance with TEM and iron absorption spectroscopy, respectively; Peter Elder and John Lewis for allowing us to use their luminometer for ATP analysis; and Chris Frampton for performing the statistical analyses.
REFERENCES
- 1.al-Aoukaty, A., V. D. Appanna, and H. Falter. 1992. Gallium toxicity and adaptation in Pseudomonas fluorescens. FEMS Microbiol. Lett. 71:265-272. [DOI] [PubMed]
- 2.Alarcon, T., D. Domingo, and M. Lopez-Brea. 1999. Antibiotic resistance problems with Helicobacter pylori. Int. J. Antimicrob. Agents 12:19-26. [DOI] [PubMed] [Google Scholar]
- 3.Andersen, L. P., H. Colding, and J. E. Kristiansen. 2000. Potentiation of the action of metronidazole on Helicobacter pylori by omeprazole and bismuth subcitrate. Int. J. Antimicrob. Agents 14:231-234. [DOI] [PubMed] [Google Scholar]
- 4.Armstrong, J. A., S. H. Wee, C. S. Goodwin, and D. H. Wilson. 1987. Response of Campylobacter pyloridis to antibiotics, bismuth and an acid-reducing agent in vitro—an ultrastructural study. J. Med. Microbiol. 24:343-350. [DOI] [PubMed] [Google Scholar]
- 5.Atherton, J. C., P. Cao, R. M. Peek, Jr., M. K. Tummuru, M. J. Blaser, and T. L. Cover. 1995. Mosaicism in vacuolating cytotoxin alleles of Helicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration. J. Biol. Chem. 270:17771-17777. [DOI] [PubMed] [Google Scholar]
- 6.Baer, W., H. Koopmann, and S. Wagner. 1993. Effects of substances inhibiting or uncoupling respiratory-chain phosphorylation of Helicobacter pylori. Zentbl. Bakteriol. 280:253-258. [DOI] [PubMed] [Google Scholar]
- 7.Beil, W., C. Birkholz, S. Wagner, and K. F. Sewing. 1995. Bismuth subcitrate and omeprazole inhibit Helicobacter pylori F1-ATPase. Pharmacology 50:333-337. [DOI] [PubMed] [Google Scholar]
- 8.Bereswill, S., S. Greiner, A. H. van Vliet, B. Waidner, F. Fassbinder, E. Schiltz, J. G. Kusters, and M. Kist. 2000. Regulation of ferritin-mediated cytoplasmic iron storage by the ferric uptake regulator homolog (Fur) of Helicobacter pylori. J. Bacteriol. 182:5948-5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bereswill, S., F. Lichte, T. Vey, F. Fassbinder, and M. Kist. 1998. Cloning and characterization of the fur gene from Helicobacter pylori. FEMS Microbiol. Lett. 159:193-200. [DOI] [PubMed] [Google Scholar]
- 10.Blaser, M. J. 1992. Helicobacter pylori: its role in disease. Clin. Infect. Dis. 15:386-391. [DOI] [PubMed] [Google Scholar]
- 11.Bode, G., F. Mauch, and P. Malfertheiner. 1993. The coccoid forms of Helicobacter pylori. Criteria for their viability. Epidemiol. Infect. 111:483-490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Censini, S., C. Lange, Z. Xiang, J. E. Crabtree, P. Ghiara, M. Borodovsky, R. Rappuoli, and A. Covacci. 1996. cag, a pathogenicity island of Helicobacter pylori, encodes type I-specific and disease-associated virulence factors. Proc. Natl. Acad. Sci. USA 93:14648-14653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Coghlan, J. G., D. Gilligan, H. Humphries, D. McKenna, C. Dooley, E. Sweeney, C. Keane, and C. O'Morain. 1987. Campylobacter pylori and recurrence of duodenal ulcers—a 12-month follow-up study. Lancet 2:1109-1111. [DOI] [PubMed] [Google Scholar]
- 14.Cover, T. L., C. P. Dooley, and M. J. Blaser. 1990. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect. Immun. 58:603-610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crabtree, J. E., R. L. Ferrero, and J. G. Kusters. 2002. The mouse colonizing Helicobacter pylori strain SS1 may lack a functional cag pathogenicity island. Helicobacter 7:139-140. [DOI] [PubMed] [Google Scholar]
- 16.de Boer, W. A. 1999. Bismuth triple therapy: still a very important drug regimen for curing Helicobacter pylori infection. Eur. J. Gastroenterol. Hepatol. 11:697-700. [PubMed] [Google Scholar]
