Significance
It is unknown how (or if) the chromatin landscape responds to transcription stalling to signal rescue of arrested RNA polymerase II (RNAPII). This study identifies a chromatin response to DNA damage-induced RNAPII arrest, the rapid deubiquitylation of histone H2B. This response is an important step for fine-tuning the chromatin landscape to allow the occurrence of DNA repair while avoiding excess RNAPII degradation. Our finding sheds light on the rescue of stalled RNAPII in the context of chromatin, illuminating the involvement of H2B deubiquitylation in coordinating different rescue pathways. Importantly, defects in RNAPII rescue are a phenotype of the human disease Cockayne syndrome, which is characterized by photosensitivity, neurological degeneration, and premature aging.
Keywords: chromatin, nucleosome, epigenetics, transcription-coupled repair
Abstract
Histone H2B monoubiquitylation plays an important role in RNA polymerase II (RNAPII) elongation. Whether this modification responds to RNAPII stalling is not yet known. We report that both yeast and human cells undergo a rapid and significant H2B deubiquitylation after exposure to UV irradiation. This deubiquitylation occurs concurrently with UV-induced transcription arrest and is significantly reduced in a DNA damage-bypassing RNAPII yeast mutant. Consistent with these results, yeast deubiquitylases Ubp8 and Ubp10 are associated with the RNAPII complex. Moreover, simultaneous deletion of Ubp8 and Ubp10 leads to a lack of H2B deubiquitylation after UV exposure. Consequently, nucleotide excision repair at an actively transcribed gene locus is decreased, whereas UV-induced RNAPII degradation is increased in ubp8Δubp10Δ mutant cells. These results indicate that eukaryotic cells respond to RNAPII arrest by deubiquitylating H2B to coordinate DNA repair and RNAPII degradation.
Elongating RNAPII frequently stalls at barriers on DNA. If RNAPII is irreversibly arrested by DNA damage such as UV-induced photoproducts, the stalled RNAPII is highly dangerous to the cell. Indeed, arrested polymerases not only block passage of subsequent RNA polymerases, they prevent exposure of damaged sites to DNA repair proteins. Cells have evolved a transcription-coupled repair (TCR) system to rapidly rescue stalled RNAPII. As a subpathway of nucleotide excision repair (NER), TCR uses the stalled RNAPII as the triggering signal for proteins such as Cockayne syndrome proteins A and B (CSA and CSB) in humans or the Rad26 protein in budding yeast (1). The recruited CSB or Rad26 can function as bridges to core NER proteins at damaged sites, to remove DNA damage, and allow continuation of transcription. However, TCR cannot always rescue stalled RNAPII, and an alternative rescue pathway is required. Such a pathway is polyubiquitylation and subsequent degradation of RNAPII to allow exposure of the damaged sites (2). Along with the two rescue pathways, stalled RNAPII is a signal for two factors, Rad26 and RNAPII degradation factor 1 (Def1) (3), to regulate TCR and degradation, respectively. Intriguingly, yeast cells preferentially rescue RNAPII with TCR, rather than destroying stalled transcription machinery (3).
Packaging of DNA in chromatin represents a barrier for factors that interact with DNA. Not surprisingly, when UV lesions are located in highly compact chromatin, access of global genomic repair (GGR; another subpathway of NER) is strongly inhibited by histones (4). Previous studies have led to the “access-repair-restore” model to explain how GGR factors access damage by first rearranging chromatin and subsequently restoring chromatin structure (4). A variety of mechanisms, such as histone modifications and ATP-dependent chromatin remodeling, have been shown to stimulate the chromatin remodeling step during NER (5). TCR occurs in actively transcribed genes and it is less understood how chromatin structure affects this pathway. Although histones are generally depleted by the transcription machinery in the promoters of active genes (6), elongating RNAPII does not always disassemble nucleosomes in the coding regions (7). In some active genes, transcription elongation through chromatin is achieved by acetylating histones with little or no net nucleosome loss (8). These studies suggest that in coding regions of active genes that exhibit high nucleosome occupancy, TCR may still be hindered by histones. Indeed, compelling evidence has indicated that chromatin remodeling is required for efficient TCR. For example, it has long been known that the human TCR protein CSB is a member of the SWI2/SNF2 chromatin remodeling family (9), and has been shown to reposition nucleosomes in vitro, in an ATP-dependent manner (10). Furthermore, it was recently shown that the ATP-dependent chromatin remodeling activity of CSB is required to rescue the high UV-sensitivity phenotype of CSB− cells (10).
Monoubiquitylation of H2B occurs at K123 in Saccharomyces cerevisiae and is catalyzed by the sequential activities of yeast E2 ubiquitin conjugase Rad6 and E3 ubiquitin ligase Bre1; this is a bulky modification and is functionally connected with transcription elongation (11). Histone H2B monoubiquitylation (H2Bub) is highly enriched in the coding regions of actively transcribed RNAPII genes and less enriched in promoters, but absent from other genomic loci such as telomeres and centromeres (12). Consistent with this observation, Rad6 travels with elongating RNAPII via the Paf1 transcription elongation complex (13). Mutations in components of the Paf1 complex, or disruption of the serine 5 CTD kinase for RNAPII (Kin28), abolish H2Bub (11). Once ubiquitin is added to H2B, H2Bub functions in stimulating transcription elongation through chromatin, likely by facilitating activity of the histone chaperone FACT (14). Another function of H2Bub in transcription is that it improves nucleosome reassembly behind elongating RNAPII (15). Several studies have now demonstrated that yeast cells lacking H2Bub (e.g., H2BK123R or H2BK123A) exhibit reduced nucleosomal occupancy and stability throughout the genome, whereas cells with higher H2Bub levels (ubp8Δ or ubp8Δubp10Δ) show the opposite phenotypes (16, 17). In addition, ubiquitylation of H2B is highly dynamic, undergoing sequential ubiquitylation and deubiquitylation cycles in a tightly controlled manner to fine-tune the chromatin landscape at different stages of transcription (18). Deubiquitylation of H2B is also important for optimal gene expression. For example, H2B deubiquitylase Ubp8 travels with elongating RNAPII and is activated at the early elongation stage to adjust H2Bub levels for recruitment of RNAPII kinase Ctk1 (19).
