Significance
In 2010, a large outbreak of poliomyelitis involving 445 laboratory-confirmed cases occurred in the Republic of Congo. The 47% case-fatality rate was unusually high. Outbreak severity was attributed to low immunization coverage but vaccine-mediated immunity against the outbreak virus was never investigated. We isolated the poliovirus type 1 responsible for the outbreak and located its evolutionary origins to Southeast Asia. Fatal cases showed evidence for previous vaccination against polioviruses and the outbreak virus was refractive against neutralization by monoclonal and vaccine-derived antibodies. This pointed to immune escape contributing to the severity of the outbreak. Sustained vaccination regimens in polio-free regions, together with clinical and environmental poliovirus surveillance will be necessary to combat antigenetically variant polioviruses in the poliomyelitis eradication endgame.
Abstract
In 2010, a large outbreak of poliomyelitis with unusual 47% lethality occurred in Pointe Noire, Republic of Congo. Vaccine-mediated immunity against the outbreak virus was never investigated. A wild poliovirus 1 (WPV1) isolated from a fatal case (termed PV1-RC2010) showed a previously unknown combination of amino acid exchanges in critical antigenic site 2 (AgS2, VP1 capsid protein positions 221SAAL→221PADL). These exchanges were also detected in an additional 11 WPV1 strains from fatal cases. PV1-RC2010 escaped neutralization by three different mAbs relevant for AgS2. Virus neutralization was tested in sera from fatal cases, who died before supplementary immunization (n = 24), Gabonese recipients of recent oral polio vaccination (n = 12), routinely vaccinated German medical students (n = 34), and German outpatients tested for antipoliovirus immunity (n = 17) on Vero, human rhabdomyosarcoma, and human epidermoid carcinoma 2 cells. Fatal poliomyelitis cases gave laboratory evidence of previous trivalent vaccination. Neutralizing antibody titers against PV1-RC2010 were significantly lower than those against the vaccine strain Sabin-1, two genetically distinct WPV1s isolated in 1965 and 2010 and two genetically distinct vaccine-derived PV strains. Of German vaccinees tested according to World Health Organization protocols, 15–29% were unprotected according to their neutralization titers (<1:8 serum dilution), even though all were protected against Sabin-1. Phylogenetic analysis of the WPV1 outbreak strains suggested a recent introduction of virus progenitors from Asia with formation of separate Angolan and Congolese lineages. Only the latter carried both critical AgS2 mutations. Antigenetically variant PVs may become relevant during the final phase of poliomyelitis eradication in populations with predominantly vaccine-derived immunity. Sustained vaccination coverage and clinical and environmental surveillance will be necessary.
The Global Polio Eradication Initiative has led to a highly efficient reduction in the global incidence of poliomyelitis (1). To date, only Nigeria, Afghanistan, and Pakistan have not been able to achieve interruption of wild poliovirus (WPV) circulation (2). In the final phase of poliomyelitis eradication, regions with WPV circulation coexist with regions from which the virus has been eradicated but where hygienic conditions prevail that favor poliovirus (PV) spread (1–3). In 2012, 223 WPV cases were reported globally, with only 6 cases in nonendemic countries. In 2013, however, there was an increase to 416 cases, including 256 from nonendemic countries (4). Recent examples of large outbreaks in previously poliomyelitis-free regions include the 2010–2011 outbreak in the Republic of Congo (ROC) with 445, the 2010 outbreak in Tajikistan with 463, and the 2011 outbreak in China with 21 laboratory-confirmed WPV1 cases, respectively (5–7).
The 2010 ROC outbreak differed from other recent outbreaks in its unusually high case-fatality rate (CFR). Of the 445 confirmed cases, 390 occurred in the city of Pointe Noire (5, 8, 9), with a CFR of 47% and a median age of 20 y for patients with paralytic disease (5, 8, 10, 11). For comparison, the CFR in the 2010 Tajikistan outbreak was only about 6% and 44% of cases of acute flaccid paralysis (AFP) were observed in children below 5 y of age (6). It has been proposed that the severity of the ROC outbreak resulted from breaches in vaccination coverage in adults, combined with the underreporting of mild cases (11, 12). However, nonpolio AFP rates in the ROC were higher than those in the neighboring countries Angola, Gabon, and the Democratic Republic of Congo (DRC) in 2010 (8.3% versus 3.9%, 5.2%, and 6.3%, respectively) (13), questioning weaker clinical surveillance and severe underreporting. Critically, in an interview-based assessment, 72% of 149 polio cases from the ROC outbreak recalled prior uptake of at least one dose of trivalent oral polio vaccine (tOPV), and 49% reported at least three doses (8). In another independent investigation of 28 laboratory-confirmed cases, 7 (25%) had more than three documented tOPV doses, and 15 (54%) had at least one dose (5). These data seem to be in conflict with current knowledge on the efficacy of OPV, as even low levels of vaccine-derived immunity should confer protection against paralytic disease (14, 15). Of note, PVs are not known to undergo any relevant antigenic drift to escape vaccine-mediated immunity, such as, e.g., influenza A viruses.
In this study, we isolated the WPV1 strain from the ROC outbreak and analyzed factors potentially associated with the higher CFR by molecular and immunologic tools.
Results
Laboratory-Confirmed Cases.
Appropriate samples to study preexisting immunity were limited in number, as they had to originate from before supplementary immunization activity (SIA). From 13 deceased patients, rectal swabs, throat swabs, and serum samples were available. PV1 RNA was detected by RT-PCR in 12 of 13 rectal swabs. The remaining patient yielded PV1 RNA in RT-PCR in a cerebrospinal fluid sample. Because of the clear clinical picture of bulbar poliomyelitis within an ongoing outbreak, the 13 cases yielding PV1 RNA were classified as laboratory-confirmed. Table S1 summarizes RT-PCR results for the 13 laboratory-confirmed cases.
