Abstract
Mature B lymphocytes undergo apoptosis when they are cultured in the absence of survival factors. Gram-negative bacterial lipopolysaccharide (LPS) prevents this spontaneous apoptosis. This study aimed to better define the signaling pathway(s) involved in the antiapoptotic activity of this endotoxin. We report here that, in addition to its effects on spontaneous apoptosis, LPS protects B cells from apoptosis induced by the broad-spectrum protein kinase inhibitor staurosporine. LPS increased cell viability and concomitantly maintained the mitochondrial transmembrane potential (ΔΨm) and high glutathione levels. Moreover, LPS inhibited cytosolic cytochrome c release and decreased caspase-9 activation. Unlike staurosporine, LPS induced the retention of Bax, a proapoptotic protein of the Bcl-2 family, in the cytosol by preventing its translocation to mitochondria. These results suggest that Bax relocalization from the cytosol to the mitochondria is an important step of mature B-cell apoptosis and that the antiapoptotic activity of LPS occurs upstream of mitochondrial events.
Lipopolysaccharide (LPS), or endotoxin, the major component of the outer membranes of gram-negative bacteria, is implicated in pathophysiological responses to infection by these microorganisms that lead to septic shock and tissue damage (15, 37). Besides its toxic manifestations, LPS exhibits beneficial effects, such as the induction of resistance to viral and bacterial infections and of immunostimulatory activity (35). LPS activates various cell types of the innate as well as adaptive immune system (14, 30, 43).
It has been known for a long time that LPS is a potent activator of B lymphocytes (1, 31). In the B-lymphocyte lineage, LPS accelerates the phenotypic maturation of pre-B and immature B lymphocytes to the mature B-cell stage (36) and induces mature B cells to proliferate and differentiate into antibody-secreting plasma cells in vitro (18). Recent studies have reported that LPS promotes the survival of immature (34, 45) and mature (34, 40) B lymphocytes by preventing apoptosis. However, the signaling pathways for the protective activity of LPS have not been identified.
Apoptosis plays a fundamental role in the development and homeostasis of the immune system and is essential for the negative selection and deletion of autoreactive cells (3, 6, 25). Apoptosis not only is used to remove cells in physiological circumstances, such as during development, but is also a common response to cell stress (41, 46). For the intrinsic pathway of apoptosis induced by chemical stimuli, abundant evidence supports an important role for mitochondria as a central control point of apoptosis (9, 16, 26, 32). Thus, changes in the mitochondrial transmembrane potential (ΔΨm) have been shown to be involved in apoptotic signaling as an early and even obligate event for apoptosis induction (44, 47). The regulation of apoptosis has been related to both antiapoptotic and proapoptotic members of the Bcl-2 family, including the Bcl-2, Bcl-XL, and Bax proteins (4, 7, 17). In particular, the proapoptotic member Bax has emerged as a mediator of the mitochondrial phase of apoptosis. It has been described for many cell types that Bax localizes principally to the cytoplasm and translocates to mitochondria in response to a wide variety of apoptotic stimuli, such as dexamethasone, staurosporine, and etoposide (20, 33). Recent studies have shown that the interleukin-7 (IL-7) treatment of thymocytes and IL-5 treatment of eosinophils inhibit Bax translocation to mitochondria, suggesting that some survival signals can suppress Bax translocation to mitochondria (10, 24). Moreover, the release of cytochrome c from mitochondria, which is an important event in this death-receptor-independent pathway, has been shown to be regulated by Bcl-2 family proteins (16, 22).
For the present study, we analyzed particularly the influence of LPS on the mitochondrial signaling pathways underlying apoptosis to better define the mechanism involved in its antiapoptotic activity in primary B cells. The results indicated that LPS rescues B cells from apoptosis, upstream of mitochondrial dysfunctioning, by inhibiting Bax translocation from the cytosol to mitochondria, caspase-9 activation, and cytochrome c release into the cytosol.
MATERIALS AND METHODS
Animals.
Female BDF1 (C57BL/6 × DBA/2; F1) mice were purchased from Charles River Laboratories (L'Arbresle, France), maintained in a sterile microisolator cage system at our central animal care facility, and used at 8 to 12 weeks of age.
Culture medium and reagents.
The culture medium used throughout was RPMI 1640 (Gibco, Paisley, United Kingdom) supplemented with 25 mM HEPES, 2 mM l-glutamine, standard antibiotics, 50 μM 2-mercaptoethanol, and 8% heat-inactivated fetal calf serum (Gibco). An anti-Thy-1.2 monoclonal antibody and Low-Tox rabbit complement were obtained from Cedarlane (Ontario, Canada). Fluorescein isothiocyanate-conjugated rabbit anti-mouse immunoglobulin (Ig) antibodies were purchased from Jackson Immunoresearch Laboratories (West Grove, Pa.). LPS from Salmonella enterica serovar Enteritidis, extracted by the phenol-water procedure, 6-diamidino-2-phenylindole (DAPI), digitonin, fluorescein diacetate (FDA), and staurosporine (STR) were purchased from Sigma Chemicals (St. Louis, Mo.). 3,3′-Dihexyloxacarbocyanine iodide [DiOC6(3)] and monochlorobimane (MCB) were purchased from Molecular Probes (Eugene, Oreg.).
Cell preparation and culture.
Mature B lymphocytes were purified as previously described (39). Briefly, spleen cells were first treated with an anti-Thy-1.2 (CD90) monoclonal antibody at 4°C for 45 min, followed by incubation with Low-Tox rabbit complement (Cedarlane) at 37°C for 1 h, and then were separated in a discontinuous Percoll gradient. More than 98% of cells recovered at the 55 to 70% interface were found to be positive for surface Ig by using fluorescein isothiocyanate-conjugated Ig antibodies. B cells were cultured in 24-well plates (Nunclon) in medium alone or in the presence of various agents, as indicated, at 5 × 105 cells/ml in 5% CO2 at 37°C.