Because the establishment of H2Bub is tightly linked to RNAPII, and this modification plays a direct role in transcription elongation, we wondered how H2Bub responds to RNAPII stalling and participates in the rescue of stalled RNAPII. Therefore, we used UV light as the DNA damaging source to induce strong RNAPII stalling. We found that UV irradiation induces a rapid deubiquitylation of H2B in both yeast and human cells, and this induction is indeed triggered by stalled RNAPII. We also found that H2B deubiquitylation facilitates TCR while inhibiting excess RNAPII degradation. Taken together, these data reveal a new mechanism at the chromatin level by which eukaryotic cells coordinate the DNA repair and RNAPII degradation pathways to rescue DNA damage-arrested RNAPII.
Results
UV Irradiation Induces Decreased Global H2Bub in Both Yeast and Mammalian Cells.
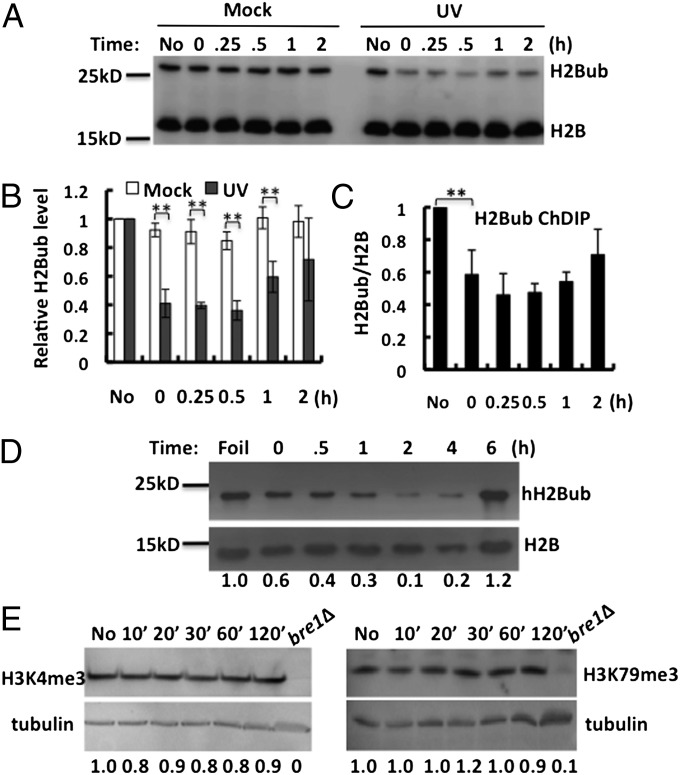
We first analyzed global H2Bub levels in intact WT yeast cells (strain Y131) at different times after UV irradiation. Mock-treated cells and UV-irradiated cells were processed in parallel by the same procedure. H2B and ubiquitylated H2B were detected simultaneously using an anti-Flag antibody, as H2B is Flag tagged. As expected, two bands are detected in WT cells and the top band is absent in the bre1Δ mutant but greatly increased in the ubp8Δubp10Δ mutant (Fig. S1A), which indicates that the top band is indeed H2Bub. Importantly, a significant reduction in H2Bub occurs almost immediately after UV irradiation (Fig. 1A; UV, 0-h lane), during the time when cell processing is conducted (∼5 min). The low level of H2Bub after UV irradiation is maintained in cells for ∼30 min and gradually recovers after 60 min, when a significant amount of cyclobutane pyrimidine dimers (CPDs) have been repaired in the genome (Fig. S2). Conversely, mock treatment does not cause any notable change in H2Bub (Fig. 1A). To investigate the impact of UV damage on H2Bub at specific genes, H2Bub levels were measured on the actively transcribed RNA polymerase II subunit B150 (RPB2) gene using a chromatin double immunoprecipitation (ChDIP) assay (20). The ChDIP data demonstrate that H2Bub levels on the RPB2 gene are also decreased and subsequently restored after UV treatment (Fig. 1C).
Fig. 1.
UV induces decrease in H2Bub in both yeast and human cells. (A) Representative Western blot of yeast monoubiquitylated H2B (Upper) and bulk H2B (Lower) at different times after mock treatment or UV irradiation (150 J/m2). Blots were probed with anti-Flag antibody. (B) Quantification of H2Bub levels shown in A, relative to the levels of no treatment (No). In each case, the unmodified H2B signal was used as loading control. Each value represents the mean ±1 SD for three independent experiments. **P < 0.02. (C) ChDIP analysis of H2Bub levels on RPB2 in response to UV irradiation. (D) Western blot of H2Bub from human fibroblast cells (hH2Bub) irradiated with UV light (12 J/m2) and allowed to repair for different times. Foil samples were collected from cells covered with aluminum foil during UV exposure. Blots were probed with human H2Bub and H2B antibodies, and numbers below the gel lanes represent H2Bub levels normalized to the H2B signal. (E) Yeast WCEs were isolated from either unirradiated cells (No) or following different times after exposure to UV light (150 J/m2). H3K4me3 (Left) and H3K79me3 (Right) were detected by Western blotting with specific antibodies. Tubulin, detected with tubulin specific antibody, was used as the loading control. WCEs isolated from bre1Δ without UV are added as a negative control to show the specificity of the methylation antibodies.
Human histone H2B is also monoubiquitylated at lysine residue 120 by the human E2 (hHR6A/hHR6B) and E3 (RNF20/RNF40) enzymes (11), and the functions of H2Bub in transcription appear to be highly conserved from yeast to human cells. To determine if the UV-induced H2Bub response also occurs in human cells, we examined histones from human fibroblasts at different times after UV irradiation using an hH2Bub-specific antibody. As shown in Fig. 1D, UV damage also induces a significant reduction in hH2Bub over mock-irradiated cells after early repair times.