Exclusion of Alternative Outbreak-Associated Etiologies.
In all 13 cases a large range of viruses was tested by (RT)-PCR to exclude possible additional outbreak-associated etiologies, including herpes-, paramyxo-, picorna-, flavi-, alpha-, bunya-, adeno-, corona-, influenza-, reo-, calici- and astroviruses (details are provided in SI Methods). None of those viruses were detected in more than one sample, suggesting PV1 as the etiology of fatalities.
Neutralizing Antibodies.
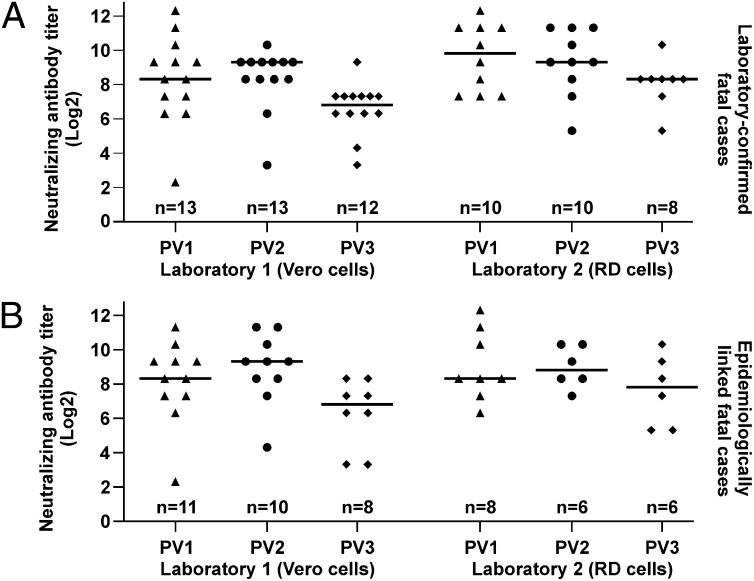
Neutralizing antibodies against Sabin-1, -2, and -3 PV reference strains were determined in sera from all 13 laboratory-confirmed cases (mean age = 19.2 y; SD = 3.4 y). Strikingly, almost all cases showed titers against all three PV types, suggestive of previous exposure to PV2 and PV3 before infection with the WPV1 outbreak strain (Fig. 1A). To further investigate possible preexisting immunity in the outbreak, sera from 11 additional fatal cases were tested (mean age = 19.6 y; SD = 4.8 y). These cases occurred during the same early phase of the outbreak and were classified as cases of bulbar poliomyelitis on clinical basis and epidemiological linkage. Only serum samples were available from these cases, preventing retrospective laboratory-confirmation of PV1. As shown in Fig. 1B, also in those epidemiologically linked cases, antibodies against PV1, PV2, and PV3 were seen. Of note, exhaustion of material prevented full titration against PV1–3 for some sera.
Fig. 1.
Neutralizing antibody titers against Sabin-1, -2, and -3 in fatal poliomyelitis cases from the RC2010 outbreak. (A) Thirteen laboratory-confirmed fatal cases. (B) Eleven additional fatal cases with clinical diagnosis of bulbar poliomyelitis and epidemiological linkage. Differences in the number of investigated sera are due to exhaustion of samples.
The above results were obtained by two separate laboratories. The first was a nonspecialized diagnostic laboratory using a neutralization test (NT) protocol with a PV challenge dose of 500 tissue culture infectious dose50 (TCID50) per assay (laboratory 1 in Fig. 1 and Tables S2–S7). This protocol can lead to slightly lower NT results, hence a more conservative estimate of preexisting immunity in the context of this study. To exclude any technical issues with these results, all tests were performed in parallel by the World Health Organization (WHO)-accredited German National Reference Center for Poliomyelitis and Enteroviruses (NRC PE) (laboratory 2 in Fig. 1 and Tables S2–S7). Even though deviations of antibody titers were observed between laboratories for some sera, the overall results were in agreement and confirmed the finding of widespread and strong concomitant anti-PV2 and -PV3 titers in fatal cases.
Virus Characterization.
The complete genome of a WPV1 strain involved in the outbreak was sequenced directly from a rectal swab sample of a fatal patient (patient code PN45). The complete genome contained 7,443 nucleotides (nt) excluding the poly-A tail. All PV genes were detected in analogous position and identical length compared with Sabin-1 and Mahoney (7,441 and 7,440 nt in genome length, respectively) (Fig. S1A). VP1 capsid protein genes were fully sequenced from individual rectal swab samples from another 11 cases (patient codes are listed in Table S1). Nucleotide sequences diverged by no more than 0.7%, indicating that the viruses obtained from the 12 patients constituted a single PV1 strain. A unique combination of mutations was observed in the discontinuous antigenic site (AgS) 2 within the viral capsid of all 12 WPV1s from fatal cases (domain 2a, VP1 amino acid residues 221SAAL changed to 221PADL). No other PV1 carrying these combined mutations was identified in GenBank (www.ncbi.nlm.nih.gov/genbank). Besides the domain 2a, no other unique exchanges were observed in AgS1–4 (Fig. S1B). For further investigation, virus isolation was attempted from all available rectal swabs. Three of the 13 rectal swabs yielded virus isolates, all identical to the sequences determined directly from clinical material. An isolate hereafter termed PV1-RC2010 obtained from the rectal swab of patient PN45 was used to investigate the mutations in domain 2a. Comparative neutralization studies were done using three different mAbs targeting the epitope. Two of those antibodies termed 14D2E9 and 12(237) were known to either affect domain 2a or 2b, as evidenced by mutations in cognate escape variants (refs. 16–18 and Table S8). Both mAbs efficiently neutralized WPV1 Mahoney, but completely failed to neutralize PV1-RC2010. Another mAb termed 14(427), known to bind a composite epitope in AgS2, conferred particularly strong neutralizing activity on a domain 2a escape mutant, but was completely escaped by PV1-RC2010 (Table S8). A structural representation of the AgS2 is given in Fig. S1C.