Quantitative apoptosis determination by DAPI staining.
After an overnight incubation, cells were harvested, fixed in ice-cold 70% ethanol, and kept at −20°C until determination of the DNA content by flow cytometry. For apoptosis analysis, the cellular DNA content was measured after fixed cells were stained with DAPI (2.5 μg/ml) at 37°C for 30 min. Cells were analyzed on a PARTEC CA II flow cytometer (Chemunex, Maisons-Alfort, France) equipped with a 100-W mercury lamp (type HBO). The fluorescence at 455 nm was recorded as a function of the DNA content. Percentages of subdiploid cells, which are considered apoptotic cells, were determined from the sub-G1 events by using DPAC software and histograms generated from at least 104 cells.
DNA fragmentation assay in agarose gels.
DNA was extracted from 106 cells that were recovered after culturing for 20 h. Briefly, cells were first incubated for 1 h at 50°C in lysis buffer (10 mM Tris-HCl [pH 8.0], 10 mM EDTA, 0.5% sodium dodecyl sulfate, 100 μg of proteinase K/ml), and then ethanol-extracted DNA was treated with 1 μg of RNase A/ml. Electrophoresis of samples in 10 μl of loading buffer (10 mM Tris, 1 mM EDTA, 5% glycerol, 0.01% bromophenol blue, 0.01% xylene cyanol FF) was performed with 1% agarose gels containing 1 μg of ethidium bromide/ml. DNAs were visualized by using UV light. A ΦX174RF-HaeIII digest was used for a DNA size marker (Gibco).
Measurements of ΔΨm, GSH levels, and cell viability by flow cytometry.
The ΔΨm, glutathione (GSH) levels, and cell viability were measured as previously described (27). The ΔΨm was determined by the retention of DiOC6(3), a cell-permeative cationic lipophilic fluorochrome which specifically accumulates in mitochondria as a function of their ΔΨm. GSH levels were assessed by using a specific cell-permeative probe, MCB, which forms a fluorescent compound upon interaction with GSH. Cell viability was assessed by FDA staining, with FDA being cleaved into green fluorescein by intracellular esterases present in living cells. Cell staining was performed by treating 5 × 105 cells/ml with 0.1 μM DiOC6(3), 50 μM MCB, or 5 μg of FDA/ml for 20 min at 37°C. Measurements were performed on an ELITE ESP flow cytometer (Coulter France). Fluorescence excitation was obtained through the blue line (488 nm) of an argon ion laser operating at 15 mW. The green fluorescence of DiOC6(3) or of FDA was collected with a 525-nm band pass filter. A 100-mW UV excitation (356 nm) from a laser (INOVA 305 coherent laser) was used for the quantification of GSH-MCB fluorescence integrated above 457 nm. Fluorescence intensity was measured on a logarithmic scale of 4 log units. Analyses were performed on 104 cells in list mode.
Isolation of whole-cell lysates and cytosolic and mitochondrial fractions.
Cells (2 × 106/sample) recovered after being cultured for 16 h were washed twice in Hanks balanced salt solution. For the isolation of whole-cell lysates, cells were lysed in a solution containing 20 mM Tris buffer (pH 8.0), 2 mM EDTA, 150 mM NaCl, 10% glycerol, 1% Triton X-100, and a cocktail of protease inhibitors, including 10 μg of aprotinin/ml, 10 μg of leupeptin/ml, 10 mM NaF, 1 mM orthovanadate, and 1 mM phenylmethylsulfonyl fluoride. After treatment for 20 min at 4°C, supernatants were recovered by centrifugation for 20 min at 15,000 × g. For the isolation of cytosolic and mitochondrial fractions, the cell pellets were resuspended in buffer A (10 mM HEPES-KOH [pH 7.5], 2 mM EDTA, a cocktail of protease inhibitors, and 250 mM sucrose) supplemented with 0.01% digitonin, as this concentration was shown to lyse cells without altering the outer mitochondrial membrane (19). After treatment for 20 min at 4°C, nuclei and cell debris were first discarded in pellets generated by centrifugation at 750 × g for 10 min, and the supernatants were then recentrifuged for 20 min at 15,000 × g to obtain cytosolic fractions in supernatants and mitochondria in the pellets. These mitochondrial fractions were washed once with buffer A and further resuspended for 1 h at 4°C in cold buffer B (10 mM HEPES-KOH [pH 7.5], 2 mM EDTA, protease inhibitor mixture) containing 1% Nonidet P-40.
Western blot analysis.
Either whole-cell lysates or the cytosolic and mitochondrial fractions were separated in 15% sodium dodecyl sulfate-polyacrylamide gels. Prestained standards (BMA, Rockland, Maine) were used to determine molecular weights. Proteins were then electroblotted onto Immobilon polyvinylidene fluoride membranes (Pharmacia Biotech). Bax, Bcl-XL, Bcl-2, caspase-9, and cytochrome c were detected after incubation for 1 h at room temperature with a rabbit polyclonal anti-Bax antibody (sc-7480; Santa Cruz Biotechnology), a monoclonal anti-Bcl-XL antibody (Stressgen), an anti-Bcl-2 antibody (Stressgen), a rabbit polyclonal anti-caspase-9 antibody (Stressgen), and an anti-cytochrome c antibody (Pharmingen), respectively. The blots were incubated with horseradish peroxidase-conjugated anti-rabbit IgG (Jackson Laboratory) or anti-mouse (Calbiochem, San Diego, CA) antibodies and then developed by enhanced chemiluminescence (ECLplus; Bio-Rad) performed according to the manufacturer's instructions. Relative amounts of proteins were determined by densitometric scanning, with the area of each peak integrated with ImageQuant software (Molecular Dynamics).