The function of H2Bub in trans-histone cross-talk has been well documented in previous studies, with H2Bub being shown to be a prerequisite for H3 K4 and K79 trimethylation (H3K4me3 and H3K79me3, respectively) (21). Therefore, we investigated whether the UV-induced change in H2Bub levels lead to a decrease in H3K4me3 and H3K79me3. Using specific antibodies, we find that neither H3K4me3 nor H3K79me3 is affected by UV irradiation (Fig. 1E), suggesting that a reduction in H2Bub by UV damage does not affect preexisting H3K4 or K79 trimethylation levels.
UV Damage-Induced RNAPII Stalling Triggers the H2Bub Decrease.
We then investigated how UV damage to DNA might induce the H2B deubiquitylation response. Because UV irradiation does not remove ubiquitin from isolated H2Bub proteins in vitro, but purified Ubp8 protein does (Fig. S1B), we reasoned that there is a mechanism that senses UV damage and activates an H2B deubiquitylation response. The tight interplay between H2Bub and elongating RNAPII suggests that UV-stalled RNAPII may be the intermediate that connects UV damage with the H2Bub response. To test this, we used RPB2 as a model and examined the potential coincidence between transcription stalling and the H2Bub response. RPB2 mRNA exhibits a sequential reduction and recovery trend after UV irradiation (Fig. 2A). Similar to changes in H2Bub, the decrease is detected almost immediately after UV irradiation, although a more significant decrease in RPB2 mRNA occurs at 15 min after UV exposure. This delay, relative to the change in H2Bub, is probably due to the time window between RNAPII stalling and degradation of preexisting mRNA. The subsequent restoration of RPB2 mRNA after UV occurs on a similar time scale as H2Bub recovery (Fig. 2A).
Fig. 2.
UV-induced H2Bub response is triggered by stalled RNAPII. (A) RPB2 mRNA levels after UV. Wild-type yeast cells were irradiated with 150 J/m2 UV, and aliquots taken at indicated times. Northern blot analysis was performed to detect RPB2 mRNA level. Numbers below the gel lanes represent RPB2 transcript levels normalized to rRNA. (B, Upper) Western blot analysis of H2Bub levels in wild-type RNAPII (WT) and UV damage-bypassing RNAPII (rpb1 E1103G) cells. Cells were irradiated with the indicated UV doses and harvested 15 min after irradiation. Western blots were performed using an anti-H2B antibody to detect both H2Bub and H2B. (B, Lower) Quantification of H2Bub levels in WT and rpb1 E1103G cells from three independent experiments. Values represent the mean ±1 SD. *P < 0.05. (C) Western blot analysis of H2Bub levels after treatment with 6-AU. Wild-type yeast cells were treated with indicated doses of 6-AU for 30 min using a 1-M 6-AU stock solution (dissolved in NH4OH). Cells without any treatment (No) or treated with NH4OH only (0) were used as the controls to indicate that any change in H2Bub was caused by 6-AU.
To more directly investigate the impact of RNAPII stalling on H2Bub, we used a yeast mutant in which RNAPII has an increased ability to bypass UV damage, thus stalling less frequently after UV irradiation. The E1103 residue of the catalytic RNAPII subunit RPB1 is located at the catalytic center and controls transcription fidelity by restricting the incorporation of incorrect nucleotides (22). An E1103G substitution results in improved transcription efficiency, at the cost of reduced transcription fidelity (22). When facing templates containing UV damage, the E1103G mutant RNAPII significantly increases translesion transcription by allowing incorporation of mispaired nucleotides, which greatly benefits UV survival in cells lacking NER activity (23). Therefore, we compared H2Bub levels in WT RPB1 and RPB1 E1103G strains upon UV irradiation. As expected, a UV dose-dependent decrease in H2Bub is seen in WT cells (Fig. 2B). Intriguingly, this decrease is less pronounced in the RPB1 E1103G mutant, especially at the higher UV dose (Fig. 2B), indicating that the increased ability of the mutant RNAPII to bypass UV damage indeed reduces the H2Bub response.
We also wondered if RNAPII stalling caused by other mechanisms leads to the H2Bub response. Hence, we treated WT yeast cells for 30 min with varying doses of the transcription elongation inhibitor 6-azauracil (6-AU) to induce transcription arrest. We found that 6-AU treatment leads to a decrease in H2Bub in a dose-dependent manner (Fig. 2C). Thus, collectively, these findings indicate that RNAPII stalling caused by either DNA damage or nucleotide starvation stimulates loss of H2Bub.
Ubp8 and Ubp10 Interact with RNAPII and Are Responsible for the UV-Induced H2Bub Decrease.
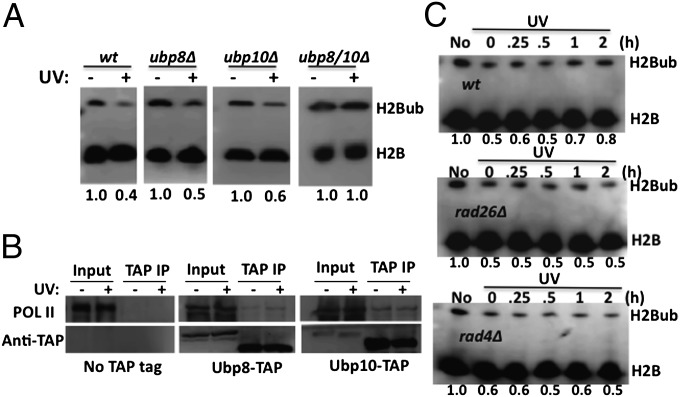
H2Bub is highly dynamic and regulated by both ubiquitylating and deubiquitylating enzymes. Taking into consideration that a decrease in H2Bub occurs almost immediately after UV irradiation in both yeast and human cells (Fig. 1), it is more likely that this change is mediated by increased deubiquitylation during the earliest time points. To determine whether Ubp8 and/or Ubp10 play a role in the UV-induced deubiquitylation, we analyzed the H2Bub levels after UV irradiation of WT, ubp8Δ, ubp10Δ, and ubp8Δubp10Δ (ubp8/10Δ) yeast strains. Notably, the UV-induced H2Bub decrease is abolished in the ubp8Δubp10Δ double mutant (Fig. 3A). In addition, this decrease still occurs in ubp8Δ and ubp10Δ single mutants (Fig. 3A), but to a lesser extent than in WT cells. These data indicate that the UV-induced H2Bub decrease is indeed a deubiquitylation process performed by both Ubp8 and Ubp10.