Immune Escape.
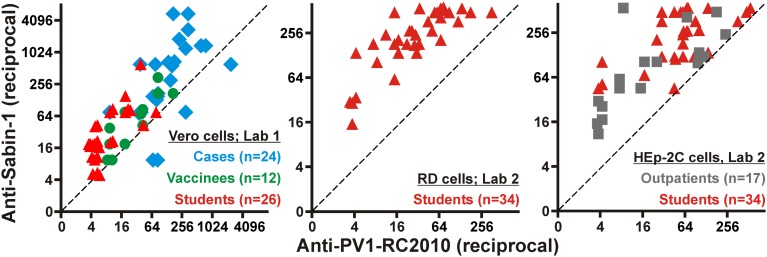
Because these results suggested a potential for PV1-RC2010 to escape antibody-dependent serum neutralization, NTs were done comparing the OPV strain Sabin-1 with PV1-RC2010. These studies involved all 24 sera from fatal outbreak cases (mean age = 19.4 y; SD = 4.0 y) and an ethnically similar control group of 12 healthy Gabonese individuals (mean age = 39.1 y; SD = 14.9 y). All Gabonese volunteers had a confirmed history of OPV within 6 mo before sampling. Another control group was composed of sera from 34 healthy German medical students (mean age = 24.5 y; SD = 3.4 y). Additionally, 17 sera from German outpatients sent to the NRC PE laboratory for confirmation of anti-PV immune status were tested (mean age = 29.4 y; SD = 18.9 y). In Germany, OPV was used before 1998 and trivalent inactivated polio vaccine (IPV) thereafter, so that almost all German vaccines received complete OPV at least once and IPV more recently, in the case of all medical students upon beginning their studies. No details on the individual vaccination histories of the German vaccinees were available. Robustness of PV1-RC2010 against serum neutralization was seen in all cohorts independent of whether Vero, human rhabdomyosarcoma (RD), or human epidermoid carcinoma 2 (HEp-2) cell lines were used for NTs (Fig. 2). All sera showed titers against Sabin-1 on at least one cell line that exceeded the threshold titer considered to be protective (generally above 1:8) (15), proving that the robustness of PV1-RC2010 was not due to low overall anti-PV1 antibody levels. Individual antibody titers against all viruses and cell lines used in this study are detailed for all involved laboratories in Tables S2–S7.
Fig. 2.
Serum neutralization against Sabin-1 compared with PV1-RC2010 on different cell culture systems. Endpoint serum dilutions still showing neutralization of PV1-RC2010 (x axis) or Sabin-1 (y axis) on Vero, RD, or HEp-2 cells. Cohorts are color-coded. Values are plotted with 10% jitter to prevent superimposition of datum points. Datum points still superimposing were shifted manually until they were distinguishable.
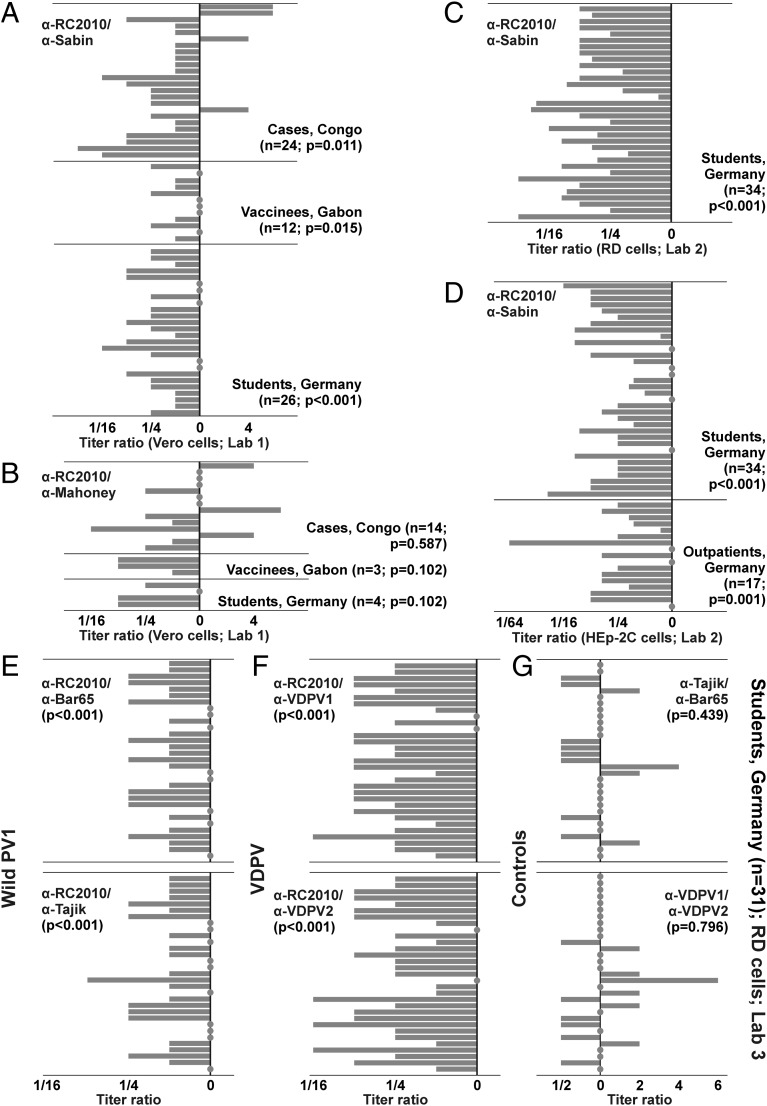
In NTs conducted on Vero cells in laboratory 1, 11 of 24 fatal cases, 3 of 12 Gabonese vaccinees, and 16 of 26 German medical students had titers against PV1-RC2010 at least fourfold lower than against Sabin-1 (48%; Wilcoxon signed rank test, P < 0.05 for all cohorts; Fig. 3A). Of note, 4 of 24 sera from fatal cases showed four- to eightfold higher titers against PV1-RC2010 than against Sabin-1. It is likely that these four sera were sampled at a later time during individual courses of infection, allowing a specific antibody response against PV1-RC2010, whereas all other cases died before this point. Unfortunately, no details on the clinical histories of the fatal cases were available. Among 26 sera from medical students included in this experiment, 8 (31%) showed undetectable titers already at the entry serum dilution of 1:5, another 7 sera showed endpoint titers of 1:5 (Tables S2–S7). Up to 15 students (58%) thus had to be considered unprotected against PV1-RC2010. Sera from 14 Congolese cases, 3 Gabonese vaccinees, and 4 medical students were also tested against WPV1 Mahoney. Limited volumes prevented testing of additional sera. Titer differences between Mahoney and PV1-RC2010 were smaller than those between Sabin-1 and PV1-RC2010, but still largely comparable to the latter. This included three of the four sera showing higher titers against PV1-RC2010 than against the vaccine viruses Sabin-1 (OPV) or Mahoney (IPV) and a Gabonese vaccinee fully protected against Sabin-1 and Mahoney but with a titer below the starting dilution of 1:10 against PV1-RC2010 (Tables S2–S7). Irrespective of sample cohorts, 9 of 21 sera included in this experiment showed at least fourfold lower titers against PV1-RC2010 than against Mahoney (43%; P > 