Statistical analysis.
Data are expressed as means ± standard deviations, determined from at least triplicates. Mean values were compared by Student's t test, and differences were considered significant at P values of <0.05.
RESULTS
LPS inhibits drug-induced apoptosis.
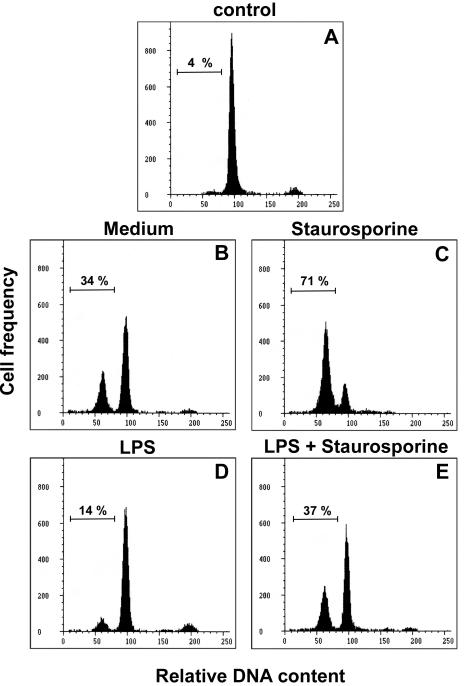
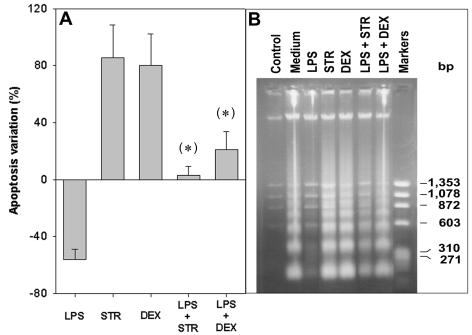
Mature B lymphocytes are dependent on the presence of survival stimuli such as cytokines or costimulatory factors for their survival in vitro. Spontaneous apoptosis occurring in B-cell cultures can be prevented by several endogenous or exogenous agents, including IL-4 (2, 21, 27), phorbol esters (2, 21), and LPS (34, 40). The results presented in Fig. 1, obtained from a flow cytometric analysis of hypodiploid DNA, show that freshly prepared B cells in control cultures exhibited <5% apoptosis at the onset (Fig. 1A), whereas after overnight culturing in medium alone, about 30% spontaneous apoptosis occurred (Fig. 1B). Apoptosis was further induced by STR, a broad-spectrum inhibitor of protein kinases (Fig. 1C). The presence of LPS greatly reduced both spontaneous (Fig. 1D) and STR-induced apoptosis (Fig. 1E) (14 versus 34% and 37 versus 71% apoptosis, respectively). As described for various cell types, such as thymocytes, hemopoietic, and endothelial cells, the results presented in Fig. 2A show that STR triggered B-cell apoptosis at a level similar to that observed with the glucocorticoid dexamethasone (DEX). Moreover, LPS significantly prevented the apoptosis induced by both drugs (P < 0.05), but with a larger effect on STR-induced than DEX-induced apoptosis. These protective effects of LPS were confirmed by the electrophoretic pattern of a DNA ladder for the classical internucleosomal degradation characteristic of apoptosis. Indeed, the intensities of the degradation bands obtained with cells exposed to STR and DEX in the presence of LPS were clearly lower than those obtained with STR and DEX alone, respectively (Fig. 2B).
FIG. 1.
Influence of LPS on B-lymphocyte apoptosis. The histograms depict the relative DNA contents of freshly prepared B cells at the onset (control) (A) or of cells recovered after an overnight culture in medium alone (B) or with 10 nM STR (C), 5 μg of S. enterica LPS/ml (D), or LPS plus STR (E). Apoptosis was determined by flow cytometry of 10,000 cells after staining with the DNA probe DAPI. The percentages of apoptotic cells, as determined by the sub-G1 DNA content, are given at the left of each histogram. These data are representative of at least three individual experiments.
FIG. 2.
Inhibitory effects of LPS on B-lymphocyte apoptosis induced by STR or DEX. B lymphocytes (5 × 105 cells/ml) were incubated for 24 h, as indicated, in medium alone or with either STR (10 nM) or DEX (5 nM), in the absence or presence of LPS (5 μg/ml). (A) Percentages of apoptosis variation were calculated by the following formula: percent apoptosis variation = 100(apoptosis with drug − apoptosis in medium)/apoptosis in medium. Data are expressed as the means ± standard deviations (SD) of seven experiments. Differences in the effects of a drug with and without LPS were considered statistically significant (*) when P values by Student's t test were <0.05. (B) Nucleosomal DNA fragmentation, which is characteristic of apoptosis, revealed by agarose gel electrophoresis. A ΦX174RF-HaeIII digest was used as a DNA size marker.
LPS increases B-cell viability and maintains ΔΨm and elevated GSH levels.
