Significance
The expression of multiple isoforms of a protein kinase in cells raises the question of which substrates are preferentially phosphorylated by each isoform. Endothelial cells (ECs) that line all blood vessels express the protein kinases, Akt1 and Akt2, and we demonstrate here that endothelial nitric oxide synthase (eNOS) is a preferential Akt1 substrate and supraphysiological overexpression of Akt2 is needed to phosphorylate eNOS and promote NO release. In mice, the endothelial loss of Akt1, but not Akt2, retards vessel growth, and quantitative assessment of the phosphoproteomes in ECs isolated from these mice shows that Akt1 uniquely phosphorylates several important proteins regulating vascular function. Thus, despite the presence of both isoforms of Akt in the endothelium, substrate phosphorylation by Akt1 is nonredundant.
Keywords: phosphoproteomics, kinase, development
Abstract
The PI3K/Akt pathway is necessary for several key endothelial cell (EC) functions, including cell growth, migration, survival, and vascular tone. However, existing literature supports the idea that Akt can be either pro- or antiangiogenic, possibly due to compensation by multiple isoforms in the EC when a single isoform is deleted. Thus, biochemical, genetic, and proteomic studies were conducted to examine isoform-substrate specificity for Akt1 vs. Akt2. In vitro, Akt1 preferentially phosphorylates endothelial nitric oxide synthase (eNOS) and promotes NO release, whereas nonphysiological overexpression of Akt2 can bypass the loss of Akt1. Conditional deletion of Akt1 in the EC, in the absence or presence of Akt2, retards retinal angiogenesis, implying that Akt1 exerts a nonredundant function during physiological angiogenesis. Finally, proteomic analysis of Akt substrates isolated from Akt1- or Akt2-deficient ECs documents that phosphorylation of multiple Akt substrates regulating angiogenic signaling is reduced in Akt1-deficient, but not Akt2-deficient, ECs, including eNOS and Forkhead box proteins. Therefore, Akt1 promotes angiogenesis largely due to phosphorylation and regulation of important downstream effectors that promote aspects of angiogenic signaling.
Angiogenesis and vascular remodeling are essential biological processes involving multiple cell types and signaling pathways. Shear stress, cytokines, and/or growth factor-mediated stimulation of receptor tyrosine kinases results in subsequent PI3K-dependent, Akt/PKB activation (1). The PI3K/Akt pathway is necessary for several key endothelial cell (EC) functions, including cell growth, migration, survival, and vascular tone.
The Akt family of serine/threonine kinases consists of three isoforms with greater than 80% sequence homology. Although the Akt isoforms have been shown to share similar mechanisms in regard to activation and even redundancy in function, emerging evidence in various cell types suggests the possibility of distinct roles for each Akt isoform. Several groups have demonstrated the importance of Akt1 in ECs, because Akt1 is a critical downstream kinase in the VEGF signaling cascade (2) and cultured Akt1−/− ECs exhibit impaired NO release, integrin activation, migration, and proliferation (3–5). We have also shown that the genetic loss of Akt1 substantially impairs ischemia-induced arteriogenesis and VEGF-induced postnatal angiogenesis, as well as wound, inflammation, and VEGF-induced vascular permeability (3, 6, 7). Despite the contribution of Akt on growth and extraembryonic placental angiogenesis (8–10), the absence of Akt1 is not embryonic-lethal, implying that additional isoforms, such as Akt2, may compensate for the loss of Akt1. In addition, because many of the cell types involved in angiogenesis and vessel remodeling express Akt1, the phenotypic contribution from specific Akt1-expressing cells is unknown.
Despite the central role of Akt in vascular signaling and function, a systematic approach elucidating the functional overlaps and differences between Akt1 and Akt2 in vascular cells has not been assessed. Oftentimes, Akt signaling is grouped together to include all Akt isoforms because most commercially available antibodies, including phosphoantibodies, do not discriminate between Akt1 and Akt2 proteins. Therefore, it is important to identify isoform-specific Akt targets, which will allow for a deeper understanding of the functional output of PI3K/Akt signaling.
To examine the roles of Akt1 and Akt2 in ECs systematically, we integrated both conditional KO mouse models and phosphoproteomic analyses. Here, we show that endothelial expression of only the Akt1 isoform is critical for normal retinal angiogenesis, because acute postnatal Akt1 deletion confers significant retinal vascular defects regardless of the presence of Akt2. Furthermore, high-throughput phosphoproteomic analyses of Akt substrates in ECs indicate that Akt1 preferentially targets substrates that mediate signaling paradigms vital for angiogenesis. Therefore, Akt1 serves as the predominant Akt isoform in the endothelium by phosphorylating multiple substrates that promote angiogenesis.
Results
Phosphorylation of Endothelial Nitric Oxide Synthase Is Preferentially Regulated by the Akt1 Isoform.
VEGF is a well-characterized angiogenic stimulus that signals through its receptors to activate Akt. Previous work from our laboratory demonstrated that VEGF stimulates Akt-mediated endothelial nitric oxide synthase (eNOS) phosphorylation at S1176 (bovine S1179), thus activating eNOS to promote NO release (3, 11). NO is an important mediator of many processes, including blood flow, vascular remodeling, and angiogenesis. Furthermore, the in vivo role of Akt1-eNOS kinase-substrate specificity is clear, where a phosphomimetic knock-in, but not a loss-of-function mutation at S1176, rescues pathologically induced angiogenesis in Akt1−/− mice (12). Given the relative EC-selective expression pattern of eNOS, we isolated mouse lung ECs (MLECs) from WT, Akt1−/−, and Akt2−/− mice to determine if Akt2 could compensate for the loss of Akt1 in the context of eNOS activation and NO release.