0.05 for all cohorts; Fig. 3B). Sera from medical students were used for confirmatory experiments at the NRC PE according to WHO protocols, using RD instead of Vero cells and using eight additional sera in comparison with laboratory 1 (total n = 34). On RD cells, 30 of 34 sera showed at least fourfold lower titers against PV1-RC2010 than against Sabin-1 (88%; P < 0.001; Fig. 3C). Five students had to be considered unprotected based on titers below 1:8 (15%). These sera were also below the 1:8 threshold on Vero cells in laboratory 1, suggesting comparable sensitivity between cell lines. These results were additionally confirmed at the NRC PE on HEp-2C cells for the same sera from medical students (n = 34) and for sera from German outpatients (n = 17). Again, PV1-RC2010 was significantly less efficiently neutralized by these sera than Sabin-1 (P < 0.001 and P = 0.001, respectively; Fig. 3D). Twenty-three of the 34 sera from medical students and 11 of the 17 sera from outpatients showed at least fourfold lower neutralizing titers against PV1-RC2010 (67%), confirming the results observed on Vero and RD cells. All five sera showing no detectable titer on RD cells also showed no detectable titer in HEp-2 cells, again suggesting comparable sensitivity of cell lines. Five of 17 outpatients (29%) had to be considered unprotected against PV1-RC2010 (titers <1:8). To compare if the lower neutralizability of PV1-RC2010 was unique among WPV1, 31 of the sera from the medical students were used for confirmatory NTs at the Russian WHO-accredited Poliovirus Reference Laboratory (laboratory 3 in Tables S2–S7). These NTs included two additional WPV1 strains. A strain isolated in 1965 (19), termed Bar65, was used because it was genetically as distinct from the vaccine strains Sabin-1 and Mahoney, as PV1-RC2010 (17.8/17.7% versus 18.0/17.8% genomic nucleotide sequence distance, respectively) and equally distinct from PV1-RC2010 (17.7%). The second WPV1 was isolated from the 2010 Tajikistan outbreak (6). This strain, termed Tajik, was selected because it was genetically more closely related to PV1-RC2010 with 11.7% genomic sequence distance than to Sabin-1 and Bar65 (18.6% and 18.4%, respectively). Neither of the two additional WPV1 strains carried the unique AgS2a exchanges of PV1-RC2010 (Fig. S1B). Antibody titers against PV1-RC2010 were significantly lower than those against Bar65 and Tajik (P < 0.001). Nine (29%) and 7 (23%) partially overlapping sera showed at least fourfold lower antibody titers. No serum showed higher titers against PV1-RC2010 than against Bar65 or Tajik (Fig. 3E). Antibody titers against each of the three WPV1 strains were lower than those against Sabin-1, but PV1-RC2010 consistently proved most robust against neutralization, yielding lower titers than either of the other two WPV1 strains in 20 of 31 sera (65%; Fig. S2). To compare whether antibody titer differences were also observed against vaccine-derived PV (VDPV) strains, two VDPV strains were tested. One strain, termed VDPV1, was isolated in 1999 in Russia (20); the other strain, termed VDPV2, was isolated in 2010 in Tajikistan during AFP surveillance. These VDPV were selected because VDPV1 differed considerably from Sabin-1 with 8.5% genomic sequence distance, whereas VDPV2 differed by only 0.7% from Sabin-1. VDPV1 and VDPV2 were equally distinct from PV1-RC2010 with 18.3% and 18.1% genomic sequence distance, respectively. Titer deviations between PV1-RC2010 and both VDPV strains were comparable to those observed between PV1-RC2010 and Sabin-1 (P < 0.001; Fig. 3F). At least fourfold lower titers against PV1-RC2010 were observed in 24 (77%) and 23 (74%) of sera, respectively. Finally, no significant differences in serum neutralizing antibody titers existed neither between the WPV1 strains Bar65 and Tajik nor between the two VDPV strains (P > 0.05; Fig. 3G). The genetic relationships between all compared viruses are illustrated in Fig. S3.