Mitochondria have been suggested to be central to the apoptotic pathway, with their dysfunctioning being implicated in the induction of apoptosis. To gain further insight into the mechanism of LPS-induced protection against apoptosis, we performed a flow cytometric analysis to examine the LPS effects on mitochondrial functions in parallel with measurements of cell viability. Depolarization of the ΔΨm was monitored by use of the fluorochrome DiOC6(3), and the reduction of GSH levels, which is associated with a ΔΨm collapse and uncoupling of the respiratory chain (29), was determined by the use of MCB. As reported in Table 1, at the initiation of culture growth (control), >95% of purified B cells presented a high ΔΨm and elevated GSH levels, and these cells were recorded as viable, as determined by FDA staining. It must be stressed that a correlation was observed between the number of viable cells and those of cells scoring positive for a high ΔΨm and for elevated GSH levels (94, 96, and 95%, respectively). After 15 h of incubation in medium, a loss of viability affected about 30% of the cells. This spontaneous apoptosis was associated with a decline in both the ΔΨm and GSH levels. STR induced a decrease in the number of cells exhibiting a high ΔΨm (42%) and high GSH levels (34%), with a parallel decrease in the number of viable cells (34%). In contrast, LPS decreased STR-induced apoptosis with a corresponding increase in cell viability (from 34 to 66%) and with a parallel increase in the percentages of cells having a high ΔΨm (42 to 69%) and elevated GSH levels (34 to 70%).
TABLE 1.
Influence of LPS on three STR-induced apoptosis-associated effects
| Cell treatment | % Fluorescent cellsa
|
||
|---|---|---|---|
| DiOC6(3)+ (ΔΨm) | MCB+ (GSH) | FDA+ (viability) | |
| Control | 96 | 95 | 94 |
| Medium | 70 | 65 | 69 |
| STR | 42 | 34 | 34 |
| LPS | 80 | 75 | 77 |
| LPS plus STR | 69 | 70 | 66 |
B cells were analyzed either before being cultured (control) or after being cultured for 15 h in medium alone or in the presence of 10 nM STR, 5 μg of LPS/ml, or LPS plus STR. An analysis of 10,000 cells was performed by flow cytometry using DiOC6(3), MCB, or FDA as a fluorochrome. Data are representative of at least three individual experiments.
Influence of LPS on antiapoptotic Bcl-2 family members.
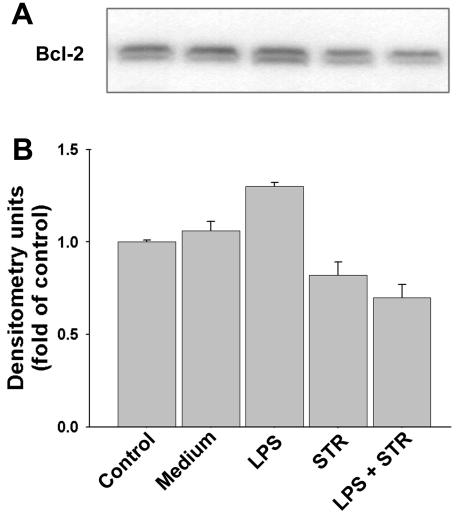
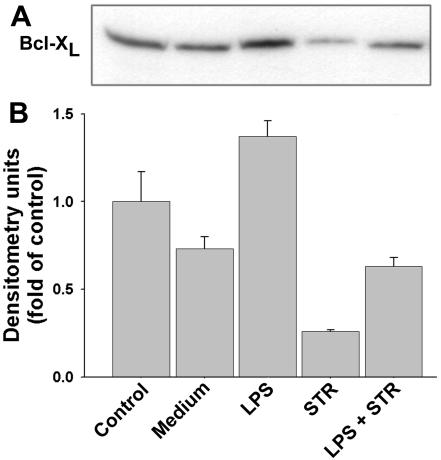
Members of the Bcl-2 family, including Bcl-2 and Bcl-XL, represent some of the most well-known down-regulators of apoptosis (4, 7, 17). The influence of LPS on the total levels of these proteins was determined in whole-cell lysates for both spontaneous and STR-induced apoptosis. The results obtained from immunoblotting analyses show that LPS induced a statistically significant increase (P < 0.05) in the levels of Bcl-2 (23% ± 6%) (Fig. 3) and Bcl-XL (89% ± 9%) (Fig. 4). In contrast, STR induced a statistically significant decrease in Bcl-2 (22% ± 3%) (Fig. 3) and Bcl-XL (65% ± 3%) (Fig. 4). It must be stressed that in primary B cells, these antiapoptotic members of the Bcl-2 family are constitutive proteins which are present in freshly purified control cells.
FIG. 3.
Expression of total Bcl-2 protein. B cells were cultured for 15 h, as indicated, in medium alone or in the presence of 5 μg of LPS/ml, 10 nM STR, or LPS plus STR. (A) The presence of Bcl-2 in total cell lysates obtained from 2 × 106 cells was detected by Western blotting. (B) Relative Bcl-2 protein levels were determined by densitometry and reported as fold changes compared to control ex vivo B cells at the initiation of cultures. Data are the means ± SD of three independent experiments.
FIG. 4.
Expression of total Bcl-XL protein. Total cell lysates were obtained from 2 × 106 cells cultured as described in the legend to Fig. 3. (A) Immunoblotting with anti-Bcl-XL antibodies. (B) Relative Bcl-XL levels as determined by densitometry, reported as fold changes compared to the control. Data are the means ± SD of three independent experiments.
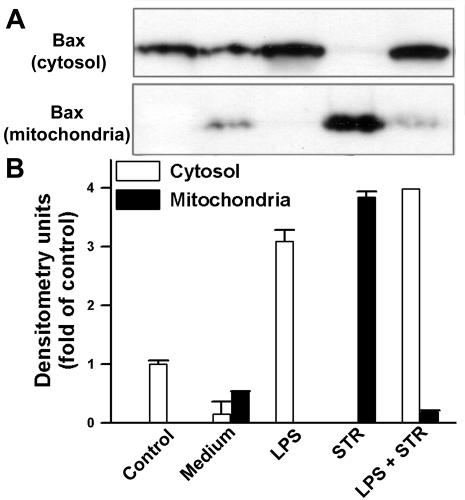
LPS prevents translocation of cytosolic Bax to mitochondria.