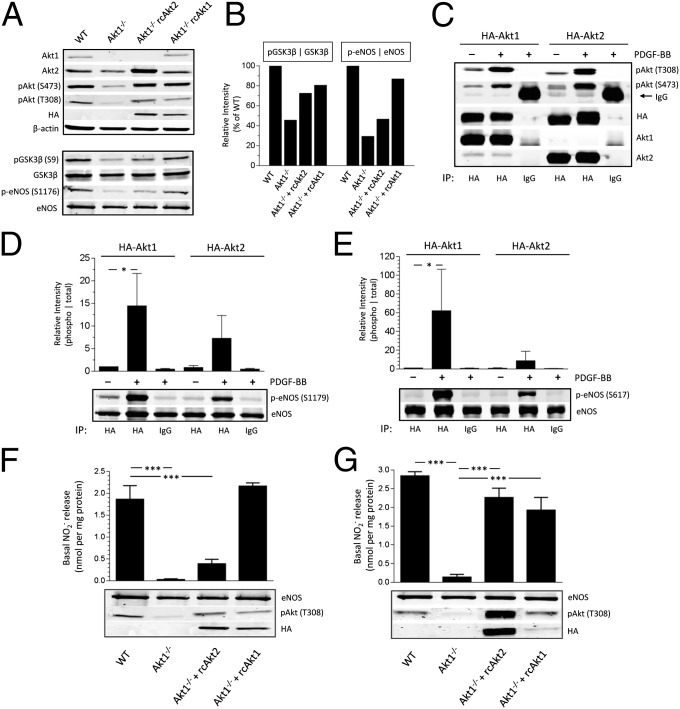
MLECs were cultured and stimulated with VEGF for 5 and 10 min. Examination of eNOS phosphorylation at the Akt phosphorylation site (S1176 or S614) showed a marked reduction in Akt1−/− cells compared with WT. Diminished phosphorylated p-eNOS levels were also seen under basal conditions in Akt1−/− cells, and the loss of Akt2 did not affect eNOS phosphorylation after VEGF stimulation (Fig. S1A). To assess the role of Akt1 vs. Akt2 on eNOS phosphorylation further, ECs were reconstituted via retroviral expression of HA-tagged versions of Akt1 or Akt2. Akt1−/− ECs demonstrated reduced Akt phosphorylation on S473 and T308, whereby reconstitution with either Akt1 or Akt2 restored Akt phosphorylation to levels comparable to that of WT (Fig. 1A). Assessment of eNOS S1176 and glycogen synthase kinase 3β (GSK3β) serine 9 (S9) phosphorylation showed reduced levels in Akt1−/− ECs, where reconstitution with a retroviral vector expressing HA-tagged Akt1 in Akt1−/− ECs restored both p-eNOS and p-GSK3β levels (Fig. 1A, Lower). Reconstitution with Akt2 partially restored p-GSK3β levels, but not p-eNOS levels, to those of WT (Fig. 1B). These observations are consistent with those seen in PDGF-BB–stimulated primary lung fibroblasts (Fig. S1 B and C), suggesting that both Akt1 and Akt2 can reconstitute S9 phosphorylation on GSK3β.
Fig. 1.
Restoration of eNOS activity in Akt1−/− ECs requires reconstitution with nonphysiological levels of Akt2. (A) Akt1−/− MLECs were infected with retroviral HA-tagged Akt1 or Akt2, and phosphospecific immunoblotting was performed. (B) Quantification of phosphorylation intensities normalized to total expression. Values are relative to WT conditions. (C) Akt1−/− MEFs were infected with retroviral, HA-tagged Akt1 or Akt2. Cells were serum-starved and stimulated with PDGF-BB (40 ng/mL) for 15 min. Immunoblots reflect pull-down of immunoprecipitates (IPs). (D and E) IP extracts were incubated with recombinant bovine eNOS as a substrate for in vitro kinase assays. Reaction products were analyzed by SDS/PAGE and immunoblotting using antibodies specific for eNOS phosphorylation at S1179 (D) and S617 (E). (F) NO release was measured in WT, Akt1−/−, and Akt1−/− MLECs reconstituted with either Akt1 or Akt2. (G) NO release was measured in Akt1−/− MLECs, where reconstitution with Akt2 is ∼10-fold greater than with Akt1. *P < 0.05; ***P < 0.001. Data are expressed as mean ± SEM (n = 3).
We next performed a series of in vitro kinase assays to investigate Akt isoform-substrate selectivity. Akt1−/− mouse embryonic fibroblasts (MEFs) were transduced with retroviral HA-tagged Akt1 or Akt2. Following serum starvation, infected MEFs were either left unstimulated or stimulated with PDGF-BB (40 ng/mL) for 15 min. Lysates were immunoprecipitated for HA-tagged Akt1 or Akt2, and HA immunoblotting showed comparable levels of isolated Akt1 and Akt2 (Fig. 1C). PDGF-BB stimulation robustly increased Akt phosphorylation levels (S473 and T308) for both Akt1 and Akt2 (Fig. 1C). The immune-isolated kinase was then incubated with substrates, either a GSK3 fusion protein or recombinant bovine eNOS, for 30 min in kinase reactions. SDS/PAGE and immunoblotting for GSK3 phosphorylation levels demonstrated that Akt activity increased six- to sevenfold higher as a result of PDGF stimulation, regardless of the reconstituted Akt isoform in the Akt1−/− MEFs (Fig. S1D). Similar analyses for eNOS phosphorylation show that activated Akt1 induced close to a 15-fold increase in eNOS S1179 phosphorylation but lower phosphorylation (approximately fivefold) with Akt2 (Fig. 1D). These isoform-substrate differences are further exemplified when evaluating other Akt-directed eNOS phosphorylation sites, because Akt1 more efficiently phosphorylates eNOS S617 compared with Akt2 (Fig. 1E). Additionally, phosphorylation of a non-Akt phosphorylation site, S116, does not occur (Fig. S1E), suggesting that at relatively equal levels of Akt1 or Akt2 activity, eNOS is preferentially phosphorylated by Akt1.
Next, we assessed the functional consequence of eNOS phosphorylation in Akt1−/− ECs via measurement of NO release. As seen in Fig. 1F, defective NO release in Akt1−/− cells was restored to WT levels with reconstitution of Akt1. Reconstitution of Akt2 to near-endogenous levels of total Akt (as seen with HA and pAkt T308 immunoblotting), however, only slightly increases basal NO accumulation. Furthermore, marked overexpression of Akt2 to nonphysiological levels (∼10-fold greater) restores NO release similar to that of WT (Fig. 1G). Therefore, Akt1 more efficiently activates eNOS compared with Akt2 when expressed at equal levels, and this preference is overcome by Akt2 overexpression.
Endothelial Akt1 Drives Developmental Retinal Angiogenesis.