Fig. 3.
Deviations between serum neutralization against PV1 strains compared with PV1-RC2010. (A) Relative reciprocal neutralization titers on Vero cells against PV1-RC2010 (“α-RC2010”) divided by those against Sabin-1 (“α-Sabin”). (B) Titers on Vero cells against PV1-RC2010 and against Mahoney. (C) Confirmation of partial results from A on RD cells. (D) Confirmation of results from A and C on HEp-2C cells. (E) Titers against PV1-RC2010 compared with those against WPV1 Bar65 and Tajik; (F) against PV1-RC2010 and against two VDPV type 1 strains; and (G) against the two WPV1 and VDPV strains, respectively. All on RD cells for 31 sera from the student cohort shown in A–D. Filled circles represent null deviations.
Virus Origin.
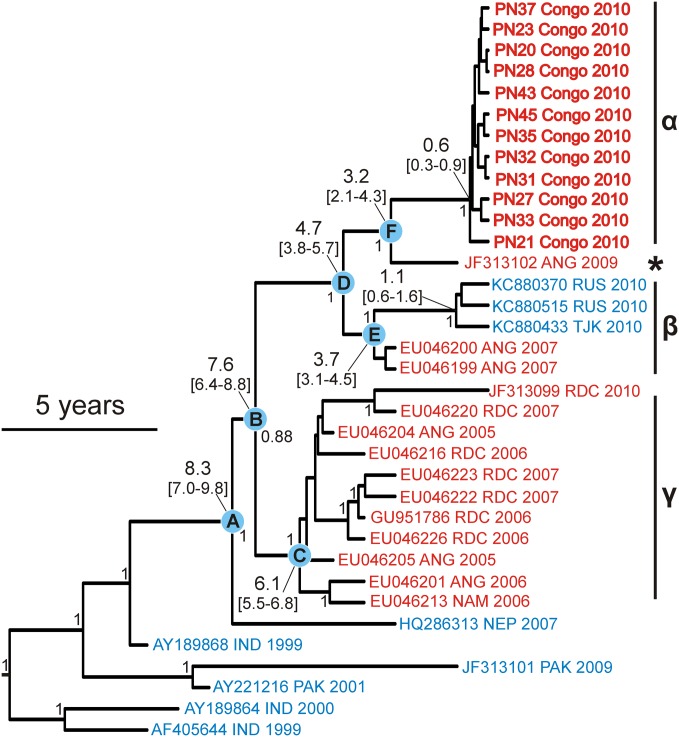
To analyze the evolutionary origin of the outbreak WPV1, a time-calibrated phylogenetic analysis of the VP1 gene was conducted (details are provided in SI Methods). The resulting phylogeny was highly robust (Fig. 4 and Fig. S4). The outbreak WPV1 (clade α) were related to an Angolan strain isolated in 2009, 1 y before the ROC outbreak (marked with a star in Fig. 4), which was unrelated to other Angolan and central African strains (clades β and γ). The common ancestor of these viruses was projected to have existed 2.1–4.3 y before the outbreak (Fig. 4, root point F). Interestingly, this Angolan virus showed the A223D mutation but lacked the S221P mutation (Fig. S1B). The next closest relatives were viruses that caused AFP outbreaks in Central African countries during 2005–2007 and in the Russian Federation during 2010. The Tajik WPV1 used in this study representing the 2010 outbreaks in the Russian Federation also carried a mutation at VP1 position 223 (A223E), but lacked the S221P exchange (Fig. S1B). The outbreak viruses and the 2009 Angolan strain shared an Asian origin, confirming conjectures communicated earlier on the basis of unpublished data (8, 21).
Fig. 4.
WPV1 complete VP1 gene phylogeny including the RC2010 viruses. (Scale bar: 5 y; indicates evolutionary timing). Numbers at tree nodes indicate posterior probabilities (only values above 0.8 are shown). Numbers connected with a line to nodes A to F indicate node heights and 95% confidence intervals. African viruses are in red and Asian in blue. Viruses from the RC2010 outbreak are given in bold with patient codes. An expanded version of this phylogeny is shown in Fig. S4.
Discussion
We provide laboratory evidence for WPV1 as the cause of the lethal poliomyelitis outbreak in Pointe Noire, ROC in 2010. The detection of closely related WPV1 sequences in almost all investigated cases, the absence of clues to any other epidemic virus from a large spectrum tested, and the rather typical clinical appearance of bulbar poliomyelitis in this outbreak made it unlikely that any other pathogen would have caused the fatalities.
We show that the outbreak WPV1 is robust against neutralization by monoclonal and vaccine-derived antibodies. This may have been a decisive factor contributing to fatalities which seem to have occurred in previously vaccinated individuals. The serological finding of strong concomitant PV2 and PV3 neutralizing antibodies indicates preexisting immunity against heterologous PV types that would have been reactivated by the outbreak WPV1 infection. The impressive height of titers against PV2 and PV3 could hardly be explained by cross-stimulation against heterologous viruses in immunologically naïve individuals, as heterologous titers in such cases would be considerably lower than homologous titers (22–24). There is a remote possibility that anti-PV3 titers might have been stimulated by unnoticed prior exposure to WPV3 circulating in other African regions, such as Nigeria and the neighboring DRC (2, 25). However, this fails to explain concomitant titers against WPV2 that was eradicated from global circulation before 1999. It must be mentioned that circulation of VDPV type 2 has been documented in Nigeria since 2005 and in DRC since 2008 (26, 27), which could theoretically explain anti-PV2 titers in the fatal cases. However, highly consistent antibody titers against all three PV types and reports of at least partial vaccination coverage in Pointe Noire (5, 8) make prior immunization with tOPV the most likely explanation for the antibody titers observed in fatal cases. This does not exclude inefficient immune protection as the necessary and most likely cause of the outbreak (5, 9, 28). However, it is intriguing that the ROC outbreak was associated with a much higher CFR than similar outbreaks in regions where WPV circulation had been interrupted and a pronounced incidence in adult patients occurred due to vaccination gaps, such as in Albania in 1996 (29), Namibia in 2000 (5) or Tajikistan in 2010 (6).