- 17.de Boer, W. A., and G. N. Tytgat. 2000. Regular review: treatment of Helicobacter pylori infection. Br. Med. J. 320:31-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dhaenens, L., F. Szczebara, and M. O. Husson. 1997. Identification, characterization, and immunogenicity of the lactoferrin-binding protein from Helicobacter pylori. Infect. Immun. 65:514-518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domenico, P., L. Baldassarri, P. E. Schoch, K. Kaehler, M. Sasatsu, and B. A. Cunha. 2001. Activities of bismuth thiols against staphylococci and staphylococcal biofilms. Antimicrob. Agents Chemother. 45:1417-1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Domenico, P., D. R. Landolphi, and B. A. Cunha. 1991. Reduction of capsular polysaccharide and potentiation of aminoglycoside inhibition in gram-negative bacteria by bismuth subsalicylate. J. Antimicrob. Chemother. 28:801-810. [DOI] [PubMed] [Google Scholar]
- 21.Domenico, P., J. Reich, W. Madonia, and B. A. Cunha. 1996. Resistance to bismuth among gram-negative bacteria is dependent upon iron and its uptake. J. Antimicrob. Chemother. 38:1031-1040. [DOI] [PubMed] [Google Scholar]
- 22.Domenico, P., J. M. Tomas, S. Merino, X. Rubires, and B. A. Cunha. 1999. Surface antigen exposure by bismuth dimercaprol suppression of Klebsiella pneumoniae capsular polysaccharide. Infect. Immun. 67:664-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emery, T. 1986. Exchange of iron by gallium in siderophores. Biochemistry 25:4629-4633. [DOI] [PubMed] [Google Scholar]
- 24.Fekete, F. A., and L. L. Barton. 1991. Effects of iron(III) analogs on growth and pseudobactin synthesis in a chromiumtolerant Pseudomonas isolate. Biol. Met. 4:211-216. [DOI] [PubMed] [Google Scholar]
- 25.George, P. M., C. Conaghan, H. B. Angus, T. A. Walmsley, and B. A. Chapman. 1996. Comparison of histological and biochemical hepatic iron indexes in the diagnosis of genetic haemochromatosis. J. Clin. Pathol. 49:159-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goodwin, C. S., P. Blake, and E. D. Blincow. 1986. The minimum inhibitory and bactericidal concentrations of antibiotics and anti-ulcer agents against Campylobacter pylordis. J. Antimicrob. Chemother. 17:309-314. [DOI] [PubMed] [Google Scholar]
- 27.Goodwin, C. S., B. J. Marshall, E. D. Blincow, D. H. Wilson, S. Blackbourn, and M. Phillips. 1988. Prevention of nitroimidazole resistance in Campylobacter pylori by coadministration of colloidal bismuth subcitrate: clinical and in vitro studies. J. Clin. Pathol. 41:207-210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang, C. T., and P. S. Stewart. 1999. Reduction of polysaccharide production in Pseudomonas aeruginosa biofilms by bismuth dimercaprol (BisBAL) treatment. J. Antimicrob. Chemother. 44:601-605. [DOI] [PubMed] [Google Scholar]
- 29.Keenan, J. I., and R. A. Allardyce. 2000. Iron influences the expression of Helicobacter pylori outer membrane vesicle-associated virulence factors. Eur. J. Gastroenterol. Hepatol. 12:1267-1273. [DOI] [PubMed] [Google Scholar]
- 30.Keenan, J. I., R. A. Allardyce, and P. F. Bagshaw. 1997. Dual silver staining to characterise Helicobacter spp. outer membrane components. J. Immunol. Methods 209:17-24. [DOI] [PubMed] [Google Scholar]
- 31.Koo, J., J. Ho, S. K. Lam, J. Wong, and G. B. Ong. 1982. Selective coating of gastric ulcer by tripotassium dicitrato bismuthate in the rat. Gastroenterology 82:864-870. [PubMed] [Google Scholar]
- 32.Lambert, J. R., and P. Midolo. 1997. The actions of bismuth in the treatment of Helicobacter pylori infection. Aliment. Pharmacol. Ther. 11(Suppl. 1):27-33. [DOI] [PubMed] [Google Scholar]
- 33.Lee, A., J. O'Rourke, M. C. De Ungria, B. Robertson, G. Daskalopoulos, and M. F. Dixon. 1997. A standardized mouse model of Helicobacter pylori infection: introducing the Sydney strain. Gastroenterology 112:1386-1397. [DOI] [PubMed] [Google Scholar]
- 34.Lee, F. I., I. M. Samloff, and M. Hardman. 1985. Comparison of tri-potassium di-citrato bismuthate tablets with ranitidine in healing and relapse of duodenal ulcers. Lancet 1:1299-1302. [DOI] [PubMed] [Google Scholar]