To study the functional importance of Akt1 in vivo, we inactivated Akt1 specifically in the endothelium using Cre/loxP technology. Briefly, Akt1flox/flox mice (13) were bred to Cdh5-Cre-ERT2 (14) mice to generate inducible, endothelial-specific Akt1 KO mice. To account for any tamoxifen-induced variability, all neonates were administered tamoxifen, and only those expressing the Cre allele underwent loxP-mediated excision. WT denotes Cre-negative, floxed littermates that received tamoxifen. These mice pose an advantage over current Akt1 mouse models in that (i) conditional deletion allows for study of ubiquitously expressed Akt1 in a single cell type and (ii) our inducible deletion strategy minimizes the possibility for compensation by other Akt isoforms and/or AGC-family kinases during in utero deletion.
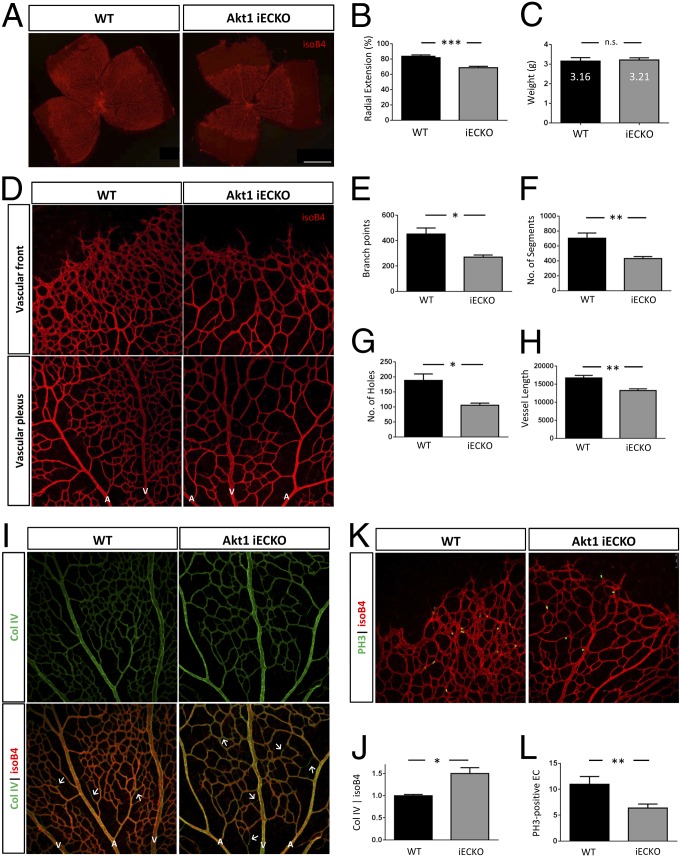
Postnatal deletion of Akt1 in the endothelium [initiated at postnatal day (P) 1 and analyzed at P6–P7, Akt1-inducible EC KO (Akt1iECKO)] impairs retinal angiogenesis, as shown by reduced endothelial coverage and delayed radial outgrowth (Fig. 2 A and B). There are also no apparent weight differences between WT and Akt1iECKO pups, thereby eliminating the possibility of a general growth defect (Fig. 2C). Quantitative analyses of the vascular plexus region indicates that Akt1iECKO retinas exhibit significantly less branching, fewer segment numbers, fewer hole numbers, and shorter vessel length (Fig. 2 E–H). The endothelial loss of Akt1, however, does not result in any significant changes in vessel thickness or tip cell/filopodia number (Fig. S2 A and B). The loss of Akt1 in ECs does not overtly affect neuron-glial antigen 2 (NG2)-positive pericyte distribution (Fig. S2C) or GFAP-positive astrocyte patterning (Fig. S2D). Furthermore, EC loss of Akt1 does not dramatically alter tip/stalk cell identity or arterial/venous specification patterns, as assessed by Delta-like 4 (dll4) staining of retinal vessels (Fig. S2E). Sprouting angiogenesis is followed by vessel remodeling and pruning to form a defined and mature, stable vascular network. Vessel regression is associated with the presence of empty basement membrane sleeves, as measured by the presence of collagen IV and the absence of endothelial markers (15). Endothelial-specific deletion of Akt1 promotes vessel regression, as seen through increased collagen IV-positive sleeves (Fig. 2 I and J). This observation is similar to previous reports, where wortmannin, a PI3K inhibitor, was injected acutely into established rat retinas (16), further supporting the role of PI3K/Akt in vessel remodeling. EC loss of Akt1 also significantly decreases endothelial proliferation, as quantified by nuclear phosphohistone 3 (PH3) staining, which contributes to the reduced retinal vascular coverage (Fig. 2 K and L).
Fig. 2.
Endothelial-specific Akt1 deletion impairs developmental retinal angiogenesis. Postnatal endothelial-specific Akt1 deletion impairs radial vessel outgrowth (injected with tamoxifen P1–P3, assessed P7) (A and B) but not overall growth at P7 (n = 11–16 per group) (C). (D) Retinal vasculature at the growing front and plexus. A, artery; V, vein. (E–H) Loss of endothelial Akt1 leads to reduced branching, segments, holes, and vessel length (WT, n = 6; iECKO, n = 8). (I) P7 retinas labeled for collagen IV (Col IV) and isolectin B4 (isoB4). Endothelial-specific loss of Akt1 increases vessel regression (arrowheads). (J) Ratio of Col IV-positive vessels to isoB4-positive vessels. P6 retinas are labeled for phosphohistone 3 (PH3) and isolectin B4 (K) and are quantified (L) (n ≥ 5 pups per group with four to five images per retina). n.s., not significant. *P < 0.05; **P < 0.01; ***P < 0.001. Representative images are shown, and data are expressed as mean ± SEM. (Scale bar: A, 5 mm. Magnification: D, I, and K, 200×.)
Our group previously showed that primary MLECs express all three Akt isoforms, with Akt1 > Akt2 >> Akt3 (3). Given the conserved sequence homology between Akt isoforms and the observation that overexpressed Akt2 can phosphorylate eNOS (Fig. 1G), it is feasible that the other Akt isoforms may compensate for the deletion of a single isoform. Global deletion of Akt2 does not confer a growth defect but rather manifests as metabolic dysfunction (17) in older mice. More importantly, retinal vascular development in Akt2−/− mice appears normal (Fig. S3 A and B), and astrocyte/pericyte coverage (Fig. S3 C and D) is unaffected. Furthermore, dll4 staining reveals normal tip/stalk cell identity and arterial/vein specification compared with WT littermates (Fig. S3 D and E).