A unique combination of mutations in the PV1-RC2010 AgS2a prompted us to investigate neutralization properties conferred by mAbs. The mutation at VP1 position 221, involving the change of a hydrophilic serine residue to a hydrophobic and conformationally different proline residue, could severely affect antibody binding (30). In a comprehensive study on PV1 antigenic structure, a virus mutant carrying a similar substitution (S221L) showed immune escape against the broadest range of all tested AgS2-specific mAbs (31). In that study, viruses escaped from all but one of the tested mAbs, but this antibody was evaded by viruses carrying mutations in an adjacent site (A223V) (31). This is exactly where PV1-RC2010 carries an additional mutation (a hydrophobic alanine to a hydrophilic aspartic acid residue, A223D) that has never appeared together with the 221 mutation in any publicly recorded PV strain to date. Positions 221 and 223 form a highly exposed part of AgS2a, one of the most prominent neutralizing epitopes of PV1 (summarized in ref. 30). We have shown that PV1-RC2010 escapes a mAb that directly binds this site, and also one that most probably acts indirectly by binding outside the AgS2a. The fact that the latter mAb’s cognate escape variant 1005 had a mutation in domain 2b (VP2 P170S), which is inaccessible to antibodies, suggests immune escape via a stabilizing effect of the mutation on the adjacent domain 2a conformation, as suggested earlier (30). PV1-RC2010 might contain additional mutations that also confer such stabilizing effects, although we could not predict such changes upon analysis of the full genomic sequence. Of note, PV1-RC2010 was able to completely escape from even a mAb with particularly strong neutralizing effects on the domain 2a escape mutant. This suggests a capability to escape a wide range of specificities present in polyclonal serum antibodies. On a cautionary note, it should be mentioned that antibodies directed against AgS2 were not found to predominate in sera of human vaccinees (32). However, the escape mutant tested in that study did not represent the unique constellation of mutations present in PV1-RC2010. Conclusive proof for the impact of the observed AgS2 exchanges on the capacity to escape serum neutralization will rely on the investigation of multiple engineered AgS2 mutants. However, our comparisons of several WPV1 and VDPV strains suggested pronounced and widespread robustness of PV1-RC2010 against neutralization. This explains why a higher fraction of patients than in other outbreaks primarily involving adults (5, 6, 29) could progress to severe disease. In contrast to strains causing those outbreaks, PV1-RC2010 may be able to escape the residual protection left by incomplete OPV particularly in young adults who have not been exposed to wild type virus during their lifetime. Lower potential for neutralization by serum antibodies causes a relatively higher availability of active virus in the blood that can enter the CNS. Together with the known higher lethality of poliomyelitis in adult patients, these specific factors may have contributed to the predominance of severe disease in the ROC 2010 outbreak. Interestingly, a recent investigation found evidence that factors elevating virus dose might have affected disease severity in the Pointe Noire outbreak, including crowded living conditions and the use of covered wells as water sources (28). Hypothetically, the WPV1 may even have circumvented the enteric route by aspiration of contaminated water in some cases. These conditions might have provided just the necessary additional virus dose to overcome a protection threshold relatively decreased against PV1-RC2010, providing an explanation for higher outbreak-associated morbidity in Pointe Noire versus surrounding communities.
The threat posed by an antigenetically variant WPV such as PV1-RC2010 is very hard to project. Based on neutralization titers done according to WHO protocols, 15–29% of German vaccinees receiving IPV since 1998 were considered unprotected against PV1-RC2010, with concomitant titers against Sabin-1 in the group of medical students suggesting immunization coverage better than average (33). Incomplete vaccination coverage in regions with cessation of WPV circulation is a common situation at the present time, but an unnatural situation in human populations in historical times. Variant viruses whose fitness is normally impaired in populations with robust immunity could evolve in this situation. Examples include a severe poliomyelitis outbreak among adult Canadian Eskimos in 1949 with an unusually high CFR following (re)introduction of a WPV (34). Similarly, a WPV3 with deviations of its major AgS caused a poliomyelitis outbreak in Finland during 1984–1985 after its introduction to a population vaccinated with low potency IPV since almost 30 y (35). Another example includes the reemergence of type 2 VDPVs as a cause of AFP in countries with suboptimal vaccination coverage such as in Nigeria (26). Finally, 13 of 463 laboratory-confirmed polio cases (2.8%) from the 2010 Tajikistan WPV1 outbreak occurred in apparently preimmunized individuals, confirming our observations (6). The more pronounced differences in the AgS2a of PV1-RC2010 compared with the WPV1s from the Tajikistan outbreak may have facilitated a higher fraction of poliomyelitis in preimmunized individuals in the ROC outbreak. Antigenic drift in PV has been suggested sporadically (36–39), but the epidemiologic and pathogenic consequences may only become apparent with the eradication of WPV in sight. High-potency IPV and OPV were effective against the divergent WPV3 causing the 1984 Finland outbreak (summarized in ref. 40). Similarly, four SIAs using monovalent OPV1 and bivalent OPV1/3 stopped the ROC 2010 outbreak (21), providing a robust population immunity that likely exceeded the relatively lower protection against PV1-RC2010. Sustained vaccination regimens potentially involving both IPV and OPV in polio-free regions aided by timely detection of WPV1 reintroductions through environmental and clinical surveillance will thus be necessary to combat the spread of variant viruses in the poliomyelitis eradication endgame (41).
