Abstract
BPI (bactericidal/permeability-increasing) is a potent antimicrobial protein that was recently reported to be expressed as a surface protein on human gastrointestinal tract epithelial cells. In this study, we investigated the resistance of Vibrio cholerae, a small-bowel pathogen that causes cholera, to a BPI-derived peptide, P2. Unlike in Escherichia coli and Salmonella enterica serovar Typhimurium, resistance to P2 in V. cholerae was not dependent on the BipA GTPase. Instead, we found that ToxR, the master regulator of V. cholerae pathogenicity, controlled resistance to P2 by regulating the production of the outer membrane protein OmpU. Both toxR and ompU mutants were at least 100-fold more sensitive to P2 than were wild-type cells. OmpU also conferred resistance to polymyxin B sulfate, suggesting that this porin may impart resistance to cationic antibacterial proteins via a common mechanism. Studies of stationary-phase cells revealed that the ToxR-repressed porin OmpT may also contribute to P2 resistance. Finally, although the mechanism of porin-mediated resistance to antimicrobial peptides remains elusive, our data suggest that the BPI peptide sensitivity of OmpU-deficient V. cholerae is not attributable to a generally defective outer membrane.
The curved, gram-negative bacterium Vibrio cholerae is the cause of cholera, a severe and potentially lethal diarrheal disease. Humans become infected with this bacterium following ingestion of contaminated food or water. After passage through the gastric barrier, V. cholerae colonizes the small intestine, a site that is relatively nonpermissive for bacterial growth. Within the small bowel, V. cholerae secretes cholera toxin. The activity of this toxin largely accounts for the clinical features of cholera (25). The fact that V. cholerae is able to colonize the human small intestine suggests that this bacterium has mechanisms to counteract enteric defense mechanisms.
The human gut possesses several types of innate defenses to fend off invading microorganisms, including gastric acid, peristalsis, mucus, and antimicrobial peptides (AP). AP are thought to protect the small bowel from a variety of microorganisms. For instance, human β-defensin 5 is secreted by intestinal crypt cells and is critical for host defense against bacterial challenge (41). BPI (for “bactericidal/permeability-increasing”) is an antimicrobial protein that is thought to be important for protection against gram-negative pathogens. BPI is a 55- to 60-kDa cationic protein found in neutrophil azurophilic granules and on the neutrophil cell surface (45). It was also recently reported to be expressed as a surface protein on human gastrointestinal tract epithelial cells (6). BPI acts specifically on gram-negative bacteria by virtue of its high affinity for the lipid A moiety of lipopolysaccharide (LPS) (7, 13). It plays several roles in host defense, and these distinct functions have been mapped to different regions of the protein. It possesses a cationic N-terminal region implicated in its antibacterial and endotoxin neutralization activities and a C-terminal region necessary for bacterial opsonization (3). Functional mapping studies have established that a peptide containing BPI residues 85 to 99 retains significant antibacterial activity. This region forms an amphipathic beta-turn and binds LPS (14, 28). It has been proposed that BPI and P2, a peptide that contains BPI residues 86 to 104, may employ the same mechanism for killing Escherichia coli since both increase outer membrane (OM) permeability to hydrophobic compounds and inhibit O2 consumption (2, 21). The cytotoxic mechanism of BPI remains poorly understood. Binding of BPI to LPS displaces calcium and magnesium ions, which normally bridge the adjacent negatively charged LPS molecules, and thereby perturbs their regular arrangement, leading to permeabilization of the membrane to small hydrophobic molecules (30). The released divalent ions are thought to activate bacterial phospholipases, leading to membrane rupture (26, 29). It is also thought that BPI may damage the cytoplasmic membranes of the target cells (2, 21, 30).
Bacteria have evolved several strategies to resist AP. These include (i) modification of LPS, the target of AP; (ii) elaboration of extracellular proteases to cleave AP; and (iii) active efflux pumps to export AP outside the cell (reviewed in reference 46). Other mechanisms of resistance seem probable since tolerance to BPI in E. coli and Salmonella spp. is mediated in part by bipA (12, 40). bipA mutant cells of E. coli were 100-fold more sensitive to BPI peptide than were wild-type (wt) cells (2). BipA is a GTPase that is thought to protect stationary-phase cells from BPI-induced cytoplasmic damage (2).
V. cholerae pathogenicity is regulated by ToxR. This transcription regulator controls the expression of a set of nearly 60 genes (the ToxR regulon), which encode products that function to promote intestinal colonization, toxin production, and survival within the host (4, 23, 33). The ToxR regulon is organized into two branches (11). In one, ToxR works as a coactivator along with another DNA-binding protein, TcpP, to activate the transcription of toxT (16, 19). ToxT directly promotes the production of cholera toxin and the pilus colonization factor, TCP, as well as other virulence factors (9). In the other branch, which is independent of ToxT, ToxR positively regulates the expression of the ompU gene encoding the OM protein (OMP) OmpU (8, 43), and negatively regulates ompT (32). OmpT production is also controlled by the cyclic AMP receptor protein (CRP) (27). CRP is a global regulator that activates ompT expression in carbon- and energy source-limiting conditions. Therefore, OmpT is expressed in stationary-phase cultures of V. cholerae.
OmpU and OmpT are porins that form water-filled channels across the OM. They are thought to function primarily as channels for entry and exit of hydrophilic low-molecular-mass (<600-Da) molecules. In the genus Vibrio, OMPs function as porins for iron, phosphate, and sugar transport and in attachment to inanimate surfaces, although their role in adhesion to host epithelia remains controversial (1). V. cholerae OMPs may play some role in pathogenicity. OmpU mediates resistance to bile and anionic detergents such as sodium dodecyl sulfate, while OmpT increases susceptibility to anionic detergents (37, 39).