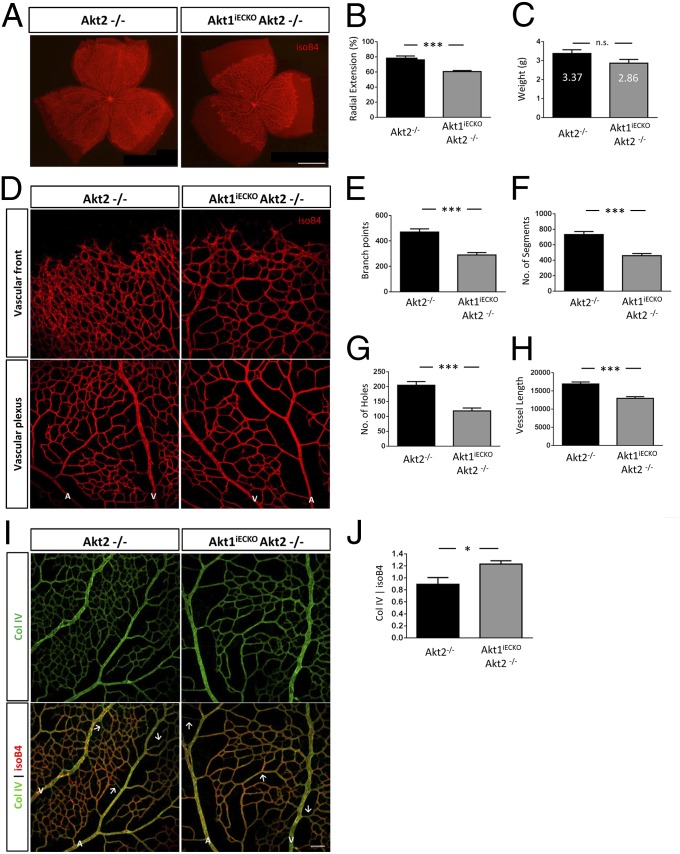
To examine if Akt2 compensates for the EC-specific loss of Akt1, Akt1flox/flox;Akt2−/− females were bred to Akt1flox/flox;Akt2−/−;Cdh5-Cre males, and resulting progeny were similarly injected with tamoxifen for P1–P3. Using this breeding strategy, littermate Akt2−/− mice were used as controls to determine the effect of dual Akt1 and Akt2 deletion in the endothelium on ensuing retinal development. As shown in Fig. 3A, dual loss of Akt1/2 in ECs results in impaired retinal outgrowth compared with control littermates (quantified in Fig. 3B) but does not significantly affect body weight (Fig. 3C). Furthermore, the retinal defects seen in Akt1iECKO mice are not worsened by the additional loss of Akt2. These observations imply that Akt1 is the predominant Akt isoform in ECs necessary for proper retinal vascular growth, because Akt2 does not compensate for its loss (Fig. S3). Quantification of vascular density at the vascular plexus regions indicates fewer branch points, holes, and segments and shorter vessel length in Akt1iECKO;Akt2−/− mice (Fig. 3 D–H), whereas vessel thickness and filopodia number were not significantly affected (Fig. S4 A and B). The dual loss of Akt1/2 also does not overtly affect NG2-positive pericyte coverage or tip/stalk and artery/vein specification [Fig. S4 C (vascular front) and D (vascular plexus)]. Vessel regression was, however, significantly increased, as measured by increased collagen IV deposition (Fig. 3 I and J). The observed disturbance in vascular network formation thereby phenocopies the conditional loss of Akt1 in ECs, as seen in Fig. 2, implying that Akt1 is the dominant functional Akt isoform in the endothelium.
Fig. 3.
Retinal vascular defects seen in Akt1iECKO mice are not worsened by the additional loss of Akt2. Loss of both endothelial Akt1 and Akt2 impairs outgrowth similar to that of endothelial-specific Akt1 loss (A and B) but not body weight (C) (n > 11 per group). (D) Retinal vasculature at the growing front and plexus regions. (E–H) Loss of endothelial Akt1 on a global Akt2-null background leads to reduced branching, segments, holes, and vessel length (Akt2−/−, n = 7; Akt1iECKO;Akt2−/−, n = 6). (I and J) Loss of both endothelial Akt1 and Akt2 (global null) increases Col IV deposition, similar to that of endothelial-specific Akt1 loss. *P < 0.05; ***P < 0.001. Representative images shown data are expressed as mean ± SEM. (Scale bar: A, 5 mm. Magnification: D and I, 200×.)
Retinal vascular development was also assessed in global Akt1-null mice to examine the relative contribution of endothelial Akt1 to the retinal phenotype. Postnatal Akt1−/− mice weigh significantly less than Akt1+/− littermates (Fig. S5A), thereby confirming previous reports of general growth retardation in Akt1-null mice (8–10). More importantly, the global loss of Akt1 phenocopies the endothelial-specific, acute postnatal deletion of Akt1 described herein (Fig. S5 B–D), and may be arguably worse than those in Akt1iECKO mice. This could, however, be attributed to the synergistic effects of Akt1 loss in other cell types critical for proper angiogenesis or incomplete deletion of the floxed allele. Nonetheless, the observation that acute, endothelial-specific Akt1 deletion yields retinal defects similar to global deletion suggests that developmental retinal angiogenesis depends largely on functional Akt1 in ECs.
Akt-Selective Phosphoproteomics Reveals a Larger Population of Akt1-Specific Substrates in ECs.