For many cells, it is becoming recognized that following exposure of the cells to apoptotic stresses, including cytokine withdrawal and treatment with glucocorticoids and cytotoxic drugs, the proapoptotic protein Bax, which localizes essentially to the cytoplasm, translocates from the cytosol to the mitochondria when cells undergo apoptosis (20, 33). An analysis of whole-cell lysates indicated that LPS did not significantly affect total Bax levels, whereas a marked increase in this protein was observed in the presence of STR (data not shown). The subcellular distribution of Bax was then examined by Western blot analyses of both cytosolic and mitochondrial fractions prepared by hypotonic lysis in the presence of digitonin. The results reported in Fig. 5 show that in primary B cells (control), the constitutive Bax protein is present predominantly in the cytosol. Upon spontaneous apoptosis after an overnight incubation of cells in medium, Bax was present in both the cytosolic and mitochondrial fractions, whereas in the presence of LPS, Bax translocation from the cytosol to the mitochondria was almost totally inhibited. Conversely, Bax completely translocated from the cytosol to the mitochondria during apoptosis induction by STR. Furthermore, in parallel with its preventive effect on STR-induced apoptosis, LPS significantly blocked the shift of cytosolic Bax into mitochondria. However, Bax was still detectable in mitochondria after the LPS treatment. This may be due to an incomplete inhibition by LPS of STR-induced apoptosis, as observed above (Fig. 1E; Table 1).
FIG. 5.
LPS inhibits Bax translocation to mitochondria. B cells were cultured as described for Fig. 3. (A) Cytosolic and mitochondrial fractions obtained by digitonin treatment were assayed for Bax by Western blotting. (B) Relative Bax levels in the cytosol (white bars) and mitochondria (black bars), as determined by densitometry and reported as fold changes compared to the control. Data are the means ± SD of three independent experiments.
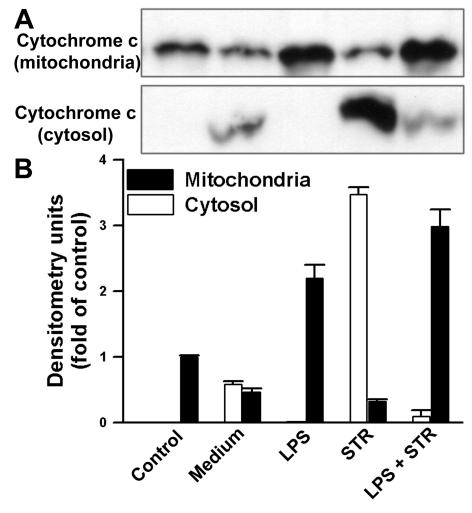
LPS inhibits release of cytochrome c from mitochondria and activation of caspase-9.
The release of cytochrome c from mitochondria is a central event in drug-induced apoptotic pathways. The present model suggests that, after Bax activation, cytochrome c is released and downstream caspases are activated (16). As was done for the Bax protein, the subcellular distribution of cytochrome c was examined by Western blot analyses of both cytosolic and mitochondrial fractions prepared by hypotonic lysis in the presence of digitonin. The results in Fig. 6 show that, in control B cells, cytochrome c was present predominantly in the mitochondrial fractions. The presence of cytochrome c was observed in both the cytosol and mitochondria when cells were cultured overnight in medium alone, suggesting a potential role for cytochrome c release in spontaneous B-cell apoptosis. In STR-induced apoptotic cells, a significant release of cytochrome c into the cytosol occurred. This cytochrome c release was almost completely inhibited by LPS for both spontaneous and STR-induced apoptosis.
FIG. 6.
LPS prevents cytochrome c release from mitochondria. B cells were cultured as described for Fig. 3. (A) Cytosolic and mitochondrial fractions obtained by digitonin treatment were assayed for cytochrome c by Western blotting. (B) Relative cytochrome c levels in cytosol (white bars) and mitochondria (black bars), as determined by densitometry and reported as fold changes compared to the control. Data are the means ± SD of three independent experiments.
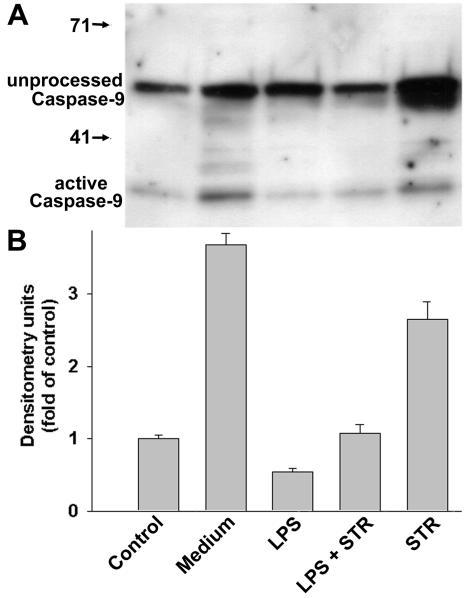
According to the current model of mitochondrion-dependent apoptosis, once cytochrome c is released into the cytosol, it forms a macromolecular complex with apoptotic protease-activating factor 1 (Apaf-1), which recruits and activates caspase-9. Accordingly, immunoblotting analyses showed that unprocessed pro-caspase-9 and active caspase-9 were found in total cell lysates (Fig. 7A). It must be stressed that the amount of unprocessed caspase-9 induced by STR was larger than that found in other samples. The relative amounts of protein presented in Fig. 7B show that, compared to control cells, increased levels of active caspase-9 were observed for both spontaneous apoptosis (medium versus control) and STR-induced apoptosis (STR versus control), whereas the presence of LPS led to a highly significant decrease in the levels of mature active caspase-9 (LPS versus medium and LPS plus STR versus STR, with P values of 2 × 10−4 and 2 × 10−3, respectively, by Student's t test).