Given the recent suggestion (6) that BPI may contribute to the innate immune defenses of the gastrointestinal tract, we examined the susceptibility of V. cholerae to the BPI-derived peptide P2. We found that V. cholerae lacking toxR is much more sensitive to P2 than is wt V. cholerae. The ToxT branch of the ToxR regulon was not important in conferring resistance to P2; instead, OmpU was found to account for the ToxR-regulated resistance to P2.
MATERIALS AND METHODS
Bacterial strains, media, and antibiotics.
The bacterial strains used in this study are listed in Table 1. To induce toxR expression, classical V. cholerae strain O395 was cultured on a roller drum at 30°C overnight as previously described (33). ToxR induction in El Tor V. cholerae strain N16961 was achieved using AKI medium as described previously (22). Antibiotics were used at the following concentrations: ampicillin, 50 μg/ml (V. cholerae) and 100 μg/ml (E. coli), and streptomycin, 200 μg/ml.
TABLE 1.
Strains and plasmids used in this work
| Strain or plasmid | Characteristics | Reference or source |
|---|---|---|
| V. cholerae | ||
| O395 | Wild-type classical biotype with lacZ deletion; Smr | M. Waldor (31) |
| O395, toxR::pVM55 | toxR insertion mutant; Smr Apr | M. Waldor (32) |
| N16961 | Wild-type El Tor biotype | M. Waldor (17) |
| N16961, toxR::pVM55 | toxR insertion mutant; Smr Apr | This study |
| O395, bipA::pJM1 | bipA insertion mutant; Smr Apr | This study |
| N16961, bipA::pJM1 | bipA insertion mutant; Smr Apr | This study |
| O395, VJ740 | toxT internal deletion mutant; Smr | V. DiRita (10) |
| O395, KKV780 | ompU deletion mutant; Smr | K. Klose (37, 38) |
| O395, KKV809 | ompT deletion mutant; Smr | K. Klose (37, 38) |
| O395, KKV884 | ompU/ompT double mutant; Smr | K. Klose (38) |
| O395, tolA::pGP704 | tolA insertion mutant; Smr Apr | D. Heilpern (18) |
| O395, tolB::pGP704 | tolB insertion mutant; Smr Apr | D. Heilpern (18) |
| O395, tolC::pGP704 | tolB insertion mutant; Smr Apr | This study |
| pKEK253 | pU-ompU; Apr | K. Klose (37, 38) |
| pKEK255 | pT-ompT; Apr | K. Klose (37, 38) |
| pKEK257 | pU-ompT; Apr | K. Klose (37, 38) |
| pBAD28 | Apr Cmr | Invitrogen |
| pVM55 | Apr; pGP704 derivative with internal fragment of toxR | V. Miller (32) |
| pJM1 | Apr; pGP704 derivative with internal fragment of bipA | This study |
Construction of insertion mutants.
Homologous recombination of pGP704-based suicide vectors (32) containing internal fragments of toxR, bipA, and tolC into their respective chromosomal genes was used to inactivate each of these genes in the V. cholerae O395 and N16961 backgrounds. These gene fragments were amplified from genomic DNA by PCR. The sequences of the primers used to amplify the bipA internal gene fragment were 5′-GGTTCTGGGCTACCTTGG-3′ and 5′-CGGTTCCATCAGTTGGC-3′. Primers 5′-CCGTGGGTCGCCAATTAG-3′ and 5′-TTGCCACCTGTGTACAGC-3′ were used to amplify the tolC internal gene fragment. Following subcloning of the PCR products into the TA cloning vector pCRII-TOPO (Invitrogen, Carlsbad, Calif.), EcoRI fragments containing the PCR products were ligated with EcoRI-digested and shrimp alkaline phosphatase-treated pGP704 (32). The resulting plasmids, along with pVM55 (32), which contains an internal fragment of toxR (Table 1), were subsequently introduced into E. coli Sm10λpir and then mobilized into V. cholerae O395 and N16961 as described previously (18). Following selection of Smr and Apr colonies, disruption of toxR, bipA, and tolC genes was confirmed by Southern and PCR analyses.
BPI peptide synthesis.
BPI peptide, consisting of residues 86 to 104 of BPI protein flanked by serine and cysteine residues (SKISGKWKAQKRFLKMSGNFGC) and referred to as P2 (2), was synthesized using standard Fmoc chemistry on an ABI model 430A synthesizer. Peptides were cleaved from solid supports with trifluoroacetic acid and purified by gel filtration and reverse-phase high-performance liquid chromatography using a C18 column and an elution gradient of 0 to 60% acetonitrile with 0.1% trifluoroacetic acid. The purity and size of the peptide were confirmed by analytical high-performance liquid chromatography and by mass spectrometry using an electrospray orthogonal-acceleration time-of-flight instrument.
Bactericidal assays.
Bacteria were cultured overnight in Luria-Bertani (LB) broth with the appropriate antibiotics at 30 or 37°C. For assays of cells from log-phase cultures, overnight cultures were diluted 1:100 in LB medium (pH 6.5) and grown to an optical density at 600 nm (OD600) of 0.5. Approximately 17-h overnight cultures were used as a source of stationary-phase cultures. Both stationary- and log-phase cells were washed twice in 20 mM sodium phosphate-buffered saline (PBS) (pH 6.0) containing 154 mM NaCl. For competition experiments, ΔlacZ wt and lacZ+ mutant strains were mixed in a 1:1 ratio and diluted to a final concentration of 5 × 108 cells/ml. A 100-μl volume of the bacterial suspension was added to 400 μl of PBS containing 0 to 40 μg of P2 dissolved in 20 mM phosphate buffer (pH 6.0). To assess the sensitivity to polymyxin B sulfate (Sigma), 0 to 6 μg of the peptide was added to the reaction mix. The cells were incubated at 37°C, and samples were taken at different times for plating on X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside) LB agar to enumerate CFUs. All assays were performed at least in duplicate and usually in triplicate. The effect of human complement (Sigma) on wt and ompU V. cholerae cells was assessed by treating cells in a fashion similar to that described above, except that the cells were incubated with 0 to 30% complement in 10 mM PIPES buffer (pH 7.4).