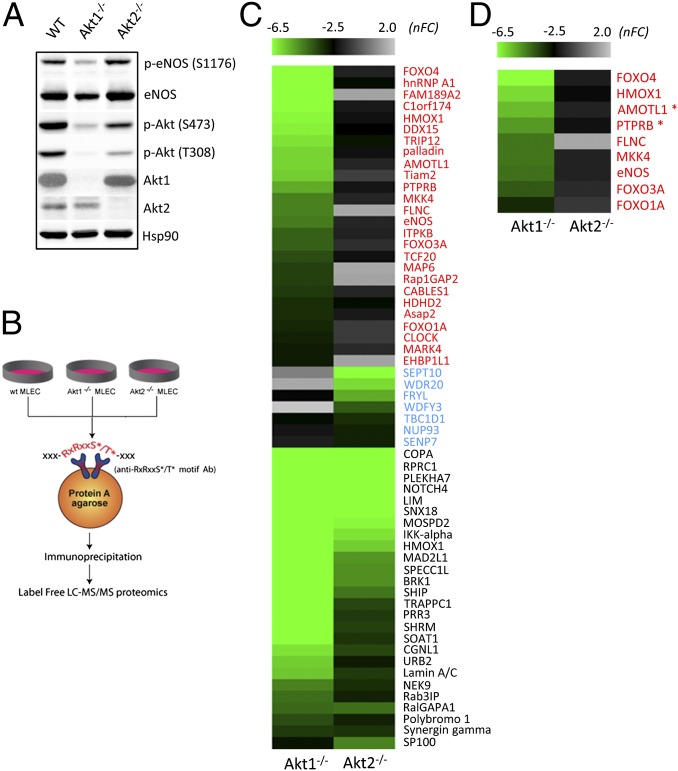
Although Akt1 and Akt2 exhibit strong similarities in sequence and function, there are subtle differences between the two isoforms. Mouse models reflecting global Akt1 or global Akt2 loss manifest in unique phenotypes (8, 9, 17). To appreciate Akt1 retinal phenotypes at the molecular level fully, a phosphoproteomics screen was performed in serum-grown ECs from Akt1−/− and Akt2−/− mice. Fig. 4A documents the total Akt/eNOS and Akt/eNOS phosphorylation levels in the starting material. Biological duplicates of protein extracts were isolated in urea and digested into peptides, and phosphopeptides were purified using an Akt phosphorylation motif-recognizing antibody (no. 23C8D2; Cell Signaling Technology), thereby allowing for the isolation of phosphopeptides containing the Akt phosphorylation motif of RXRXXS*/T* (18) (* indicates phosphorylated amino acid) (Fig. 4B). The immunoisolated peptides were subjected to MS analysis and label-free quantification, as previously described (19), to identify Akt1- and Akt2-dependent substrates (i.e., decreased phosphopeptides in KO ECs relative to WT ECs). To identify those phosphopeptides that are most likely Akt substrates, we considered only phosphopeptides with the following conditions as being significantly down-regulated relative to WT: (i) They contain the Akt phosphorylation motif, and (ii) they exceeded a −2.5-fold directional cutoff (Materials and Methods). The resultant phosphopeptides from this analysis are depicted as a heat map in Fig. 4C and detailed in Dataset S1, where 59 unique phosphosites in 58 different proteins (HMOX1 was identified to have two distinct phosphosites) were identified. Among these sites, 26 phosphoproteins were down-regulated in both Akt1−/− and Akt2−/− cells (black text in Dataset S1), 26 phosphoproteins were down-regulated exclusively in Akt1−/− cells (red highlighted text in Dataset S1), and seven phosphopeptides were decreased solely in the Akt2−/− cells (blue text in Dataset S1). The heat map clustering portrays a larger population of Akt1-sensitive substrates in ECs, which likely contributes to the observed EC phenotypes. Furthermore, the identification of mutually exclusive substrates indicates Akt isoform-substrate specificity, and hence distinct downstream functions.
Fig. 4.
Phosphoproteomic analysis reveals a larger population of Akt1-dependent substrates in endothelial lysates. (A) Characterization of the analyzed protein lysates. (B) Schematic illustrates the phosphoproteomic approach in identifying Akt1- vs. Akt2-targeted substrates. MS/MS, tandem MS. (C) Heat map of the phosphorylation events identified to be down-regulated in either Akt1−/− or Akt2−/− EC lysates. The heat map scale indicates normalized fold change (nFC) relative to WT. The green color denotes a decrease in the corresponding Akt motif phosphorylation compared with the WT condition. The heat map contains proteins that presented fold changes greater than −2.5 exclusively for the Akt1 KO (red font), fold changes greater than −2.5 exclusively for the Akt2 KO (blue font), and fold changes greater than −2.5 for both Akt1 and Akt2 KO (black font) ECs. (D) Network of proteins with phosphoproteomic changes that comprise the Akt1-exclusive, high-scoring category associated with cardiovascular disease and development (from Fig. S6A). Asterisks (*) indicate novel phosphosites identified in screens.
Phosphoproteomic Pathway Analysis Indicates Akt1-Associated Substrates, Not Akt2-Associated Substrates, Are Significantly Correlated with Cardiovascular Development and Function.
In an effort to understand the biological relevance of the phosphoscreen, the generated lists were subjected to pathway analysis using the Ingenuity Pathway Analysis (IPA; Ingenuity) database. The phosphoprotemic results were compared against global molecular networks to identify associated diseases/biological function and canonical pathways affected by loss of either Akt1 or Akt2 activity. The absence of the “lower expressed” Akt2 isoform significantly affects broad cellular function, namely, “cell signaling” and “cell function and maintenance” (Fig. S6A and Table S1). Ablation of Akt1, however, is associated with nine additional biological functions. More importantly, the changes in protein phosphorylation due to the loss of Akt1 significantly correlate with “cardiovascular system development and function,” as represented by over one-third (nine of 26 proteins) of the identified mutually exclusive Akt1-specific substrates (Fig. 4D). Furthermore, the nine identified proteins are implicated in angiogenesis and/or endothelial function.
Affected canonical pathways were also modeled using the IPA database to clarify the phenotypic differences between Akt1- and Akt2-null cells further. The loss of Akt2 did not significantly influence canonical pathway signaling, whereas the loss of Akt1 is associated with several known pathways, all of which are necessary for developmental angiogenesis (Fig. S6B). The parallel output between canonical pathways and biological function suggests Akt1-specific substrates dictate growth and development in the endothelium, whereas Akt2 likely plays a minor role.
Discussion
Our study identifies Akt1 as the predominant Akt isoform responsible for eNOS phosphorylation and developmental angiogenesis in the endothelium. The in vivo conditional models paired with an unbiased, high-throughput phosphoproteomics analysis demonstrate that endothelial Akt1, as opposed to Akt2, influences multiple signaling networks critical for vascular development and function. Interestingly, we identified common and isoform-specific Akt substrates via proteomics, providing evidence for isoform selectivity in vivo. These data reporting the consequential developmental vascular phenotypes with conditional deletion of Akt1 in ECs and characterization of the substrates preferred by Akt1 in ECs are consistent with the importance of Akt downstream of VEGF signaling (2).