Fig. 3.
UV-induced H2Bub response is mediated by RNAPII-associated Ubp8 and Ubp10. (A) The UV-induced H2Bub response is absent in ubp8Δubp10Δ cells. Yeast cells were either mock treated (−) or UV irradiated (+; 150 J/m2), and WCEs were isolated from WT, ubp8Δ, ubp10Δ, and ubp8Δ10Δ, and subjected to Western blotting using an anti-Flag antibody. Independent experiments were repeated at least three times with similar results. (B) Ubp8 and Ubp10 proteins associate with RNAPII. Ubp8 and Ubp10 were purified from Ubp8-TAP and Ubp10-TAP strains, respectively. Western blotting was performed using anti-RNAPII and anti-TAP antibodies. The protein A domain of the TAP tag was removed by TEV protease during the purification process, which led to the faster migration of Ubp8 and Ubp10 in TAP IP samples. (C) H2Bub recovery after UV irradiation is dependent on NER. Yeast WT (BY4741), rad26Δ mutant, and rad4Δ mutant cells were UV irradiated, and aliquots were taken at the indicated times. WCEs were subjected to Western blotting using an anti-H2B antibody. Numbers below each panel indicate the relative H2Bub levels.
Because Ubp8 and Ubp10 are responsible for the rapid H2B deubiquitylation upon UV irradiation, we investigated how they may respond to UV damage. Previously, it was shown that Ubp8 physically associates with RNAPII in the absence of DNA damage to regulate transcription (19). This physical interaction provides a potential explanation for the rapid response of Ubp8 to UV damage, because UV-arrested RNAPII may signal its associated Ubp8 immediately. Therefore, we performed pull-down experiments using either a Ubp8-TAP or Ubp10-TAP strain with IgG beads (24). We found that RNAPII copurifies with both Ubp8 and Ubp10, and is not present in a control strain where neither enzyme is TAP tagged (Fig. 3B), indicating that both Ubp8 and Ubp10 physically interact with RNAPII. Additionally, UV irradiation does not affect the association of Ubp8 or Ubp10 with RNAPII (Fig. 3B), indicating that both Ubp8 and Ubp10 are associated with RNAPII before UV irradiation and their interaction is not affected by UV damage.
We also examined the dependence of H2Bub recovery on DNA repair of UV damage, using the NER mutants rad26Δ and rad4Δ. Rad26 is required for TCR in yeast, and Rad4 (an ortholog to human XPC) recognizes helix-distorting DNA damage in the genome and initiates GGR (25). Notably, deletion of either Rad26 or Rad4 genes does not affect the initial H2Bub reduction following UV exposure (Fig. 3C), indicating that the loss of H2Bub occurs before NER. Intriguingly, no clear recovery of H2Bub levels is observed in either the rad26Δ or rad4Δ mutant cells even after 2-h incubation (Fig. 3C). Taken together, these data suggest that NER is not required for the initial H2Bub loss, although both subpathways of NER are required for the subsequent recovery of H2Bub.
H2B Deubiquitylation Is Important for Coordinating TCR and Degradation of Stalled RNAPII.
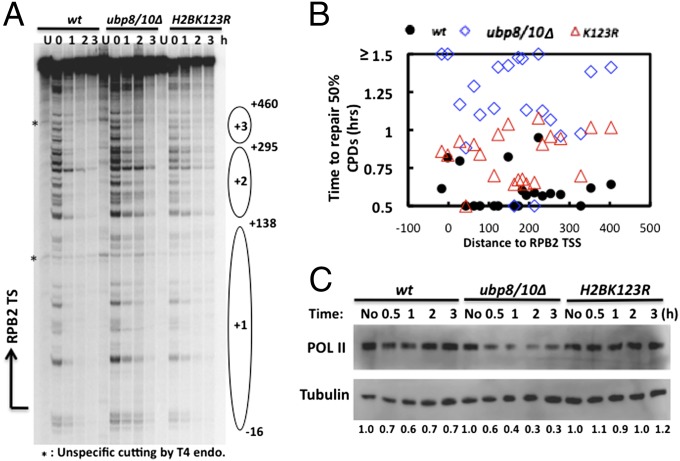
Histone H2B deubiquitylation following RNAPII stalling indicates this response may participate in the rescue of stalled RNAPII. Because TCR is a major pathway for the rescue of stalled RNAPII, we measured TCR directly by examining repair of UV-induced CPDs in the transcribed strand (TS) of the transcriptionally active RPB2 gene at single-nucleotide resolution using a method described previously (26). As TCR occurs over time, there will be fewer CPDs in the TS, resulting in fewer single-strand breaks caused by digestion with T4 endonuclease V (T4 endo V); this is reflected on DNA sequencing gels by weaker bands at individual CPD sites following increased repair. As shown in Fig. 4 A and B, TCR occurs rapidly in WT cells, with most CPDs removed within 1 h. Importantly, repair in the ubp8Δubp10Δ mutant is much slower, because the average time required to repair 50% of the CPDs at each site in the mutant is significantly longer than in WT (Fig. 4B). Additionally, TCR is impaired in an H2BK123R mutant, which has no H2Bub, although the repair deficiency is not as severe as in ubp8Δubp10Δ cells. Together, these results indicate that both deubiquitylation and ubiquitylation of H2B are important for proficient TCR in the RPB2 gene.
Fig. 4.
Lack of H2B deubiquitylation leads to TCR deficiency and excess RNAPII degradation. (A) Representative sequencing gel showing CPDs along the TS of RPB2 at different repair times. Arrow on the left represents the transcription start site (TSS), and ovals represent nucleosome positions (with +1 being the first nucleosome in ORF region). Nucleotide positions are assigned relative to the transcription start site of RPB2. (B) Plot showing the time required to repair 50% of the initial CPDs at individual sites in WT, ubp8Δubp10Δ, and H2BK123R cells. Sites repaired slowly (T0.5 ≥ 1.5 h) are shown at the same level on the graph. Abscissa values are nucleotide positions relative to RPB2 TSS. (C) Yeast cells (WT, ubp8Δubp10Δ, H2BK123R) were UV irradiated (150 J/m2 in SC medium), and aliquots taken at indicated times. Western blotting was performed to detect RNAPII levels.