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis and Western blotting.
Whole-cell lysates were boiled for 5 min in NuPage LDS sample buffer (Invitrogen) and separated on a 12% polyacrylamide gel (NuPage). The gels were subsequently stained with Coomassie brilliant blue for visualization. Protein gels were transferred to nitrocellulose for Western blotting using a transblotter (Invitrogen). The blots were probed with rabbit polyclonal antisera against V. cholerae OmpU and OmpT (the kind gift of J. Kaper) and developed with the enhanced chemiluminescence detection system (Amersham).
Enzyme-linked immunosorbent assay.
ToxR induction was validated by detection of cholera toxin in cell supernatants by GM1 enzyme-linked immunosorbent assay using rabbit polyclonal antiserum against the purified B subunit of cholera toxin as described previously (20).
Measurement of OM permeability.
The permeability of the outer membrane was assayed by measuring the penetration of the chromogenic probe, 3-(2,4-dinitrostyryl)-(6 R,7 R)-7-(2-thienylacetamido)-ceph-3-em-4-carboxyl acid (nitrocefin; Calbiochem). Cells were grown to stationary and/or mid-exponential phase in LB medium, washed twice in 20 mM PBS (pH 6.0), and resuspended in the same buffer at a concentration of 5 × 107 cells/ml. A 50-μl volume of the bacterial suspension was added to 175 μl of PBS buffer and 25 μl of the nitrocefin stock solution (500 μg/ml prepared as specified by the manufacturer). A 5-μg portion of P2 was added to the solution and the increase in OD490 was immediately monitored in a plate reader (Victor II; Perkin-Elmer) maintained at 37°C and programmed to shake for 10 s every minute. The negative control lacked P2.
RESULTS
Dose-dependent killing of V. cholerae by BPI peptide P2.
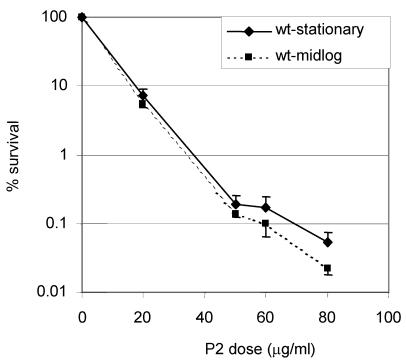
We utilized a synthetic peptide containing BPI residues 84 to 106 (previously referred to as P2 [2]) to test the susceptibility of V. cholerae to BPI, since previous studies (14) with E. coli showed that this peptide retains most of the cytotoxic activity of the full-length protein. Both stationary-phase and mid-log-phase cultures of V. cholerae classical biotype strain O395 exhibited dose-dependent killing in the presence of this BPI peptide, as has been reported for E. coli and S. enterica serovar Typhimurium (Fig. 1).
FIG. 1.
Dose-dependent killing of V. cholerae by the BPI peptide P2. Mid-log-phase V. cholerae (O395 ΔlacZ) and stationary-phase V. cholerae O395 lacZ+ cells were incubated with the indicated concentrations of P2 for 90 min. The numbers of CFU of the cells were determined by plating on LB agar containing X-Gal, and the percent survival of the cells was calculated by normalizing to the control (0 P2) at each time point. The means of three independent experiments and corresponding standard errors of the means (SEMs) are presented.
Since stationary-phase S. enterica serovar Typhimurium cells and enteropathogenic E. coli cells lacking the bipA (BPI-inducible protein A) gene were found to be hypersusceptible to BPI (2, 12), we tested whether this GTPase also promotes resistance to BPI in V. cholerae. We identified an orthologue of the S. enterica serovar Typhimurium bipA in the V. cholerae N16961 genome by using BLAST. This gene, vc2744, encodes a protein with 74% identity and 83% similarity to S. enterica serovar Typhimurium BipA. We generated insertion mutations in vc2744 in strains O395 and N16961 and assessed the P2 sensitivity of these cells in mid-log and stationary phase. Under all growth conditions tested, these mutants displayed identical P2 sensitivity to that shown by the wt strains (data not shown). Reverse transcription-PCR was used to confirm vc2744 expression under stationary-phase growth conditions. These observations suggest that a bipA-independent mechanism of P2 resistance exists in V. cholerae.
ToxR modulates resistance to P2 via its control of OmpU.
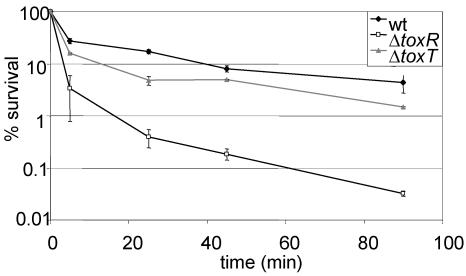
Since BipA did not contribute to V. cholerae survival in the presence of P2, we tested whether the well-characterized virulence regulator ToxR mediated resistance to P2. We found that toxR derivatives of O395 (Fig. 2) and N16961 (data not shown) grown to log phase were both nearly 100-fold more sensitive to P2 than were the corresponding wt cells. ToxR induction at this stage of growth was validated by detection of cholera toxin in supernatants of wt cells.
FIG. 2.
ToxR, but not ToxT, contributes to resistance to BPI peptide in V. cholerae. The percent survival of mid-log-phase wt, toxR, and toxT V. cholerae cells as a function of time of incubation with 40 mg of P2 per ml is shown. Shown here are the means of three experiments with SEMs.