Previous studies investigating the consequence of Akt1 loss used global KO mice. Global Akt1 deletion results in sub-Mendelian birth ratios and increased neonatal mortality, where surviving Akt1−/− mice are viable despite growth retardation. Although these global-null studies have been instrumental in moving the field forward, the ubiquitous nature of Akt1 limits the ability to study isoform-specific effects in particular cell types. Additionally, the viability of Akt1−/− mice suggests compensation from other Akt isoforms. Several groups have generated double-null and even triple-null Akt mouse models to address the possibility of functional overlap. Interestingly, both Akt1−/−;Akt2−/− double KO (DKO) and Akt1−/−;Akt3−/− DKO result in postnatal and embryonic lethality, respectively (20, 21). Furthermore, triple Akt1+/−;Akt2−/−;Akt3−/− mice are viable with a severe growth impairment, suggesting a single Akt1 allele is sufficient for successful embryonic development and postnatal survival (22). Akt1 is critical for postnatal angiogenesis, because Akt1 is downstream of several proangiogenic and survival factors, including VEGF (23, 24), angiopoietins (25), and sphingosine 1-phosphate (26). The global loss of Akt1 in mice, however, reduces lymphangiogenesis (27) and can reduce (3, 5, 6) or increase (4) angiogenesis depending on the context and model used, thereby supporting the possibility that remaining Akt isoforms compensate for Akt1 loss by phosphorylating its substrates. Therefore, we used an inducible, endothelial-targeted Akt1 deletion mouse model in an effort to minimize long-term compensation and allow for examination of acute, cell-targeted Akt1 loss on vascular development. Endothelial-specific Akt1 loss results in significant retinal vascular defects that, based on proteomic analysis, may be attributed to an improper balance in signaling cascades regulating angiogenesis and vessel remodeling. Moreover, Akt2 appears to play a minimal role in vascular development, because the observed Akt1iECKO retinal defects described herein do not display obvious exacerbation of phenotype when placed on an Akt2-null background. However, we cannot rule out the role of Akt3 in our system, despite very low mRNA and protein levels of this isoform in MLECs (4).
Proper remodeling of the retinal vascular network requires a balance and intricate regulation of Akt signaling. The remodeling behavior is affected by numerous stimuli (i.e., VEGF, flow dynamics, apoptosis, pericyte recruitment), all of which are mediated, in part, through PI3K-Akt signaling. Endothelial-specific Akt1 loss of function significantly increases vessel regression in retinal vascular beds, supporting the prominent role of Akt in developing and maintaining vascular networks. This may occur via an intrinsic EC defect in growth, survival, and/or migration, or, alternatively, through the influence of ECs on mural cell recruitment, all phenotypes potentially influenced by Akt1 and its substrates. Not only does this parallel the effects of pharmacological PI3K inhibition on vessel regression (16) or the loss of PI3K p100α (28), but endothelial-specific Akt overexpression exhibits inverse effects on vascular remodeling (29).
Our phosphoproteomics screen aimed to elucidate the differences in endothelial Akt1 and Akt2 substrate preference to provide insight on the observed retinal phenotypes in the Akt1iECKO mice. Although previous work has illustrated clear examples of Akt isoform-substrate selectivity, the molecular basis for specificity is not yet known (30). In the present study using ECs from different strains of mice, more Akt1-affected substrates were reduced compared with those affected by the loss of Akt2, whereas similar numbers of substrates were equally reduced by the loss of either Akt1 or Akt2. Pathway analyses on the output substrates identify angiogenic proteins that are significantly affected by the loss of Akt1, thus paralleling the retinal phenotypes in Akt1iECKO. As expected, the loss of Akt1 affected substrates that were found to be associated with the PI3K, phosphatase and tensin homolog, and VEGF signaling modules, whereas the loss of Akt2 had negligible effects on known canonical pathways. Substrate differences, however, may reflect the fact that the MLECs used for the analysis express higher levels of Akt1 than Akt2. Nonetheless, despite these expression differences, exclusive Akt1 vs. Akt2 substrates were identified. Collectively, these data indicate that Akt2 activity is negligible for vascular retinal development.
Several of the Akt phosphosites identified from our screen as Akt1-dependent and cardiovascular-associated have previously been described as substrates, thereby validating the proteomic methodology. The phosphoscreen detected eNOS S1176, a well-characterized Akt substrate, as an Akt1-specific substrate (11, 31), and eNOS is the predominant Akt1 substrate necessary for angiogenesis, blood flow, and tissue repair (12). These in vivo data are supported by in vitro data, where Akt1, but not Akt2, optimally phosphorylates eNOS and promotes NO release. However, massive overexpression of Akt2 can rescue the loss of Akt1, implying that the levels of Akt1 are critical for the fidelity of eNOS phosphorylation and NO release. PI3K/Akt-mediated phosphorylation of the Forkhead box (FOXO) subclass of transcription factors has been described to inactivate all 4 FOXO family members, three of which were detected in our screen (FOXO1A, FOXO3A, and FOXO4). Dephosphorylation and subsequent activation of FOXO-dependent transcription stimulates several key cellular aspects, including apoptosis, cell-cycle arrest, and oxidative-stress resistance. FOXO1 activity is critical for development and viability, because global deletion of FOXO1 results in vasculogenesis defects and embryonic lethality (32). Analysis of the FOXO transcriptome suggests FOXO regulates the expression of genes related to angiogenesis and vascular remodeling, including angiopoietins and matrix metalloproteinases (33). FOXO1 has also been studied as a transcriptional effector of angiopoietin1/Akt signaling and related vessel stability, further supporting the role of Akt (via FOXO) in vessel remodeling (34).
Other interesting molecules detected as Akt substrates in the phosphoscreen that may influence vascular function are filamin C (FLNC), vascular endothelial tyrosine phosphatase (PTPRB/VE-PTP) and angiomotin-like protein 1 (AMOTL1). FLNC has been shown to interact with several signaling-related proteins, including SHIP-2 and RasGAP, suggesting FLNC may harness functions related to signal transduction and may play a role in EC migration and trafficking events (35, 36) PTPRB activity appears to be nonessential for initial blood vessel formation but vital for maintenance and vascular remodeling (37, 38). AMOTL1 governs endothelial tip cell formation during sprouting angiogenesis and aspects of junctional stability to mediate paracellular permeability (39, 40). Additional studies characterizing the Akt-dependent phosphorylation of these proteins in the context of growth factor signaling and angiogenic functions are ongoing.