- 35.Leunk, R. D., P. T. Johnson, B. C. David, W. G. Kraft, and D. R. Morgan. 1988. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J. Med. Microbiol. 26:93-99. [DOI] [PubMed] [Google Scholar]
- 36.Markwell, M. A., S. M. Haas, L. L. Bieber, and N. E. Tolbert. 1978. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal. Biochem. 87:206-210. [DOI] [PubMed] [Google Scholar]
- 37.Marshall, B. J., J. A. Armstrong, G. J. Francis, N. T. Nokes, and S. H. Wee. 1987. Antibacterial action of bismuth in relation to Campylobacter pyloridis colonization and gastritis. Digestion 37:16-30. [DOI] [PubMed] [Google Scholar]
- 38.McGowan, C. C., T. L. Cover, and M. J. Blaser. 1997. Analysis of F1F0-ATPase from Helicobacter pylori. Infect. Immun. 65:2640-2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McNulty, C. A., J. Dent, and R. Wise. 1985. Susceptibility of clinical isolates of Campylobacter pyloridis to 11 antimicrobial agents. Antimicrob. Agents Chemother. 28:837-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Midolo, P. D., J. R. Lambert, T. G. Kerr, and W. Tee. 1999. In vitro synergy between ranitidine bismuth citrate and tetracycline or clarithromycin against resistant strains of Helicobacter pylori. Eur. J. Clin. Microbiol. Infect. Dis. 18:832-834. [DOI] [PubMed] [Google Scholar]
- 41.Odenbreit, S., J. Puls, B. Sedlmaier, E. Gerland, W. Fischer, and R. Haas. 2000. Translocation of Helicobacter pylori CagA into gastric epithelial cells by type IV secretion. Science 287:1497-1500. [DOI] [PubMed] [Google Scholar]
- 42.Ratledge, C., and L. G. Dover. 2000. Iron metabolism in pathogenic bacteria. Annu. Rev. Microbiol. 54:881-941. [DOI] [PubMed] [Google Scholar]
- 43.Rauws, E. A., W. Langenberg, H. J. Houthoff, H. C. Zanen, and G. N. Tytgat. 1988. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology 94:33-40. [PubMed] [Google Scholar]
- 44.Sox, T. E., and C. A. Olson. 1989. Binding and killing of bacteria by bismuth subsalicylate. Antimicrob. Agents Chemother. 33:2075-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stoltenberg, M., M. Martiny, K. Sorensen, J. Rungby, and K. A. Krogfelt. 2001. Histochemical tracing of bismuth in Helicobacter pylori after in vitro exposure to bismuth citrate. Scand. J. Gastroenterol. 36:144-148. [PubMed] [Google Scholar]
- 46.Stratton, C. W. 1996. Mechanisms of action for antimicrobial agents: general principles and mechanisms for selected classes of antibiotics, p. 579-603. In V. Lorian (ed.), Antibiotics in laboratory medicine. Lippincott, Williams & Wilkins Co., Baltimore, Md.
- 47.Stratton, C. W., R. R. Warner, P. E. Coudron, and N. A. Lilly. 1999. Bismuth-mediated disruption of the glycocalyx-cell wall of Helicobacter pylori: ultrastructural evidence for a mechanism of action for bismuth salts. J. Antimicrob. Chemother. 43:659-666. [DOI] [PubMed] [Google Scholar]
- 48.Stryer, L. 1988. Biochemistry, 3rd ed. W. H. Freeman & Co., New York, N.Y.
- 49.Tytgat, G. N. J., A. T. R. Axon, and M. F. Dixon. 1990. Helicobacter pylori: causal agent in peptic ulcer? Working Party Reports of the World Congress of Gastroenterology. Blackwell Scientific Publications Ltd., Oxford, England.
- 50.Vaara, M., and T. Vaara. 1983. Polycations sensitize enteric bacteria to antibiotics. Antimicrob. Agents Chemother. 24:107-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Van Caekenberghe, D. L., and J. Breyssens. 1987. In vitro synergistic activity between bismuth subcitrate and various antimicrobial agents against Campylobacter pyloridis (C. pylori). Antimicrob. Agents Chemother. 31:1429-1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Worst, D. J., B. R. Otto, and J. de Graaff. 1995. Iron-repressible outer membrane proteins of Helicobacter pylori involved in heme uptake. Infect. Immun. 63:4161-4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeo, H. J., S. N. Savvides, A. B. Herr, E. Lanka, and G. Waksman. 2000. Crystal structure of the hexameric traffic ATPase of the Helicobacter pylori type IV secretion system. Mol. Cell 6:1461-1472. [DOI] [PubMed] [Google Scholar]