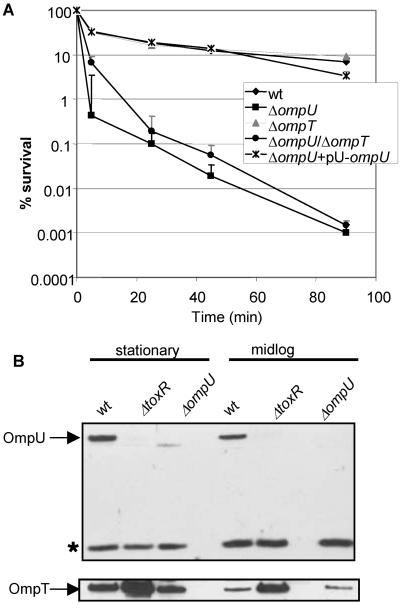
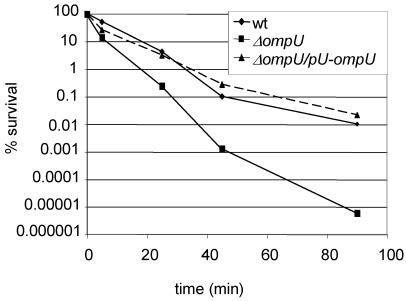
To determine whether the ToxT-dependent or ToxT-independent branches of the ToxR regulon impart P2 resistance, we compared the P2 susceptibilities of O395 and O395 toxT (PVJ740) cells under ToxR-inducing conditions. Mid-log-phase cultures (OD600 = 0.5) of O395 and O395 toxT were nearly equally resistant to P2, indicating that ToxT-independent, ToxR-dependent factors impart P2 resistance (Fig. 2). O395 ompU (KKV780) cells were even more sensitive to P2 than were O395 toxR cells (Fig. 3A). This observation suggests that ToxR activation of ompU transcription accounts for the ToxR control of resistance to P2. Complementation of the ompU mutant with a low-copy-number plasmid encoding OmpU under the control of its native promoter restored P2 resistance to wt levels (Fig. 3A). Thus, in mid-log-phase cultures, ToxR-regulated resistance to P2 appears to be conferred primarily by OmpU. OmpT does not play a significant role in resistance to P2 in mid-log-phase cultures since O395 ΔompT (KKV 809) exhibited a similar P2 resistance to that of O395 (Fig. 3A). Furthermore, the O395 ΔompU ΔompT mutant exhibited similar P2 sensitivity to that of the ΔompU mutant (Fig. 3A). This was not an unexpected finding since OmpT was present at low levels in lysates from wt mid-log-phase cultures by Western blotting (Fig. 3B). However, as discussed below, under different conditions, OmpT may contribute to P2 resistance.
FIG. 3.
OmpU confers BPI peptide resistance in mid-log-phase cells. (A) The percent survival of mid-log-phase wt O395 and ompU, ompT, ompU/ompT, and ompU+pU−ompU cells as a function of time of incubation with 40 μg of P2 per ml is shown. Means of three independent experiments and corresponding SEMs are presented. (B) Western blots comparing OmpU and OmpT levels in stationary- and mid-log-phase cultures of the indicated strains. The asterisk denotes a cross-reacting band used as a loading control.
Both OmpU and OmpT can confer resistance to BPI peptide.
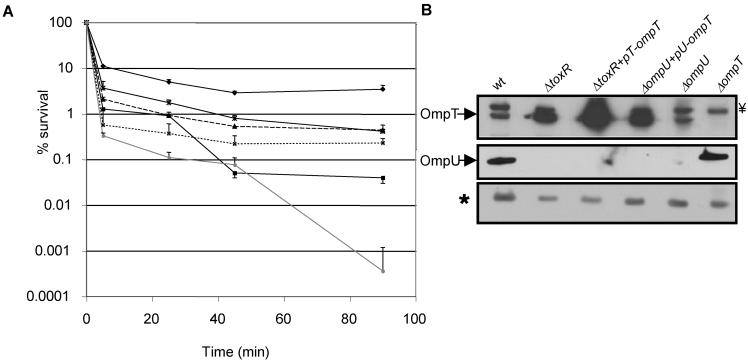
Since cells from stationary-phase cultures of E. coli and S. enterica serovar Typhimurium were found to be more resistant to P2 than were cells from log-phase cultures (2), we investigated if the resistance of V. cholerae to P2 was also modulated by growth phase. We found that stationary-phase O395 was only two- to threefold more resistant to P2 than were log-phase cells (Fig. 1). However, studies of stationary-phase cells revealed that OmpT may possess the potential to contribute to P2 resistance, especially in the absence of OmpU. First, ectopic expression of OmpT from the ompU promoter in KKV780 cells (ΔompU/pU-ompT) partially relieved the marked sensitivity of an ompU strain (Fig. 4A). Second, stationary-phase ompT cells were about five- to sevenfold more sensitive to P2 than were wt cells (Fig. 4a). Lastly, to further test the idea that P2 resistance was not a unique attribute of OmpU, the P2 sensitivity of stationary-phase JM2 cells (ΔtoxR/pT-ompT) was measured. This toxR null strain harbors a plasmid-borne OmpT that is expressed from its native promoter. Since OmpT is maximally produced in the stationary phase and its expression is negatively controlled by ToxR, the P2 resistance of stationary-phase JM2 cells provides another means of assessing whether OmpT can contribute to P2 resistance in the absence of OmpU. Indeed, JM2 was 1 log unit more resistant than toxR cells to P2 (Fig. 4A). Western blot analyses of OmpT protein levels confirmed that P2 resistance in the ompU and toxR backgrounds could generally be correlated with the amount of OmpT (Fig. 4B). These findings indicate that both OmpU and OmpT can independently mediate P2 resistance. Thus, although OmpT is less effective at mediating P2 resistance, the total cellular levels of OmpU and OmpT contribute to P2 resistance.