The important roles for each of the identified Akt1-specific substrates highlight the possible contributions of multiple substrates to the observed Akt1iECKO retinal phenotype. Furthermore, although many well-characterized Akt substrates were identified in the phosphoscreen, other known targets did not appear in the final substrate list. This observation can be explained by several reasons. First, our initial analysis used fairly high stringencies, because previously identified substrates (i.e., Sin1, TBC1D4) emerged in the output when we relieve the fold-change requirements (from −2.5- to −2.0-fold directional cutoff). In addition, phosphorylation events are dynamic and spatially regulated, because Akt has been shown to have differential effects on substrate phosphorylation depending on its subcellular localization (41, 42), and its activity is, in turn, regulated by phosphatases, such as protein phosphatase 2A and PH domain and leucine rich repeat protein phosphatase (30). Considering the phosphoscreen-reflected cells under basal serum-grown conditions, agonist stimulation poststarvation may have yielded a larger cohort of known Akt substrates (2). Collectively, this study clearly shows the critical role of Akt1 in angiogenesis and begins to decipher the complexity of Akt isoform selectivity in the endothelium.
Materials and Methods
Mice.
The Akt1−/−, Akt2−/−, and Akt1flox/flox mice were generated as previously described (9, 13, 17). Conditional pups were injected with 50 μg of tamoxifen per day (P1–P3) via i.p. delivery. All mouse experiments were approved by the Institutional Animal Care Use Committee at the Yale School of Medicine.
Phosphoproteomic Analysis of RxRxxS*/T* Substrates.
Immortalized MLECs were grown to 70–80% confluency and harvested in appropriate lysis buffer for each experimental condition. The Akt isoform-specific phosphoproteome of MLEC lysates was characterized using PTMScan technology (Cell Signaling Technology) based on LC/tandem MS, as described previously (19).
Statistics.
Data are expressed as mean ± SEM. Comparisons between groups were made using a two-tailed Student t test or ANOVA with the Bonferroni post hoc test.
Supplementary Material
Acknowledgments
We thank Dr. Morris Birnbaum for the Akt mouse lines (Akt1−/−, Akt2−/−, and Akt1flox/flox). This work was supported by Grants R01 HL64793, R01 HL61371, R01 HL081190, and P01 HL70295 from the National Institutes of Health (to W.C.S.), Grant F32 HL119147 (to M.Y.L.), Grant T32 GM007324 (to A.K.L.) and Grant BA11/00016 from the Instituto de Salud Carlos III and Grant SAF2013-41840-R from the Ministerio de Economía y Competitividad (MINECO) (to M.M.-R.). J.R.-V. is the recipient of a BIOTRACK Postdoctoral Fellowship supported by the European Community’s Seventh Framework Programme and the MINECO (Contract 229673).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408472111/-/DCSupplemental.
References
- 1.Shiojima I, Walsh K. Role of Akt signaling in vascular homeostasis and angiogenesis. Circ Res. 2002;90(12):1243–1250. doi: 10.1161/01.res.0000022200.71892.9f. [DOI] [PubMed] [Google Scholar]
- 2.Zhuang G, et al. Phosphoproteomic analysis implicates the mTORC2-FoxO1 axis in VEGF signaling and feedback activation of receptor tyrosine kinases. Sci Signal. 2013;6(271):ra25. doi: 10.1126/scisignal.2003572. [DOI] [PubMed] [Google Scholar]
- 3.Ackah E, et al. Akt1/protein kinase Balpha is critical for ischemic and VEGF-mediated angiogenesis. J Clin Invest. 2005;115(8):2119–2127. doi: 10.1172/JCI24726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen J, et al. Akt1 regulates pathological angiogenesis, vascular maturation and permeability in vivo. Nat Med. 2005;11(11):1188–1196. doi: 10.1038/nm1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Somanath PR, Chen J, Byzova TV. Akt1 is necessary for the vascular maturation and angiogenesis during cutaneous wound healing. Angiogenesis. 2008;11(3):277–288. doi: 10.1007/s10456-008-9111-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ju R, et al. Angiopoietin-2 secretion by endothelial cell exosomes: Regulation by the phosphatidylinositol 3-kinase (PI3K)/Akt/endothelial nitric oxide synthase (eNOS) and syndecan-4/syntenin pathways. J Biol Chem. 2014;289(1):510–519. doi: 10.1074/jbc.M113.506899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Lorenzo A, Fernández-Hernando C, Cirino G, Sessa WC. Akt1 is critical for acute inflammation and histamine-mediated vascular leakage. Proc Natl Acad Sci USA. 2009;106(34):14552–14557. doi: 10.1073/pnas.0904073106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang ZZ, et al. Protein kinase B alpha/Akt1 regulates placental development and fetal growth. J Biol Chem. 2003;278(34):32124–32131. doi: 10.1074/jbc.M302847200. [DOI] [PubMed] [Google Scholar]
- 9.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem. 2001;276(42):38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- 10.Chen WS, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15(17):2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fulton D, et al. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399(6736):597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schleicher M, et al. The Akt1-eNOS axis illustrates the specificity of kinase-substrate relationships in vivo. Sci Signal. 2009;2(82):ra41. doi: 10.1126/scisignal.2000343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wan M, et al. Loss of Akt1 in mice increases energy expenditure and protects against diet-induced obesity. Mol Cell Biol. 2012;32(1):96–106. doi: 10.1128/MCB.05806-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Benedito R, et al. The notch ligands Dll4 and Jagged1 have opposing effects on angiogenesis. Cell. 2009;137(6):1124–1135. doi: 10.1016/j.cell.2009.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Bray SJ. Notch signalling: A simple pathway becomes complex. Nat Rev Mol Cell Biol. 2006;7(9):678–689. doi: 10.1038/nrm2009. [DOI] [PubMed] [Google Scholar]