FIG. 7.
Influence of LPS on caspase-9 activation. B cells were cultured as described for Fig. 3. (A) Total cell lysates were subjected to immunoblotting with an anti-caspase-9 antibody which recognizes both unprocessed pro-caspase-9 and cleaved active caspase-9. (B) Relative levels of active caspase-9, as determined by densitometry and reported as fold changes compared to the control. Data are the means ± SD of three independent experiments.
DISCUSSION
As described for a number of cell types (9, 16, 26, 32), mitochondria appear to play a key role in both spontaneous and drug-induced apoptosis of mature B cells. Accordingly, the present study indicates that LPS can effectively preserve cell viability in parallel with the maintenance of the ΔΨm and high intracellular GSH levels. Unlike STR, LPS was found to retain the Bax protein, a proapoptotic member of the Bcl-2 family, in the cytosol, thus preventing its translocation to mitochondria. Furthermore, we observed that LPS blocks the release of cytochrome c from mitochondria into the cytosol. These results indicate that the rescue from apoptosis occurs upstream of mitochondrial perturbations.
Mitochondrial dysfunctioning has been shown to participate in the induction of apoptosis, and a decrease in the ΔΨm is considered to be an early event in the apoptotic pathway (28, 47). Previous findings have shown that IL-4 has antiapoptotic activity by countering early mitochondrial perturbations (27). However, in the present study, the effects of LPS on the maintenance of the ΔΨm occurred later than those observed for IL-4 (at 15 versus 8 h). These apoptotic events could be detected after an overnight incubation, i.e., from 15 to 18 h, suggesting that changes in the ΔΨm could be detected concurrently with Bax translocation and cytochrome c release as a late occurrence in the chain of apoptotic events. Consistent with these observations, there is accumulating evidence that in some apoptotic systems, a decrease in ΔΨm can be a late event in the apoptotic pathway. For example, it has been reported that rat thymocytes treated with DEX undergo DNA fragmentation prior to any mitochondrial alteration (8). Similarly, HL-60 cells, which have been induced to undergo apoptosis in response to actinomycin D, etoposide, or STR, show no significant early changes in ΔΨm (12). In addition, it has been reported that during etoposide-induced apoptosis of L929 fibroblasts, a decrease in the ΔΨm occurs as a late event following Bax translocation to mitochondria (23). Therefore, the dissipation of ΔΨm may or may not be an early event in the apoptotic pathway, depending on the apoptotic stimuli used and the cell system under investigation, with discrepancies between various reports even being attributed to the fluorochromes used to detect ΔΨm (28).
Both anti- and proapoptotic Bcl-2 family proteins, such as Bcl-2, Bcl-XL, and Bax, were constitutively present in ex vivo-purified mature B cells, suggesting an effective machinery for apoptosis which could thus explain why these cells undergo spontaneous apoptosis after being cultured in vitro. The Bax protein was found to be preferentially localized in the cytosol in mature B cells, and its translocation to mitochondria was observed after apoptosis. Bax translocation from the cytosol to mitochondria and cytochrome c release into the cytosol were both almost totally achieved by exposure to STR. Conversely, LPS was found to retain Bax in the cytosol, thereby almost completely preventing Bax translocation to mitochondria, for both spontaneous and STR-induced apoptosis. While many studies indicate that in response to apoptotic stimuli, cytosolic Bax redistributes to mitochondria, where it induces cytochrome c release (7, 22), others show that Bax translocation to mitochondria occurs downstream of cytochrome c release, as reported for STR-induced apoptosis in cardiomyocytes (5). In agreement with our findings, it was recently reported that in apoptotic ML-1 human leukemia cells treated with anisomycin or STR, Bax translocation to mitochondria is concurrent with cytochrome c release and is inhibited by the antiapoptotic Bcl-XL protein of the Bcl-2 family (13). Further studies should address how LPS can block Bax relocalization from the cytosol to mitochondria, by examining either inactivation of its active form or prevention of the activation process of this protein. This last hypothesis may require the overexpression of Ku70, an endogenous protein involved in DNA repair which binds to the inactive form of Bax and suppresses its translocation to mitochondria (38). It would also be interesting to determine if the LPS-induced blockade of Bax translocation that we observed in primary B lymphocytes involves the phosphatidylinositol 3-kinase pathway, which has been reported to play an important role in the regulation of Bax translocation to mitochondria (42), and more recently, in B-cell survival (11).
In conclusion, the present study indicates that Bax redistribution from the cytosol to mitochondria is an important step in the apoptotic process in primary B cells. Moreover, LPS was shown to prevent mitochondrial dysfunctions and Bax translocation to mitochondria, thus suggesting its involvement upstream of mitochondrial events for antiapoptotic activity.
Acknowledgments
We thank Nicole Esquirol for her excellent technical assistance.
This work was supported by the Centre National de la Recherche Scientifique (CNRS) and by the Paris-Sud University (UPS).