FIG. 4.
Ectopic expression of OmpU as well as OmpT imparts resistance to BPI peptide in stationary-phase O395 cells. (A) The percent survival of stationary-phase wt O395 (⧫) and isogenic toxR (▪), ompT (▴), ompU+pU−ompT (×), toxR + pT−ompT (✻), and ompU (•) cells is shown as a function of time of incubation with 40 μg of P2 per ml. The means of three independent experiments and corresponding SEMs are presented. (B) Western blots showing OmpT and OmpU levels in these strains at stationary phase. The asterisk denotes a cross-reacting band used as a loading control. Y= denotes another cross-reactive band just above the OmpT band.
OmpU confers resistance to polymyxin B sulfate.
OmpU conferred resistance to another cationic peptide in addition to P2. The ompU O395 mutant was nearly 100 times more sensitive to 12 μg of polymyxin B sulfate per ml than to the wt strain, when logarithimically growing cells were assayed. The defect could be complemented by using a plasmid carrying OmpU (Fig. 5). These observations suggest that OmpU may impart resistance to cationic antibacterial proteins via a common mechanism.
FIG. 5.
OmpU also confers resistance to polymyxin B sulfate. The percent survival of mid-log-phase O395 wt, ompU, and ompU+pU−ompU cells is shown as a function of time of incubation with 12 μg of polymyxin B sulfate per ml. Shown here are means of two independent experiments.
Mechanism of sensitivity of OmpU mutants.
We wondered if the ompU mutant was more sensitive to P2 because it possessed a more fragile OM than did wt cells. We assessed the OM integrity of wt and ompU cells by measuring the hydrolysis of nitrocefin, a chromogenic β-lactamase substrate. In these experiments, nitrocefin was added to wt and ompU cells transformed with a plasmid (pBAD28) encoding bla and its hydrolysis was monitored by measuring the development of color at OD490. β-Lactamase is a periplasmic enzyme; therefore, to detect nitrocefin hydrolysis, this substrate must first traverse the OM. The extents and rates of nitrocefin hydrolysis were nearly identical in ompU and wt cells (Fig. 6). Furthermore, levels of sensitivity to actinomycin D, an antibiotic to which V. cholerae cells with an intact OM are impermeant, were similar in ompU and wt cells (data not shown). These observations suggest that the sensitivity of ompU V. cholerae to P2 does not reflect a nonspecific destabilization of the OM in this mutant. Conversely, P2 sensitivity does not appear to result from OM defects that are independent of OmpU production. Although the integrity of the OM in tolA and tolB V. cholerae mutants is reported to be compromised (18), these mutant cells had nearly identical P2 resistance to that of wt cells (data not shown).
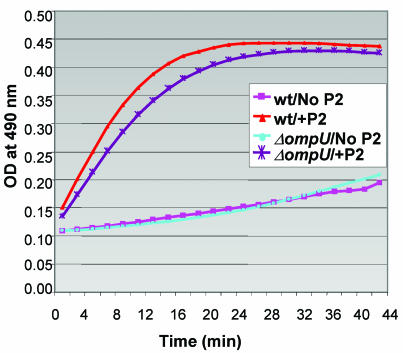
FIG. 6.
BPI peptide increases OM permeability in wt and ompU O395 to similar extents. Nitrocefin hydrolysis was monitored at an OD490 as a function of time and used as a measure of OM permeability. In the absence of P2, there was minimal nitrocefin hydrolysis in wt and ompU cells. Data are representative of five independent experiments.
In E. coli, BPI increases the permeability of the OM (30). We wondered if ompU cells were more susceptible to P2-mediated OM damage. Therefore, we compared P2-induced outer membrane permeability changes in wt and ompU V. cholerae cells by using the nitrocefin hydrolysis assay discussed above. Addition of P2 led to rapid and similar increases in nitrocefin hydrolysis in both wt and ompU cells (Fig. 6). Overall, the similarity of the P2-induced hydrolysis of nitrocefin in ompU and wt cells suggests that OM perturbations may not account for P2-mediated cell killing and that the cytotoxic target of P2 resides elsewhere in the cell.
DISCUSSION
We found that ToxR, the master regulator of V. cholerae pathogenicity, also regulates the resistance of this enteric pathogen to a BPI-derived peptide, an important effector of innate immunity. The ToxR-mediated control of resistance to P2 in log-phase cells is independent of ToxT and, instead, is mediated by the ToxR activation of expression of OmpU, a major V. cholerae porin. Since BPI is thought to be present on the epithelial lining of most of the human gastrointestinal tract (6), ToxR activation of OmpU expression during in vivo growth could facilitate V. cholerae intestinal colonization. While OmpU has been reported not to enhance intestinal colonization in suckling mice (38), BPI is not thought to be present in the gastrointestinal tract of suckling mice. Therefore, it is possible that this porin plays a role in V. cholerae intestinal colonization in humans.
Previous studies have not implicated porins in resistance to BPI, but our work indicates that in V. cholerae the total cellular content of OmpU and OmpT at least partially determines resistance to this cationic peptide. Analysis of BPI resistance in E. coli suggested that OmpR (a ToxR analog) is important for resistance (36). However, the OmpR-regulated porins OmpC and OmpF were not thought to be involved in this phenomenon (36). This observation suggests that resistance to BPI-derived peptides and other AP may be an intrinsic property of only certain OMPs.