Methods
Clinical Specimens.
Details on clinical samples, ethics, and viral diagnostics are provided in SI Methods.
Cell Culture.
Virus isolation and neutralization assays were done on Vero, RD, and HEp-2 cells. Details are provided in SI Methods.
Phylogenetic Analysis.
Bayesian phylogenetic analyses were done as described in SI Methods.
Supplementary Material
Acknowledgments
We thank P. Yaba, P. Engandja, A. Délicat, M. Caron, G. Maganga, M. Eschbach-Bludau, M. Frank, U. Skali-Lami, S. Brünink, and T. Bleicker for technical assistance. We also thank the Ministry of Health of the Republic of Congo and the World Health Organization. This work was supported by European Union Seventh Framework Programme Contracts 223498 (European Management Platform for Emerging and Re-Emerging Infectious Disease Entities, EMPERIE) and 228292 (European Virus Archive, EVA); and through an infrastructural grant from the German Federal Ministry of Education and Research through the German Centre for Infection Research (to C.D.) and the Government of Gabon, Total-Fina-Elf Gabon, and the French Ministère des Affaires Etrangères et Européennes (to E.M.L.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The nucleotide sequences reported in this paper have been deposited in the GenBank database (accession nos. JF838278–JF838290).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1323502111/-/DCSupplemental.
References
- 1.Nathanson N, Kew OM. From emergence to eradication: The epidemiology of poliomyelitis deconstructed. Am J Epidemiol. 2010;172(11):1213–1229. doi: 10.1093/aje/kwq320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Centers for Disease Control and Prevention (CDC) Progress toward interrupting wild poliovirus circulation in countries with reestablished transmission—Africa, 2009-2010. MMWR Morb Mortal Wkly Rep. 2011;60(10):306–311. [PubMed] [Google Scholar]
- 3.Patriarca PA. Research and development and the polio eradication initiative: Too much, too soon...too little, too late? Clin Infect Dis. 2012;55(10):1307–1311. doi: 10.1093/cid/cis720. [DOI] [PubMed] [Google Scholar]
- 4. The Global Polio Eradication Initiative (2014) Wild Poliovirus (WPV) cases. Available at www.polioeradication.org/Dataandmonitoring/Poliothisweek.aspx. Accessed May 21, 2014.
- 5.Patel MK, et al. An outbreak of wild poliovirus in the Republic of Congo, 2010-2011. Clin Infect Dis. 2012;55(10):1291–1298. doi: 10.1093/cid/cis714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yakovenko ML, et al. The 2010 outbreak of poliomyelitis in Tajikistan: Epidemiology and lessons learnt. Euro Surveill. 2014;19(7):20706. doi: 10.2807/1560-7917.es2014.19.7.20706. [DOI] [PubMed] [Google Scholar]
- 7.Luo HM, et al. Identification and control of a poliomyelitis outbreak in Xinjiang, China. N Engl J Med. 2013;369(21):1981–1990. doi: 10.1056/NEJMoa1303368. [DOI] [PubMed] [Google Scholar]
- 8.Centers for Disease Control and Prevention (CDC) Poliomyelitis outbreak—Republic of the Congo, September 2010-February 2011. MMWR Morb Mortal Wkly Rep. 2011;60(10):312–313. [PubMed] [Google Scholar]
- 9.Roberts L. Infectious disease. Polio outbreak breaks the rules. Science. 2010;330(6012):1730–1731. doi: 10.1126/science.330.6012.1730. [DOI] [PubMed] [Google Scholar]
- 10.Grard G, et al. Type 1 wild poliovirus and putative enterovirus 109 in an outbreak of acute flaccid paralysis in Congo, October-November 2010. Euro Surveill. 2010;15(47):19723. doi: 10.2807/ese.15.47.19723-en. [DOI] [PubMed] [Google Scholar]
- 11.Le Menach A, et al. Poliomyelitis outbreak, Pointe-Noire, Republic of the Congo, September 2010-February 2011. Emerg Infect Dis. 2011;17(8):1506–1509. doi: 10.3201/eid1708.110195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford CL. No mystery about the polio outbreak. Science. 2011;331(6018):674. doi: 10.1126/science.331.6018.674-b. [DOI] [PubMed] [Google Scholar]
- 13. World Health Organization (2014) Acute flaccid paralysis (AFP) surveillance data. http://apps.who.int/immunization_monitoring/en/diseases/poliomyelitis/case_count.cfm. Accessed May 30, 2014.