- 16.Ueda K, et al. Differential effects of LY294002 and wortmannin on neurons and vascular endothelial cells in the rat retina. Pharmacol Rep. 2013;65(4):854–862. doi: 10.1016/s1734-1140(13)71066-1. [DOI] [PubMed] [Google Scholar]
- 17.Cho H, et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta) Science. 2001;292(5522):1728–1731. doi: 10.1126/science.292.5522.1728. [DOI] [PubMed] [Google Scholar]
- 18.Lawlor MA, Alessi DR. PKB/Akt: A key mediator of cell proliferation, survival and insulin responses? J Cell Sci. 2001;114(Pt 16):2903–2910. doi: 10.1242/jcs.114.16.2903. [DOI] [PubMed] [Google Scholar]
- 19.Moritz A, et al. Akt-RSK-S6 kinase signaling networks activated by oncogenic receptor tyrosine kinases. Sci Signal. 2010;3(136):ra64. doi: 10.1126/scisignal.2000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peng XD, et al. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang ZZ, et al. Dosage-dependent effects of Akt1/protein kinase Balpha (PKBalpha) and Akt3/PKBgamma on thymus, skin, and cardiovascular and nervous system development in mice. Mol Cell Biol. 2005;25(23):10407–10418. doi: 10.1128/MCB.25.23.10407-10418.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dummler B, et al. Life with a single isoform of Akt: Mice lacking Akt2 and Akt3 are viable but display impaired glucose homeostasis and growth deficiencies. Mol Cell Biol. 2006;26(21):8042–8051. doi: 10.1128/MCB.00722-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morales-Ruiz M, et al. Vascular endothelial growth factor-stimulated actin reorganization and migration of endothelial cells is regulated via the serine/threonine kinase Akt. Circ Res. 2000;86(8):892–896. doi: 10.1161/01.res.86.8.892. [DOI] [PubMed] [Google Scholar]
- 24.Gerber HP, et al. Vascular endothelial growth factor regulates endothelial cell survival through the phosphatidylinositol 3′-kinase/Akt signal transduction pathway. Requirement for Flk-1/KDR activation. J Biol Chem. 1998;273(46):30336–30343. doi: 10.1074/jbc.273.46.30336. [DOI] [PubMed] [Google Scholar]
- 25.Kim I, et al. Angiopoietin-1 regulates endothelial cell survival through the phosphatidylinositol 3′-Kinase/Akt signal transduction pathway. Circ Res. 2000;86(1):24–29. doi: 10.1161/01.res.86.1.24. [DOI] [PubMed] [Google Scholar]
- 26.Lee MJ, et al. Akt-mediated phosphorylation of the G protein-coupled receptor EDG-1 is required for endothelial cell chemotaxis. Mol Cell. 2001;8(3):693–704. doi: 10.1016/s1097-2765(01)00324-0. [DOI] [PubMed] [Google Scholar]
- 27.Zhou F, et al. Akt/Protein kinase B is required for lymphatic network formation, remodeling, and valve development. Am J Pathol. 2010;177(4):2124–2133. doi: 10.2353/ajpath.2010.091301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Graupera M, et al. Angiogenesis selectively requires the p110alpha isoform of PI3K to control endothelial cell migration. Nature. 2008;453(7195):662–666. doi: 10.1038/nature06892. [DOI] [PubMed] [Google Scholar]
- 29.Sun JF, et al. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc Natl Acad Sci USA. 2005;102(1):128–133. doi: 10.1073/pnas.0403198102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toker A. Achieving specificity in Akt signaling in cancer. Adv Biol Regul. 2012;52(1):78–87. doi: 10.1016/j.advenzreg.2011.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dimmeler S, et al. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399(6736):601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 32.Furuyama T, et al. Abnormal angiogenesis in Foxo1 (Fkhr)-deficient mice. J Biol Chem. 2004;279(33):34741–34749. doi: 10.1074/jbc.M314214200. [DOI] [PubMed] [Google Scholar]
- 33.Potente M, et al. Involvement of Foxo transcription factors in angiogenesis and postnatal neovascularization. J Clin Invest. 2005;115(9):2382–2392. doi: 10.1172/JCI23126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Daly C, et al. Angiopoietin-1 modulates endothelial cell function and gene expression via the transcription factor FKHR (FOXO1) Genes Dev. 2004;18(9):1060–1071. doi: 10.1101/gad.1189704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sverdlov M, Shinin V, Place AT, Castellon M, Minshall RD. Filamin A regulates caveolae internalization and trafficking in endothelial cells. Mol Biol Cell. 2009;20(21):4531–4540. doi: 10.1091/mbc.E08-10-0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Valle-Pérez B, et al. Filamin B plays a key role in vascular endothelial growth factor-induced endothelial cell motility through its interaction with Rac-1 and Vav-2. J Biol Chem. 2010;285(14):10748–10760. doi: 10.1074/jbc.M109.062984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bäumer S, et al. Vascular endothelial cell-specific phosphotyrosine phosphatase (VE-PTP) activity is required for blood vessel development. Blood. 2006;107(12):4754–4762. doi: 10.1182/blood-2006-01-0141. [DOI] [PubMed] [Google Scholar]
- 38.Dominguez MG, et al. Vascular endothelial tyrosine phosphatase (VE-PTP)-null mice undergo vasculogenesis but die embryonically because of defects in angiogenesis. Proc Natl Acad Sci USA. 2007;104(9):3243–3248. doi: 10.1073/pnas.0611510104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bratt A, et al. Angiomotin belongs to a novel protein family with conserved coiled-coil and PDZ binding domains. Gene. 2002;298(1):69–77. doi: 10.1016/s0378-1119(02)00928-9. [DOI] [PubMed] [Google Scholar]
- 40.Zheng Y, et al. Angiomotin-like protein 1 controls endothelial polarity and junction stability during sprouting angiogenesis. Circ Res. 2009;105(3):260–270. doi: 10.1161/CIRCRESAHA.109.195156. [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez E, McGraw TE. Insulin-modulated Akt subcellular localization determines Akt isoform-specific signaling. Proc Natl Acad Sci USA. 2009;106(17):7004–7009. doi: 10.1073/pnas.0901933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosner M, Hanneder M, Freilinger A, Hengstschläger M. Nuclear/cytoplasmic localization of Akt activity in the cell cycle. Amino Acids. 2007;32(3):341–345. doi: 10.1007/s00726-007-0509-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.