An alternative rescue pathway for stalled RNAPII is proteasomal degradation of the polymerase. Therefore, we examined degradation of RNAPII in WT, ubp8Δubp10Δ, and H2BK123R cells. As shown in Fig. 4C, ∼40% of RNAPII is degraded by 1 h after UV irradiation, and polymerase levels recover at later times in WT cells. In ubp8Δubp10Δ cells, however, RNAPII degradation increases, with no clear recovery, during the duration of our repair experiments (Fig. 4C). In contrast, only a small fraction of RNAPII is degraded in H2BK123R cells following UV irradiation. These results suggest a positive role for H2Bub in stimulating degradation of stalled RNAPII. Consistent with the altered TCR and RNAPII degradation, we found that both H2BK123R and ubp8Δubp10Δ cells show significant UV sensitivity (Fig. S3).
The impact of H2B deubiquitylation on GGR was also examined in the nontranscribed strand (NTS) of RPB2 as well as the TS and NTS of the repressed GAL1 gene. As shown in Fig. S4, repair of the NTS in both RPB2 and GAL1, and TS in GAL1, is strikingly reduced in the ubp8ubp10Δ cells. This repair deficiency is further supported by the finding that repair of CPDs in the genome overall is impaired in this double mutant (Fig. S2), indicating that H2B deubiquitylation is important for both TCR and GGR.
H2B Deubiquitylation Is Important for Nucleosome Disruption During Repair.
To elucidate the mechanism of H2Bub inhibition of both TCR and GGR in ubp8Δubp10Δ mutant cells, we examined the role of H2Bub in histone eviction during NER. Initially, we examined the presence of nucleosomes in the coding region of RPB2 by ChIP analysis before UV irradiation. The data demonstrate that H2B occupancy in the RPB2 ORF region (see schematic in Fig. 5A) is significantly higher than the GAL1 upstream activation sequence, which is nucleosome-free (27), and slightly higher than the nucleosome-loaded GAL1 promoter region (Fig. S5). This observation suggests that nucleosomes are indeed present in the RPB2 ORF region, which is consistent with a previous study using yeast genome-wide nucleosome mapping (28).
Fig. 5.
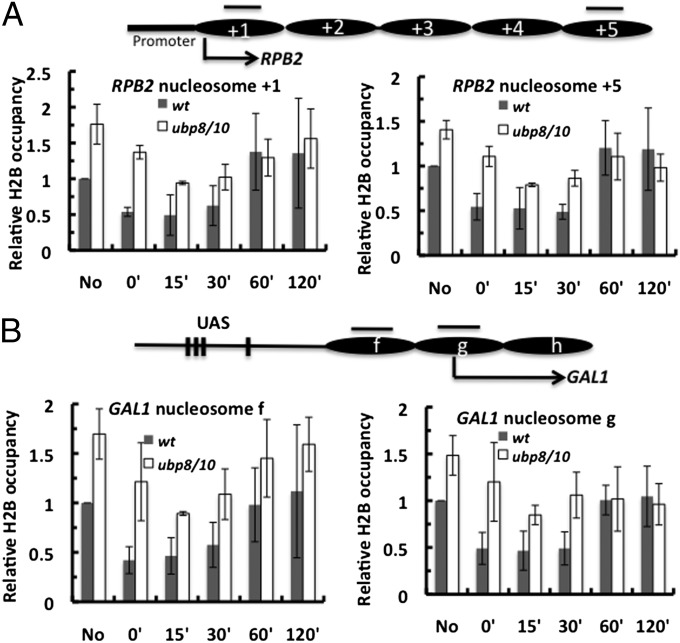
Lack of H2B deubiquitylation inhibits nucleosome disruption during NER. (A) ChIP analysis of H2B occupancy in the RPB2 gene locus. The upper diagram represents nucleosome occupancy in RPB2 ORF mapped by Jiang et al. (28). Arrow and ovals are the same as described in Fig. 4A. Black bars represent PCR amplification regions. Yeast cells (WT and ubp8Δubp10Δ) were irradiated with 200 J/m2 UV. DNA precipitated by the antibody was analyzed by quantitative PCR. Data represent the mean ±1 SD of three independent experiments. (B) ChIP analysis of H2B occupancy during NER in the repressed GAL1 promoter region. The upper diagram represents nucleosome positions (ovals) and nucleosome designations mapped by Li et al. (38).
For postirradiation, we modified the traditional ChIP procedure as described by Sarkar et al. (29) to allow analysis of histone occupancy at specific chromatin loci containing UV photolesions. This procedure involves removing UV photoproducts in the immunoprecipitated DNA using a mixture of CPD and 6-4 photolyases, ensuring that the thermostable DNA polymerase is not blocked by these photoproducts during PCR. We examined histone H2B occupancy in RPB2 (ORF) and GAL1 (promoter), both of which showed decreased repair efficiency in ubp8Δubp10Δ cells (Fig. 4 and Fig. S4). Importantly, we found H2B occupancy levels are higher in ubp8Δubp10Δ cells than in WT before UV irradiation in both genes (Fig. 5), which is consistent with reports on other chromatin loci (16, 17). In WT, depletion of H2B is seen almost immediately after UV irradiation (Fig. 5), indicating that H2B depletion occurs during the first few minutes when cells are irradiated and processed, a time frame similar to UV-induced H2B deubiquitylation. The low H2B occupancy is maintained for ∼30 min after UV and is restored by ∼60 min.