- 14.Pallansch MA, Roos RP. Enteroviruses: Polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th Ed. Vol 1. Philadelphia: Lippincott-Raven; 2001. pp. 724–743. [Google Scholar]
- 15.Diedrich S, Claus H, Schreier E. Immunity status against poliomyelitis in Germany: determination of cut-off values in International Units. BMC Infect Dis. 2002;2:2. doi: 10.1186/1471-2334-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Osterhaus AD, et al. Monoclonal antibodies to polioviruses. Comparison of intratypic strain differentiation of poliovirus type 1 using monoclonal antibodies versus cross-absorbed antisera. Intervirology. 1983;20(2-3):129–136. doi: 10.1159/000149381. [DOI] [PubMed] [Google Scholar]
- 17.Minor PD, Ferguson M, Evans DM, Almond JW, Icenogle JP. Antigenic structure of polioviruses of serotypes 1, 2 and 3. J Gen Virol. 1986;67(Pt 7):1283–1291. doi: 10.1099/0022-1317-67-7-1283. [DOI] [PubMed] [Google Scholar]
- 18.Herremans MM, et al. Evaluation of a poliovirus-binding inhibition assay as an alternative to the virus neutralization test. Clin Diagn Lab Immunol. 1997;4(6):659–664. doi: 10.1128/cdli.4.6.659-664.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Korotkova EA, et al. Retrospective analysis of a local cessation of vaccination against poliomyelitis: A possible scenario for the future. J Virol. 2003;77(23):12460–12465. doi: 10.1128/JVI.77.23.12460-12465.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cherkasova EA, et al. Long-term circulation of vaccine-derived poliovirus that causes paralytic disease. J Virol. 2002;76(13):6791–6799. doi: 10.1128/JVI.76.13.6791-6799.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kew O. Reaching the last one per cent: Progress and challenges in global polio eradication. Curr Opinion Virol. 2012;2(2):188–198. doi: 10.1016/j.coviro.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Sabin AB. Transitory appearance of type 2 neutralizing antibody in patients infected with type 1 poliomyelitis virus. J Exp Med. 1952;96(1):99–106. doi: 10.1084/jem.96.1.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Danes L, Sladká E, Hancil J, Horácek J. Cross reactivity among human enterovirus serotypes as revealed by microneutralization assay technique. J Hyg Epidemiol Microbiol Immunol. 1988;32(2):233–238. [PubMed] [Google Scholar]
- 24.Uytdehaag FG, et al. Human peripheral blood lymphocytes from recently vaccinated individuals produce both type-specific and intertypic cross-reacting neutralizing antibody on in vitro stimulation with one type of poliovirus. J Immunol. 1985;135(5):3094–3101. [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention (CDC) Progress toward interruption of wild poliovirus transmission—worldwide, January 2010-March 2011. MMWR Morb Mortal Wkly Rep. 2011;60(18):582–586. [PubMed] [Google Scholar]
- 26.Wassilak S, et al. Outbreak of type 2 vaccine-derived poliovirus in Nigeria: Emergence and widespread circulation in an underimmunized population. J Infect Dis. 2011;203(7):898–909. doi: 10.1093/infdis/jiq140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Minor PD. The polio-eradication programme and issues of the end game. J Gen Virol. 2012;93(Pt 3):457–474. doi: 10.1099/vir.0.036988-0. [DOI] [PubMed] [Google Scholar]
- 28.Gregory CJ, et al. Investigation of elevated case-fatality rate in poliomyelitis outbreak in Pointe Noire, Republic of Congo, 2010. Clin Infect Dis. 2012;55(10):1299–1306. doi: 10.1093/cid/cis715. [DOI] [PubMed] [Google Scholar]
- 29.Prevots DR, et al. Outbreak of paralytic poliomyelitis in Albania, 1996: High attack rate among adults and apparent interruption of transmission following nationwide mass vaccination. Clin Infect Dis. 1998;26(2):419–425. doi: 10.1086/516312. [DOI] [PubMed] [Google Scholar]
- 30.Minor PD. Antigenic structure of picornaviruses. Curr Top Microbiol Immunol. 1990;161:121–154. doi: 10.1007/978-3-642-75602-3_5. [DOI] [PubMed] [Google Scholar]
- 31.Page GS, et al. Three-dimensional structure of poliovirus serotype 1 neutralizing determinants. J Virol. 1988;62(5):1781–1794. doi: 10.1128/jvi.62.5.1781-1794.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rezapkin G, et al. Repertoire of antibodies against type 1 poliovirus in human sera. J Virol Methods. 2010;169(2):322–331. doi: 10.1016/j.jviromet.2010.07.037. [DOI] [PubMed] [Google Scholar]
- 33.Reinheimer C, Friedrichs I, Rabenau HF, Doerr HW. Deficiency of immunity to poliovirus type 3: A lurking danger? BMC Infect Dis. 2012;12:24. doi: 10.1186/1471-2334-12-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peart AF, et al. An outbreak of poliomyelitis in Canadian Eskimos in wintertime. Can J Public Health. 1949;40(10):405–419. [PubMed] [Google Scholar]
- 35.Hovi T, et al. Outbreak of paralytic poliomyelitis in Finland: Widespread circulation of antigenically altered poliovirus type 3 in a vaccinated population. Lancet. 1986;1(8495):1427–1432. doi: 10.1016/s0140-6736(86)91566-7. [DOI] [PubMed] [Google Scholar]
- 36.Huovilainen A, Kinnunen L, Ferguson M, Hovi T. Antigenic variation among 173 strains of type 3 poliovirus isolated in Finland during the 1984 to 1985 outbreak. J Gen Virol. 1988;69(Pt 8):1941–1948. doi: 10.1099/0022-1317-69-8-1941. [DOI] [PubMed] [Google Scholar]
- 37.Huovilainen A, et al. Evolution of poliovirus during an outbreak: Sequential type 3 poliovirus isolates from several persons show shifts of neutralization determinants. J Gen Virol. 1987;68(Pt 5):1373–1378. doi: 10.1099/0022-1317-68-5-1373. [DOI] [PubMed] [Google Scholar]
- 38.Hovi T, Lindholm N, Savolainen C, Stenvik M, Burns C. Evolution of wild-type 1 poliovirus in two healthy siblings excreting the virus over a period of 6 months. J Gen Virol. 2004;85(Pt 2):369–377. doi: 10.1099/vir.0.19518-0. [DOI] [PubMed] [Google Scholar]
- 39.Shulman LM, et al. Molecular and antigenic characterization of a highly evolved derivative of the type 2 oral poliovaccine strain isolated from sewage in Israel. J Clin Microbiol. 2000;38(10):3729–3734. doi: 10.1128/jcm.38.10.3729-3734.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morrison EG, Embil JA. Poliomyelitis in North America: The disease is not dead yet. CMAJ. 1987;137(12):1085–1087. [PMC free article] [PubMed] [Google Scholar]
- 41.Ehrenfeld E, Modlin J, Chumakov K. Future of polio vaccines. Expert Rev Vaccines. 2009;8(7):899–905. doi: 10.1586/erv.09.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.