Editor: A. D. O'Brien
REFERENCES
- 1.Andersson, J., G. Moller, and O. Sjoberg. 1972. Selective induction of DNA synthesis in T and B lymphocytes. Cell. Immunol. 4:381-393. [DOI] [PubMed] [Google Scholar]
- 2.Ashman, R. F., D. Peckham, and L. L. Stunz. 1996. Fc receptor off-signal in the B cell involves apoptosis. J. Immunol. 157:5-11. [PubMed] [Google Scholar]
- 3.Baixeras, E., L. Bosca, C. Stauber, A. Gonzalez, A. C. Carrera, J. A. Gonzalo, and C. Martineza. 1994. From apoptosis to autoimmunity: insights from the signaling pathways leading to proliferation or to programmed cell death. Immunol. Rev. 142:53-91. [DOI] [PubMed] [Google Scholar]
- 4.Borner, C. 2003. The Bcl-2 protein family: sensors and checkpoints for life-or-death decisions. Mol. Immunol. 39:615-647. [DOI] [PubMed] [Google Scholar]
- 5.Capano, M., and M. Crompton. 2002. Biphasic translocation of Bax to mitochondria. Biochem. J. 367:169-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen, J. J. 1999. Apoptosis: mechanisms of life and death in the immune system. J. Allergy Clin. Immunol. 103:548-554. [DOI] [PubMed] [Google Scholar]
- 7.Cory, S., and J. M. Adams. 2002. The BCL2 family: regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2:647-656. [DOI] [PubMed] [Google Scholar]
- 8.Cossarizza, A., G. Kalashnikova, E. Grassilli, F. Chiappelli, S. Salvioli, M. Capri, D. Barbieri, L. Troiano, D. Monti, and C. Franceschi. 1994. Mitochondrial modifications during rat thymocyte apoptosis: a study at the single cell level. Exp. Cell Res. 214:323-330. [DOI] [PubMed] [Google Scholar]
- 9.Desagher, S., and J. C. Martinou. 2000. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 10:369-377. [DOI] [PubMed] [Google Scholar]
- 10.Dewson, G., G. M. Cohen, and A. J. Wardlaw. 2001. Interleukin-5 inhibits translocation of Bax to the mitochondria, cytochrome c release, and activation of caspases in human eosinophils. Blood 98:2239-2247. [DOI] [PubMed] [Google Scholar]
- 11.Donahue, A. C., and D. A. Fruman. 2003. Proliferation and survival of activated B cells requires sustained antigen receptor engagement and phosphoinositide 3-kinase activation. J. Immunol. 170:5851-5860. [DOI] [PubMed] [Google Scholar]
- 12.Finucane, D. M., N. J. Waterhouse, G. P. Amarante-Mendes, T. G. Cotter, and D. R. Green. 1999. Collapse of the inner mitochondrial transmembrane potential is not required for apoptosis of HL60 cells. Exp. Cell Res. 251:166-174. [DOI] [PubMed] [Google Scholar]
- 13.Ganju, N., and A. Eastman. 2002. Bcl-X-L and calyculin A prevent translocation of Bax to mitochondria during apoptosis. Biochem. Biophys. Res. Commun. 291:1258-1264. [DOI] [PubMed] [Google Scholar]
- 14.Girard, R., T. Pedron, and R. Chaby. 1997. Functional lipopolysaccharide receptors of low affinity are constitutively expressed on mouse bone marrow cells. Immunology 91:391-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glauser, M. P., G. Zanetti, J. D. Baumgartner, and J. Cohen. 1991. Septic shock: pathogenesis. Lancet 338:732-736. [DOI] [PubMed] [Google Scholar]
- 16.Green, D. R., and J. C. Reed. 1998. Mitochondria and apoptosis. Science 281:1309-1312. [DOI] [PubMed] [Google Scholar]
- 17.Gross, A., J. M. McDonnell, and S. J. Korsmeyer. 1999. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 13:1899-1911. [DOI] [PubMed] [Google Scholar]
- 18.Hammerling, U., A. F. Chin, and J. Abbott. 1976. Ontogeny of murine B lymphocytes: sequence of B-cell differentiation from surface-immunoglobulin-negative precursors to plasma cells. Proc. Natl. Acad. Sci. USA 73:2008-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hofhaus, G., R. M. Shakeley, and G. Attardi. 1996. Use of polarography to detect respiration defects in cell cultures. Methods Enzymol. 264:476-483. [DOI] [PubMed] [Google Scholar]
- 20.Hsu, Y. T., K. G. Wolter, and R. J. Youle. 1997. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc. Natl. Acad. Sci. USA 94:3668-3672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Illera, V. A., C. E. Perandones, L. L. Stunz, D. A. Mower, Jr., and R. F. Ashman. 1993. Apoptosis in splenic B lymphocytes. Regulation by protein kinase C and IL-4. J. Immunol. 151:2965-2973. [PubMed] [Google Scholar]
- 22.Jurgensmeier, J. M., Z. Xie, Q. Deveraux, L. Ellerby, D. Bredesen, and J. C. Reed. 1998. Bax directly induces release of cytochrome c from isolated mitochondria. Proc. Natl. Acad. Sci. USA 95:4997-5002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karpinich, N. O., M. Tafani, R. J. Rothman, M. A. Russo, and J. L. Farber. 2002. The course of etoposide-induced apoptosis from damage to DNA and p53 activation to mitochondrial release of cytochrome c. J. Biol. Chem. 277:16547-16552. [DOI] [PubMed] [Google Scholar]