Immediately following UV irradiation, H2B is also depleted from RPB2 and GAL1 chromatin in ubp8Δubp10Δ cells (Fig. 5). However, UV-induced H2B depletion is reduced in the mutant at the first time point (0 min) after irradiation. For example, in RPB2 nucleosome +1, there is ∼45% H2B depletion in WT and only ∼20% depletion in the mutant (Fig. S5B), suggesting that H2Bub-containing nucleosomes are more resistant to UV-induced chromatin disruption in the mutant at early times after UV damage. Although H2B depletion by percentage in ubp8Δubp10Δ cells is comparable to WT cells at later repair times, the absolute level of H2B occupancy in both RPB2 and GAL1 is higher in the mutant cells (Fig. 5, compare 15′ and 30′ between WT and mutant); this indicates that both TCR and GGR in ubp8Δubp10Δ mutant cells can occur in chromatin with higher nucleosome occupancy, although the higher occupancy may partially occlude access to repair enzymes. It is also possible that other mechanisms (e.g., direct inhibition of recruitment of repair factors by excess H2Bub) may play a role in this process.
Discussion
Here, we report a previously unidentified and interesting finding that both yeast and human cells respond to UV damage by rapidly and significantly reducing their H2Bub levels. The deubiquitylation event occurs quickly, being seen almost immediately after UV irradiation in both cell types (Fig. 1). The high similarity between yeast and human suggests that the H2Bub response is conserved and may play similar roles in various species upon UV damage. Regarding the mechanism, we provide several lines of evidence showing that UV damage-arrested RNAPII plays a critical role in stimulating H2B deubiquitylation. First, UV-induced H2B deubiquitylation and subsequent ubiquitylation occur concurrently with transcription stalling and resumption, respectively. Second, in a UV damage-bypassing yeast strain (RPB1 E1103G), reduced H2B deubiquitylation is observed. Third, treatment with the transcription elongation inhibitor 6-AU results in loss of H2Bub in yeast, indicating that the reduction of H2Bub is a generic response to RNAPII stalling, regardless of what causes this stalling. Finally, the recovery of H2Bub after UV irradiation is dependent on proficient NER (Fig. 3C).
We also show that yeast H2B deubiquitylases Ubp8 and Ubp10 are both involved in UV-induced H2B deubiquitylation, as revealed by the complete absence of an immediate H2Bub decrease upon UV irradiation in a ubp8Δubp10Δ double mutant (Fig. 3A). An intriguing question is how Ubp8 and Ubp10 respond to UV-arrested RNAPII and deubiquitylate H2B in such a rapid fashion. Interestingly, Ubp8 has been found to interact with elongating RNAPII (19). We further show that both Ubp8 and Ubp10 proteins are associated with RNAPII (Fig. 3B), independently of UV irradiation. Therefore, we propose that upon RNAPII arrest, the stalled RNAPII activates its associated Ubp8 or Ubp10 immediately to deubiquitylate H2B in neighboring nucleosomes (Fig. S6). It is not known how stalled RNAPII triggers the activity of Ubp8 or Ubp10; however, it is unlikely that H2B deubiquitylation is activated by “recruiting” more Ubp8 or Ubp10, because UV damage does not increase the amount of Ubp8 or Ubp10 that associates with RNAPII (Fig. 3B). It is possible that RNAPII stalling stimulates a conformational change in Ubp8 or Ubp10 to activate these enzymes. Furthermore, the histone acetyltransferase Gcn5 coexists with Ubp8 in the Spt-Ada-Gcn5 acetyltransferase (SAGA) complex in yeast (18), and the human SAGA complex also contains hGcn5 and H2B deubiquitylase hUSP22 (30). Similar to H2B deubiquitylation, UV damage stimulates rapid histone H3 acetylation by Gcn5 in both yeast and human cells (31, 32). Thus, because UV damage induces both H3 acetylation and H2B deubiquitylation, it is conceivable that stalled RNAPII activates its associated SAGA complex, leading to simultaneous H3 acetylation and H2B deubiquitylation via Gcn5 and Ubp8 (or hUSP22) activities, respectively.
Our data reveal that deubiquitylation of H2B is an important regulatory mechanism at the chromatin level to optimize DNA repair and RNAPII degradation (Fig. S6). It has been proposed that H2Bub stabilizes nucleosomes (16) and promotes their (re)assembly in the coding regions of active genes (17). By reducing H2Bub levels through the activity of deubiquitylases, the nucleosome density and/or stability can be effectively reduced, possibly via enhanced eviction of the H2A–H2B dimer (16). In the coding regions of active genes where there is high nucleosome occupancy, such as in the RPB2 gene (Fig. S5A), the reduced nucleosome occupancy promoted by Ubp8 and Ubp10 presumably stimulates the processes of GGR and TCR. Conversely, the lack of both Ubp8 and Ubp10 inevitably leads to higher and persistent nucleosome occupancy during NER (Fig. 5), which results in reduced TCR and GGR. Furthermore, our data indicate that H2Bub levels play a role in the degradation of stalled RNAPII, as shown by a positive correlation between H2Bub and RNAPII degradation (Fig. 4C). Thus, H2Bub may act as a signal for the enzyme(s) responsible for polyubiquitylation of stalled RNAPII. Indeed, reverses Spt− phenotype 5 (Rsp5) (33) is an essential E3 ligase for RNAPII ubiquitylation and degradation (34), and contains a ubiquitin-binding site that is crucial for Rsp5 to add ubiquitin to its substrates (35). Our results provide compelling data for future studies on the potential role of H2Bub in the retention of Rsp5 in chromatin.
Materials and Methods
Yeast Strains.
All yeast strains used in this study are listed in Table S1.
Western Blotting of H2Bub.
Yeast cells were grown to early log phase in synthetic complete (SC) medium (yeast nitrogen base, glucose, dropout mix, and amino acids) and irradiated with 150 J/m2 UV in SC medium, which is equivalent to ∼75 J/m2 UV in PBS based on measurement of CPD per kilobase yields. To isolate whole-cell extracts (WCEs), yeast cells were treated with 500 µL of 0.1 M NaOH for 5 min at room temperature. After centrifugation, cells were resuspended in 60 µL of 1× SDS loading buffer [50 mM Tris-Cl pH 6.8, 2% (wt/vol) SDS, 6% (vol/vol) glycerol, 0.1 M DTT, 0.004% Bromophenol blue] and boiled for 5 min, and after centrifugation, the supernatant examined by SDS/PAGE (13.5% gel) and Western blotting with either (i) anti-Flag antibody (M2; Sigma) or (ii) anti-H2B antibody (Active Motif), depending on the experiment.