The amounts of OmpU and OmpT are regulated by the V. cholerae growth phase. Expression of toxR is apparent from early log phase to stationary phase in V. cholerae O395 cells grown at 30°C in LB broth (pH 6.5) (33, 35). In mid-log-phase cultures, ompU is expressed and ompT is repressed, so that the OmpT protein is virtually absent. In this state, toxR cells were found to be nearly 100-fold more sensitive to BPI than were wt cells. ompU mutants were even more sensitive to BPI than were toxR mutants (Fig. 2 and 3A). The greater susceptibility of the ompU mutant than of the toxR mutant can be explained by the fact that under these growth conditions, while ompU mutants produce no OmpU and negligible OmpT, toxR cells synthesized some OmpT (because of alleviation of ToxR-mediated repression of ompT transcription) (Fig. 3B). In cells from stationary-phase cultures, OmpT may also contribute to BPI resistance, although ToxR is still present. ompT transcription is activated by CRP, which accumulates under the glucose-limiting conditions typical of stationary-phase growth (27). It does appear that OmpU is better suited to combating the lethal action of BPI than is OmpT. Both the greater abundance of OmpU than of OmpT in the cell (8) and the distinct channel properties of these porins (42) may account for this difference.
OmpU mediates resistance to other cationic peptides besides BPI peptide. Absence of OmpU increased the susceptibiliy of mid-log-phase V. cholerae to polymyxin B sulfate-mediated killing by nearly 1,000-fold relative to the wt. Surprisingly, stationary-phase V. cholerae strains lacking ompU were previously reported to have wt resistance to polymyxin B nonapeptide (38). Two potential explanations for this discrepancy are that (i) Provenzano et al. (38) used lower concentrations of the nonapeptide form of polymyxin B and we used the decapeptide form instead and (ii) Provenzano et al. analyzed only cells taken from stationary-phase cultures of V. cholerae O395, which possess intrinsically higher levels of resistance.
The question of why the ompU mutant is hypersensitive to BPI-mediated killing remains unanswered; however, our work counters the hypothesis that the ompU mutant has a gross defect in its OM. The OM permeability of ompU cells was very similar to that of wt cells as measured by actinomycin D susceptibility as well as by nitrocefin hydrolysis in the absence of P2. Another observation that supports this idea was our finding that human complement killed both wt and ompU cells to equal degrees by the alternative pathway. Complement generates macromolecular pores on the OM, and resistance to complement may depend on certain OM components (24). These findings are also consistent with those of Provenzano et al., who reported that the ompU and ompT mutants have uncompromised OM integrity as measured by leakage of periplasmic proteins and possess wt-like LPS structure (38).
The OM permeabilities of ompU and wt cells were similar when measured as a function of time of incubation with BPI peptide. Thus, at least as measured by nitrocefin hydrolysis, our work suggests that BPI-mediated increases in OM permeability can be dissociated from cytotoxicity. This conclusion is corroborated by similar findings with E. coli. While E. coli cells lacking OmpR are susceptible to killing by rBPI21 (recombinant BPI containing amino acid residues 1 to 193), the peptide-induced OM permeability change exhibited by OmpR+ and OmpR− cells was the same (36). The fact that BPI peptide did not kill tolB and tolA V. cholerae mutants, which have multiple characterized OM defects, any more effectively than it killed wt cells supports the idea that OM perturbation can be uncoupled from cell cytotoxicity.
If the absence of certain porins does not cause hyperpermeability of the OM, then perhaps there is defective efflux of the BPI peptide or some other substance that ameliorates BPI cytotoxicity in ompU cells. Precedents for resistance arising out of active efflux of AP-like protegrins and LL-37 exist in Neisseria gonorrhoeae and in Salmonella spp. (15, 34). The efflux porins of Pseudomonas aeruginosa, which are known to confer antibiotic resistance, are the best-characterized examples of porin-mediated drug resistance. While OmpU and OmpT do not bear any obvious sequence homology to this family of porins, it is possible that these OM porins may be involved in an active efflux mechanism. It is also possible that the OMPs act in concert with other efflux proteins. The best-studied OM efflux channel protein is the E. coli TolC protein. The vc2436 gene is the closest V. cholerae homolog of E. coli tolC (70.6% similarity). TolC in V. cholerae N16961 is involved in efflux of bile acids and hydrophobic antibiotics (5). However, we found that a mutant with an insertion mutation in tolC in the O395 strain did not show enhanced sensitivity to P2 (data not shown). Hence, TolC does not appear to be involved in the export of P2.
Although it has been suggested that certain cationic peptides pass through the OM and subsequently disrupt the cytoplasmic membrane, it seems unlikely that holo-BPI (a 55-kDa protein) can traverse the OM. A scenario in which the interaction of BPI peptide with lipid A triggers a signaling pathway that depends in some way on OmpU and OmpT seems more plausible. We speculate that the porin activity of OmpU may facilitate a cellular response that ameliorates BPI cytotoxicity.
Acknowledgments
We are grateful to Karl Klose for providing us with several strains, James Kaper for providing OmpU and OmpT antisera, Ferric Fang for providing synthetic BPI peptide, and John Mekalanos for providing antisera to cholera toxin. We thank Anne Kane and the New England Medical Center GRASP Center for preparation of media. We thank Brigid Davis, Anne Kane, and John Beaber for their helpful suggestions and critical reading of the manuscript.
This work was supported by an NIH grant (AI-42347) and the Howard Hughes Medical Institute.