- 24.Khaled, A. R., K. Kim, R. Hofmeister, K. Muegge, and S. K. Durum. 1999. Withdrawal of IL-7 induces Bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc. Natl. Acad. Sci. USA 96:14476-14481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krammer, P. H., I. Behrmann, P. Daniel, J. Dhein, and K. M. Debatin. 1994. Regulation of apoptosis in the immune system. Curr. Opin. Immunol. 6:279-289. [DOI] [PubMed] [Google Scholar]
- 26.Kroemer, G., and J. C. Reed. 2000. Mitochondrial control of cell death. Nat. Med. 6:513-519. [DOI] [PubMed] [Google Scholar]
- 27.Lemaire, C., K. Andréau, C. Sidoti-deFraisse, A. Adam, and V. Souvannavong. 1999. IL-4 inhibits apoptosis and prevents mitochondrial damage without inducing the switch to necrosis observed with caspase inhibitors. Cell Death Differ. 6:813-820. [DOI] [PubMed] [Google Scholar]
- 28.Ly, J. D., D. R. Grubb, and A. Lawen. 2003. The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 8:115-128. [DOI] [PubMed] [Google Scholar]
- 29.Macho, A., T. Hirsch, I. Marzo, P. Marchetti, B. Dallaporta, S. A. Susin, N. Zamzami, and G. Kroemer. 1997. Glutathione depletion is an early and calcium elevation is a late event of thymocyte apoptosis. J. Immunol. 158:4612-4619. [PubMed] [Google Scholar]
- 30.Medzhitov, R., and C. A. Janeway, Jr. 1997. Innate immunity: impact on the adaptive immune response. Curr. Opin. Immunol. 9:4-9. [DOI] [PubMed] [Google Scholar]
- 31.Melchers, F., V. Braun, and C. Galanos. 1975. The lipoprotein of the outer membrane of Escherichia coli: a B-lymphocyte mitogen. J. Exp. Med. 142:473-482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mignotte, B., and J. L. Vayssiere. 1998. Mitochondria and apoptosis. Eur. J. Biochem. 252:1-15. [DOI] [PubMed] [Google Scholar]
- 33.Murphy, K. M., V. Ranganathan, M. L. Farnsworth, M. Kavallaris, and R. B. Lock. 2000. Bcl-2 inhibits Bax translocation from cytosol to mitochondria during drug-induced apoptosis of human tumor cells. Cell Death Differ. 7:102-111. [DOI] [PubMed] [Google Scholar]
- 34.Norvell, A., L. Mandik, and J. G. Monroe. 1995. Engagement of the antigen-receptor on immature murine B lymphocytes results in death by apoptosis. J. Immunol. 154:4404-4413. [PubMed] [Google Scholar]
- 35.Nowotny, A. 1987. Review of the molecular requirements of endotoxic actions. Rev. Infect. Dis. 9(Suppl. 5):S503-S511. [DOI] [PubMed] [Google Scholar]
- 36.Paige, C. J., P. W. Kincade, and P. Ralph. 1981. Independent control of immunoglobulin heavy and light chain expression in a murine pre-B-cell line. Nature 292:631-633. [DOI] [PubMed] [Google Scholar]
- 37.Parillo, J., M. Parker, C. Natanson, A. Suffredini, R. Danner, R. Cunnion, and F. Ognibene. 1990. Septic shock in humans. Advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann. Intern. Med. 113:227-241. [DOI] [PubMed] [Google Scholar]
- 38.Sawada, M., W. Sun, P. Hayes, K. Leskov, D. A. Boothman, and S. Matsuyama. 2003. Ku70 suppresses the apoptotic translocation of Bax to mitochondria. Nat. Cell Biol. 5:320-329. [DOI] [PubMed] [Google Scholar]
- 39.Souvannavong, V., K. Andreau, A. Adam, and R. Chaby. 1999. Effect of synthetic lipids on apoptosis and expression of alkaline phosphatase in B-lymphocytes: influence on lipopolysaccharide action. FEMS Immunol. Med. Microbiol. 26:37-47. [DOI] [PubMed] [Google Scholar]
- 40.Souvannavong, V., S. Brown, M. Sarih, and A. Adam. 1994. Expression and visualization during cell cycle progression of alkaline phosphatase in B lymphocytes from C3H/HeJ mice. J. Leukoc. Biol. 55:626-632. [DOI] [PubMed] [Google Scholar]
- 41.Strasser, A., L. O'Connor, and V. M. Dixit. 2000. Apoptosis signaling. Annu. Rev. Biochem. 69:217-245. [DOI] [PubMed] [Google Scholar]
- 42.Tsuruta, F., N. Masuyama, and Y. Gotoh. 2002. The phosphatidylinositol 3-kinase (PI3K)-Akt pathway suppresses Bax translocation to mitochondria. J. Biol. Chem. 277:14040-14047. [DOI] [PubMed] [Google Scholar]
- 43.Ulevitch, R. J., and P. S. Tobias. 1999. Recognition of gram-negative bacteria and endotoxin by the innate immune system. Curr. Opin. Immunol. 11:19-22. [DOI] [PubMed] [Google Scholar]
- 44.Vayssiere, J. L., P. X. Petit, Y. Risler, and B. Mignotte. 1994. Commitment to apoptosis is associated with changes in mitochondrial biogenesis and activity in cell lines conditionally immortalized with simian virus 40. Proc. Natl. Acad. Sci. USA 91:11752-11756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wechsler-Reya, R. J., and J. G. Monroe. 1996. Lipopolysaccharide prevents apoptosis and induces responsiveness to antigen receptor cross-linking in immature B cells. Immunology 89:356-362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wyllie, A. H., J. F. Kerr, and A. R. Currie. 1980. Cell death: the significance of apoptosis. Int. Rev. Cytol. 68:251-306. [DOI] [PubMed] [Google Scholar]
- 47.Zamzami, N., P. Marchetti, M. Castedo, C. Zanin, J. L. Vayssiere, P. X. Petit, and G. Kroemer. 1995. Reduction in mitochondrial potential constitutes an early irreversible step of programmed lymphocyte death in vivo. J. Exp. Med. 181:1661-1672. [DOI] [PMC free article] [PubMed] [Google Scholar]