To investigate the UV-induced H2Bub response in human cells, histones were isolated from NHF1-hTERT cells collected after UV irradiation as previously described (36). Histone extracts were then run in SDS loading buffer on a 15% SDS/PAGE gel and examined via Western blot using monoclonal anti-ubiquityl-histone H2B antibody (clone 56; Millipore) or polyclonal anti-histone H2B antibody (Abcam).
Northern Blotting.
Northern blots were performed as described in ref. 37.
In Vivo Protein Interaction.
Yeast cells (Ubp8-TAP, Ubp10-TAP, and untagged control) were grown to an A600 of ∼1.0 and spit into two equal parts, one for UV irradiation (75 J/m2 in PBS) and another for mock treatment. Cells were allowed to repair for 15 min after UV irradiation. To purify TAP-tagged Ubp8 and Ubp10 proteins, we followed a previously described protocol (24). Proteins eluted by tobacco etch virus (TEV) protease were loaded to an 8% SDS gel and subjected to Western blotting using anti-RPB1 (8WG16; Santa Cruz Biotechnology) and anti-TAP (Open Biosystems) antibodies.
High-Resolution Mapping of CPDs and Repair.
High-resolution mapping of CPDs was performed as described in ref. 26.
RNAPII Degradation Assay.
Yeast cells were irradiated with 150 J/m2 UV in SC medium and aliquots taken at different time points as indicated. Cells were spun down and stored at −80 °C. WCEs extracts were isolated and loaded onto a 6% gel for Western blot analysis using an anti-Rpb1 antibody (8WG16; Santa Cruz Biotechnology).
ChIP.
Yeast cells were grown to midlog phase (OD600 ≅1.0) and irradiated with 200 J/m2 UV in PBS. After centrifugation, cells were resuspended in prewarmed yeast extract peptone dextrose for repair incubation. A 50-mL aliquot was taken at each time point. Cell cross-linking, chromatin isolation, sonication, and immunoprecipitation with the anti-Flag antibody were conducted as described in ref. 20. Before real-time PCR, the DNA was incubated with a mixture of CPD photolyase and 6-4 photolyase (a gift from Aziz Sancar, University of North Carolina at Chapel Hill, Chapel Hill, NC) to remove UV photolesions, as described in ref. 29.
Supplementary Material
Acknowledgments
We thank Drs. Zu-Wen Sun (Vanderbilt University) and Mikhail Kashlev (NCI Center for Cancer Research) for yeast strains. This study was made possible by National Institutes of Health Grant ES002614 from the National Institute of Environmental Health Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1403901111/-/DCSupplemental.
References
- 1.Hanawalt PC, Spivak G. Transcription-coupled DNA repair: Two decades of progress and surprises. Nat Rev Mol Cell Biol. 2008;9(12):958–970. doi: 10.1038/nrm2549. [DOI] [PubMed] [Google Scholar]
- 2.Somesh BP, et al. Multiple mechanisms confining RNA polymerase II ubiquitylation to polymerases undergoing transcriptional arrest. Cell. 2005;121(6):913–923. doi: 10.1016/j.cell.2005.04.010. [DOI] [PubMed] [Google Scholar]
- 3.Woudstra EC, et al. A Rad26-Def1 complex coordinates repair and RNA pol II proteolysis in response to DNA damage. Nature. 2002;415(6874):929–933. doi: 10.1038/415929a. [DOI] [PubMed] [Google Scholar]
- 4.Groth A, Rocha W, Verreault A, Almouzni G. Chromatin challenges during DNA replication and repair. Cell. 2007;128(4):721–733. doi: 10.1016/j.cell.2007.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Czaja W, Mao P, Smerdon MJ. The emerging roles of ATP-dependent chromatin remodeling enzymes in nucleotide excision repair. Int J Mol Sci. 2013;13(9):11954–11973. doi: 10.3390/ijms130911954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee CK, Shibata Y, Rao B, Strahl BD, Lieb JD. Evidence for nucleosome depletion at active regulatory regions genome-wide. Nat Genet. 2004;36(8):900–905. doi: 10.1038/ng1400. [DOI] [PubMed] [Google Scholar]
- 7.Selth LA, Sigurdsson S, Svejstrup JQ. Transcript elongation by RNA polymerase II. Annu Rev Biochem. 2010;79:271–293. doi: 10.1146/annurev.biochem.78.062807.091425. [DOI] [PubMed] [Google Scholar]
- 8.Kristjuhan A, Svejstrup JQ. Evidence for distinct mechanisms facilitating transcript elongation through chromatin in vivo. EMBO J. 2004;23(21):4243–4252. doi: 10.1038/sj.emboj.7600433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eisen JA, Sweder KS, Hanawalt PC. Evolution of the SNF2 family of proteins: Subfamilies with distinct sequences and functions. Nucleic Acids Res. 1995;23(14):2715–2723. doi: 10.1093/nar/23.14.2715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cho I, Tsai PF, Lake RJ, Basheer A, Fan HY. ATP-dependent chromatin remodeling by Cockayne syndrome protein B and NAP1-like histone chaperones is required for efficient transcription-coupled DNA repair. PLoS Genet. 2013;9(4):e1003407. doi: 10.1371/journal.pgen.1003407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weake VM, Workman JL. Histone ubiquitination: Triggering gene activity. Mol Cell. 2008;29(6):653–663. doi: 10.1016/j.molcel.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 12.Schulze JM, et al. Splitting the task: Ubp8 and Ubp10 deubiquitinate different cellular pools of H2BK123. Genes Dev. 2011;25(21):2242–2247. doi: 10.1101/gad.177220.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xiao T, et al. Histone H2B ubiquitylation is associated with elongating RNA polymerase II. Mol Cell Biol. 2005;25(2):637–651. doi: 10.1128/MCB.25.2.637-651.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pavri R, et al. Histone H2B monoubiquitination functions cooperatively with FACT to regulate elongation by RNA polymerase II. Cell. 2006;125(4):703–717. doi: 10.1016/j.cell.2006.04.029. [DOI] [PubMed] [Google Scholar]