Editor: V. J. DiRita
REFERENCES
- 1.Aeckersberg, F., C. Lupp, B. Feliciano, and E. G. Ruby. 2001. Vibrio fischeri outer membrane protein OmpU plays a role in normal symbiotic colonization. J. Bacteriol. 183:6590-6597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barker, H. C., N. Kinsella, A. Jaspe, T. Friedrich, and C. D. O'Connor. 2000. Formate protects stationary-phase Escherichia coli and Salmonella cells from killing by a cationic antimicrobial peptide. Mol. Microbiol. 35:1518-1529. [DOI] [PubMed] [Google Scholar]
- 3.Beamer, L. J., S. F. Carroll, and D. Eisenberg. 1997. Crystal structure of human BPI and two bound phospholipids at 2.4 angstrom resolution. Science 276:1861-1864. [DOI] [PubMed] [Google Scholar]
- 4.Bina, J., J. Zhu, M. Dziejman, S. Faruque, S. Calderwood, and J. Mekalanos. 2003. ToxR regulon of Vibrio cholerae and its expression in vibrios shed by cholera patients. Proc. Natl. Acad. Sci. USA 100:2801-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bina, J. E., and J. J. Mekalanos. 2001. Vibrio cholerae tolC is required for bile resistance and colonization. Infect. Immun. 69:4681-4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Canny, G., O. Levy, G. T. Furuta, S. Narravula-Alipati, R. B. Sisson, C. N. Serhan, and S. P. Colgan. 2002. Lipid mediator-induced expression of bactericidal/permeability-increasing protein (BPI) in human mucosal epithelia. Proc. Natl. Acad. Sci. USA 99:3902-3907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capodici, C., S. Chen, Z. Sidorczyk, P. Elsbach, and J. Weiss. 1994. Effect of lipopolysaccharide (LPS) chain length on interactions of bactericidal/permeability-increasing protein and its bioactive 23-kilodalton NH2-terminal fragment with isolated LPS and intact Proteus mirabilis and Escherichia coli. Infect. Immun. 62:259-265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chakrabarti, S. R., K. Chaudhuri, K. Sen, and J. Das. 1996. Porins of Vibrio cholerae: purification and characterization of OmpU. J. Bacteriol. 178:524-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.DiRita, V. J. 1992. Co-ordinate expression of virulence genes by ToxR in Vibrio cholerae. Mol. Microbiol. 6:451-458. [DOI] [PubMed] [Google Scholar]
- 10.DiRita, V. J., M. Neely, R. K. Taylor, and P. M. Bruss. 1996. Differential expression of the ToxR regulon in classical and El Tor biotypes of Vibrio cholerae is due to biotype-specific control over toxT expression. Proc. Natl. Acad. Sci. USA 93:7991-7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DiRita, V. J., C. Parsot, G. Jander, and J. J. Mekalanos. 1991. Regulatory cascade controls virulence in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 88:5403-5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farris, M., A. Grant, T. B. Richardson, and C. D. O'Connor. 1998. BipA: a tyrosine-phosphorylated GTPase that mediates interactions between enteropathogenic Escherichia coli (EPEC) and epithelial cells. Mol. Microbiol. 28:265-279. [DOI] [PubMed] [Google Scholar]
- 13.Gazzano-Santoro, H., J. B. Parent, L. Grinna, A. Horwitz, T. Parsons, G. Theofan, P. Elsbach, J. Weiss, and P. J. Conlon. 1992. High-affinity binding of the bactericidal/permeability-increasing protein and a recombinant amino-terminal fragment to the lipid A region of lipopolysaccharide. Infect. Immun. 60:4754-4761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gray, B. H., and J. R. Haseman. 1994. Bactericidal activity of synthetic peptides based on the structure of the 55-kilodalton bactericidal protein from human neutrophils. Infect. Immun. 62:2732-2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagman, K. E., W. Pan, B. G. Spratt, J. T. Balthazar, R. C. Judd, and W. M. Shafer. 1995. Resistance of Neisseria gonorrhoeae to antimicrobial hydrophobic agents is modulated by the mtrRCDE efflux system. Microbiology 141:611-622. [DOI] [PubMed] [Google Scholar]
- 16.Hase, C. C., and J. J. Mekalanos. 1998. TcpP protein is a positive regulator of virulence gene expression in Vibrio cholerae. Proc. Natl. Acad. Sci. USA 95:730-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Heidelberg, J. F., J. A. Eisen, W. C. Nelson, R. A. Clayton, M. L. Gwinn, R. J. Dodson, D. H. Haft, E. K. Hickey, J. D. Peterson, L. Umayam, S. R. Gill, K. E. Nelson, T. D. Read, H. Tettelin, D. Richardson, M. D. Ermolaeva, J. Vamathevan, S. Bass, H. Qin, I. Dragoi, P. Sellers, L. McDonald, T. Utterback, R. D. Fleishmann, W. C. Nierman, and O. White. 2000. DNA sequence of both chromosomes of the cholera pathogen Vibrio cholerae. Nature 406:477-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heilpern, A. J., and M. K. Waldor. 2000. CTXφ infection of Vibrio cholerae requires the tolQRA gene products. J. Bacteriol. 182:1739-1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higgins, D. E., E. Nazareno, and V. J. DiRita. 1992. The virulence gene activator ToxT from Vibrio cholerae is a member of the AraC family of transcriptional activators. J. Bacteriol. 174:6974-6980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holmgren, J., A. M. Svennerholm, J. Clemens, D. Sack, R. Black, and M. Levine. 1987. An oral B subunit-whole cell vaccine against cholera: from concept to successful field trial. Adv. Exp. Med. Biol. 216B:1649-1660. [PubMed] [Google Scholar]
- 21.Hovde, C. J., and B. H. Gray. 1986. Physiological effects of a bactericidal protein from human polymorphonuclear leukocytes on Pseudomonas aeruginosa. Infect. Immun. 52:90-95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Iwanaga, M., K. Yamamoto, N. Higa, Y. Ichinose, N. Nakasone, and M. Tanabe. 1986. Culture conditions for stimulating cholera toxin production by Vibrio cholerae O1 El Tor. Microbiol. Immunol. 30:1075-1083. [DOI] [PubMed] [Google Scholar]
- 23.Kovach, M. E., K. J. Hughes, K. D. Everiss, and K. M. Peterson. 1994. Identification of a ToxR-activated gene, tagE, that lies within the accessory colonization factor gene cluster of Vibrio cholerae O395. Gene 148:91-95. [DOI] [PubMed] [Google Scholar]
- 24.Kroll, H. P., S. Bhakdi, and P. W. Taylor. 1983. Membrane changes induced by exposure of Escherichia coli to human serum. Infect. Immun. 42:1055-1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Levine, M. M., J. B. Kaper, R. E. Black, and M. L. Clements. 1983. New knowledge on pathogenesis of bacterial enteric infections as applied to vaccine development. Microbiol. Rev. 47:510-550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy, O. 2000. A neutrophil-derived anti-infective molecule: bactericidal/permeability-increasing protein. Antimicrob. Agents Chemother. 44:2925-2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li, C. C., D. S. Merrell, A. Camilli, and J. B. Kaper. 2002. ToxR interferes with CRP-dependent transcriptional activation of ompT in Vibrio cholerae. Mol. Microbiol. 43:1577-1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Little, R. G., D. N. Kelner, E. Lim, D. J. Burke, and P. J. Conlon. 1994. Functional domains of recombinant bactericidal/permeability increasing protein (rBPI23). J. Biol. Chem. 269:1865-1872. [PubMed] [Google Scholar]
- 29.Mannion, B. A., E. S. Kalatzis, J. Weiss, and P. Elsbach. 1989. Preferential binding of the neutrophil cytoplasmic granule-derived bactericidal/permeability increasing protein to target bacteria. Implications and use as a means of purification. J. Immunol. 142:2807-2812. [PubMed] [Google Scholar]
- 30.Mannion, B. A., J. Weiss, and P. Elsbach. 1990. Separation of sublethal and lethal effects of the bactericidal/permeability increasing protein on Escherichia coli. J. Clin. Investig. 85:853-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mekalanos, J. J. 1983. Duplication and amplification of toxin genes in Vibrio cholerae. Cell 35:253-263. [DOI] [PubMed] [Google Scholar]
- 32.Miller, V. L., and J. J. Mekalanos. 1988. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J. Bacteriol. 170:2575-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, V. L., R. K. Taylor, and J. J. Mekalanos. 1987. Cholera toxin transcriptional activator toxR is a transmembrane DNA binding protein. Cell 48:271-279. [DOI] [PubMed] [Google Scholar]
- 34.Parra-Lopez, C., R. Lin, A. Aspedon, and E. A. Groisman. 1994. A Salmonella protein that is required for resistance to antimicrobial peptides and transport of potassium. EMBO J. 13:3964-3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peterson, K. M., and J. J. Mekalanos. 1988. Characterization of the Vibrio cholerae ToxR regulon: identification of novel genes involved in intestinal colonization. Infect. Immun. 56:2822-2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Prohinar, P., S. A. Forst, D. Reed, I. Mandic-Mulec, and J. Weiss. 2002. OmpR-dependent and OmpR-independent responses of Escherichia coli to sublethal attack by the neutrophil bactericidal/permeability increasing protein. Mol. Microbiol. 43:1493-1504. [DOI] [PubMed] [Google Scholar]
- 37.Provenzano, D., and K. E. Klose. 2000. Altered expression of the ToxR-regulated porins OmpU and OmpT diminishes Vibrio cholerae bile resistance, virulence factor expression, and intestinal colonization. Proc. Natl. Acad. Sci. USA 97:10220-10224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Provenzano, D., C. M. Lauriano, and K. E. Klose. 2001. Characterization of the role of the ToxR-modulated outer membrane porins OmpU and OmpT in Vibrio cholerae virulence. J. Bacteriol. 183:3652-3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Provenzano, D., D. A. Schuhmacher, J. L. Barker, and K. E. Klose. 2000. The virulence regulatory protein ToxR mediates enhanced bile resistance in Vibrio cholerae and other pathogenic Vibrio species. Infect. Immun. 68:1491-1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qi, S. Y., Y. Li, A. Szyroki, I. G. Giles, A. Moir, and C. D. O'Connor. 1995. Salmonella typhimurium responses to a bactericidal protein from human neutrophils. Mol. Microbiol. 17:523-531. [DOI] [PubMed] [Google Scholar]
- 41.Salzman, N. H., M. M. Chou, H. de Jong, L. Liu, E. M. Porter, and Y. Paterson. 2003. Enteric Salmonella infection inhibits Paneth cell antimicrobial peptide expression. Infect. Immun. 71:1109-1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Simonet, V. C., A. Basle, K. E. Klose, and A. H. Delcour. 2003. The Vibrio cholerae porins OmpU and OmpT have distinct channel properties. J. Biol. Chem. 278:17539-17545. [DOI] [PubMed] [Google Scholar]
- 43.Sperandio, V., J. A. Giron, W. D. Silveira, and J. B. Kaper. 1995. The OmpU outer membrane protein, a potential adherence factor of Vibrio cholerae. Infect. Immun. 63:4433-4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stevenson, G., B. Neal, D. Liu, M. Hobbs, N. H. Packer, M. Batley, J. W. Redmond, L. Lindquist, and P. Reeves. 1994. Structure of the O antigen of Escherichia coli K-12 and the sequence of its rfb gene cluster. J. Bacteriol. 176:4144-4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weiss, J., P. Elsbach, I. Olsson, and H. Odeberg. 1978. Purification and characterization of a potent bactericidal and membrane active protein from the granules of human polymorphonuclear leukocytes. J. Biol. Chem. 253:2664-2672. [PubMed] [Google Scholar]
- 46.Zasloff, M. 2002. Antimicrobial peptides of multicellular organisms. Nature 415:389-395. [DOI] [PubMed] [Google Scholar]