- 15.Fleming AB, Kao CF, Hillyer C, Pikaart M, Osley MA. H2B ubiquitylation plays a role in nucleosome dynamics during transcription elongation. Mol Cell. 2008;31(1):57–66. doi: 10.1016/j.molcel.2008.04.025. [DOI] [PubMed] [Google Scholar]
- 16.Chandrasekharan MB, Huang F, Sun ZW. Ubiquitination of histone H2B regulates chromatin dynamics by enhancing nucleosome stability. Proc Natl Acad Sci USA. 2009;106(39):16686–16691. doi: 10.1073/pnas.0907862106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Batta K, Zhang Z, Yen K, Goffman DB, Pugh BF. Genome-wide function of H2B ubiquitylation in promoter and genic regions. Genes Dev. 2011;25(21):2254–2265. doi: 10.1101/gad.177238.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry KW, et al. Transcriptional activation via sequential histone H2B ubiquitylation and deubiquitylation, mediated by SAGA-associated Ubp8. Genes Dev. 2003;17(21):2648–2663. doi: 10.1101/gad.1144003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wyce A, et al. H2B ubiquitylation acts as a barrier to Ctk1 nucleosomal recruitment prior to removal by Ubp8 within a SAGA-related complex. Mol Cell. 2007;27(2):275–288. doi: 10.1016/j.molcel.2007.01.035. [DOI] [PubMed] [Google Scholar]
- 20.Chandrasekharan MB, Huang F, Sun ZW. Decoding the trans-histone crosstalk: Methods to analyze H2B ubiquitination, H3 methylation and their regulatory factors. Methods. 2011;54(3):304–314. doi: 10.1016/j.ymeth.2011.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nakanishi S, et al. Histone H2BK123 monoubiquitination is the critical determinant for H3K4 and H3K79 trimethylation by COMPASS and Dot1. J Cell Biol. 2009;186(3):371–377. doi: 10.1083/jcb.200906005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kireeva ML, et al. Transient reversal of RNA polymerase II active site closing controls fidelity of transcription elongation. Mol Cell. 2008;30(5):557–566. doi: 10.1016/j.molcel.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walmacq C, et al. Mechanism of translesion transcription by RNA polymerase II and its role in cellular resistance to DNA damage. Mol Cell. 2012;46(1):18–29. doi: 10.1016/j.molcel.2012.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Puig O, et al. The tandem affinity purification (TAP) method: A general procedure of protein complex purification. Methods. 2001;24(3):218–229. doi: 10.1006/meth.2001.1183. [DOI] [PubMed] [Google Scholar]
- 25.Min JH, Pavletich NP. Recognition of DNA damage by the Rad4 nucleotide excision repair protein. Nature. 2007;449(7162):570–575. doi: 10.1038/nature06155. [DOI] [PubMed] [Google Scholar]
- 26.Li S, Waters R, Smerdon MJ. Low- and high-resolution mapping of DNA damage at specific sites. Methods. 2000;22(2):170–179. doi: 10.1006/meth.2000.1058. [DOI] [PubMed] [Google Scholar]
- 27.Bryant GO, et al. Activator control of nucleosome occupancy in activation and repression of transcription. PLoS Biol. 2008;6(12):2928–2939. doi: 10.1371/journal.pbio.0060317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang C, Pugh BF. A compiled and systematic reference map of nucleosome positions across the Saccharomyces cerevisiae genome. Genome Biol. 2009;10(10):R109. doi: 10.1186/gb-2009-10-10-r109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sarkar S, Kiely R, McHugh PJ. The Ino80 chromatin-remodeling complex restores chromatin structure during UV DNA damage repair. J Cell Biol. 2010;191(6):1061–1068. doi: 10.1083/jcb.201006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao Y, et al. A TFTC/STAGA module mediates histone H2A and H2B deubiquitination, coactivates nuclear receptors, and counteracts heterochromatin silencing. Mol Cell. 2008;29(1):92–101. doi: 10.1016/j.molcel.2007.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Yu Y, Teng Y, Liu H, Reed SH, Waters R. UV irradiation stimulates histone acetylation and chromatin remodeling at a repressed yeast locus. Proc Natl Acad Sci USA. 2005;102(24):8650–8655. doi: 10.1073/pnas.0501458102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo R, Chen J, Mitchell DL, Johnson DG. GCN5 and E2F1 stimulate nucleotide excision repair by promoting H3K9 acetylation at sites of damage. Nucleic Acids Res. 2010;39(4):1390–1397. doi: 10.1093/nar/gkq983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Happel AM, Winston F. A mutant tRNA affects delta-mediated transcription in Saccharomyces cerevisiae. Genetics. 1992;132(2):361–374. doi: 10.1093/genetics/132.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huibregtse JM, Yang JC, Beaudenon SL. The large subunit of RNA polymerase II is a substrate of the Rsp5 ubiquitin-protein ligase. Proc Natl Acad Sci USA. 1997;94(8):3656–3661. doi: 10.1073/pnas.94.8.3656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.French ME, Kretzmann BR, Hicke L. Regulation of the RSP5 ubiquitin ligase by an intrinsic ubiquitin-binding site. J Biol Chem. 2009;284(18):12071–12079. doi: 10.1074/jbc.M901106200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smerdon MJ, Lan SY, Calza RE, Reeves R. Sodium butyrate stimulates DNA repair in UV-irradiated normal and xeroderma pigmentosum human fibroblasts. J Biol Chem. 1982;257(22):13441–13447. [PubMed] [Google Scholar]
- 37.Li S, et al. The roles of Rad16 and Rad26 in repairing repressed and actively transcribed genes in yeast. DNA Repair (Amst) 2007;6(11):1596–1606. doi: 10.1016/j.dnarep.2007.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li S, Smerdon MJ. Nucleosome structure and repair of N-methylpurines in the GAL1-10 genes of Saccharomyces cerevisiae. J Biol Chem. 2002;277(47):44651–44659. doi: 10.1074/jbc.M206623200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.